Abstract
Status epilepticus is a clinical emergency that can lead to the development of acquired epilepsy following neuronal injury. Understanding the pathophysiological changes that occur between the injury itself and the expression of epilepsy is important in the development of new therapeutics to prevent epileptogenesis. Currently, no anti-epileptogenic agents exist; thus, the ability to treat an individual immediately after status epilepticus to prevent the ultimate development of epilepsy remains an important clinical challenge. In the Sprague–Dawley rat pilocarpine model of status epilepticus-induced acquired epilepsy, intracellular calcium has been shown to increase in hippocampal neurons during status epilepticus and remain elevated well past the duration of the injury in those animals that develop epilepsy. This study aimed to determine if such changes in calcium dynamics exist in the hippocampal culture model of status epilepticus-induced acquired epilepsy and, if so, to study whether manipulating the calcium plateau after status epilepticus would prevent epileptogenesis. The in vitro status epilepticus model resembled the in vivo model in terms of elevations in neuronal calcium concentrations that were maintained well past the duration of the injury. When used following in vitro status epilepticus, dantrolene, a ryanodine receptor inhibitor, but not the N-methyl-d-aspartic acid channel blocker MK-801 inhibited the elevations in intracellular calcium, decreased neuronal death and prevented the expression of spontaneous recurrent epileptiform discharges, the in vitro correlate of epilepsy. These findings offer potential for a novel treatment to prevent the development of epileptiform discharges following brain injuries.
Keywords: calcium dynamics, cultured hippocampal neurons, epileptogenesis, Fura-2, patch-clamp electrophysiology, rat
Introduction
Epilepsy is a common neurological condition that is characterized by spontaneous, recurrent seizures and affects 1–2% of the population worldwide (Hauser & Hesdorffer, 1990; Duncan et al., 2006). Acquired epilepsy (AE) is one form of epilepsy that occurs following a brain injury such as status epilepticus (SE), traumatic brain injury, stroke or infections of the central nervous system (Willmore, 1990; Delorenzo et al., 2005). AE represents approximately 40% of all cases of epilepsy (Hauser & Hesdorffer, 1990); thus, it remains an important public health concern.
The transformation of healthy brain tissue into hyperexcitable networks of neurons that manifest epileptiform discharges is called epileptogenesis. In order to better understand the development of AE, it can be described as the progression through three stages: the injury itself, a period of latency or epileptogenesis and finally the maintenance of chronic epilepsy (Klitgaard et al., 2002; Pitkanen et al., 2007). The period between the injury and the expression of epilepsy could provide a therapeutic window of opportunity wherein pharmacological agents could be administered to prevent epileptogenesis. Currently, only anti-epileptic drugs (AEDs) exist, which limit the occurrence of seizures once an individual has developed epilepsy. Thus, characterization of this process may help in elucidating potential targets to which anti-epileptogenic agents can be directed to prevent the process of epileptogenesis.
In the pilocarpine model of SE-induced AE, significant changes in intracellular Ca2+([Ca2+]i) and Ca2+ homeostatic mechanisms occur in hippocampal neurons following SE (Raza et al., 2004). As much as 1 year after SE, animals that demonstrated epilepsy showed significant elevations in hippocampal neuronal Ca2+ levels (Ca2+ plateau), in contrast to those animals that failed to develop epilepsy after SE (Raza et al., 2004). As a ubiquitous second messenger, changes in [Ca2+]i can affect everything from basic neurotransmission to gene transcription. Therefore, studying these alterations in Ca2+ and the underlying regulatory systems that control these changes is important not only in understanding plasticity but also in developing anti-epileptogenic strategies.
It was also demonstrated in the rat pilocarpine model of SE-induced AE that the N-methyl-d-aspartic acid receptors (NMDAR) are involved in initiating the Ca2+ plateau during SE (Raza et al., 2004). However, little is known regarding what systems contribute to the long-term maintenance of these elevations in [Ca2+]i and whether inhibiting this elevation after the injury could prevent epileptogenesis. We hypothesized that the Ca2+ -induced Ca2+ release (CICR) system was activated by the influx of Ca2+ into the cell through NMDAR. Ryanodine receptors (RyR), along with inositol triphosphate receptors, are part of the CICR mechanisms and control release of Ca2+ from intracellular stores. Therefore, these channels can either amplify or dampen the Ca2+ signal coming from outside the cell (Friel, 2004; Bardo et al., 2006). In these studies, dantrolene, a pharmacological inhibitor of RyR, was used to better elucidate the role of these receptors in the development of the Ca2+ plateau following in vitro SE.
Materials and methods
Reagents
All the reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise noted. Cell culture media were purchased from Invitrogen (Carlsbad, CA, USA).
Hippocampal neuronal culture preparation
All animal use procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Virginia Commonwealth University’s Institutional Animal Care and Use Committee. As described previously (Sombati & Delorenzo, 1995), hippocampal neurons were harvested from 2-day postnatal Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) and grown on a glial support layer that was previously plated onto poly-l-lysine- (0.05 mg / mL) coated plates. The cultures were maintained at 37°C in a 5% CO2 / 95% air atmosphere and were fed twice a week with minimal essential media containing Earle’s salts, 25 mm HEPES, 2 mm l-glutamine, 3 mm glucose, 100 µg / mL transferrin, 5 µg / mL insulin, 100 µm putrescine, 3 nm sodium selenite, 200 nm progesterone, 1 mm sodium pyruvate, 0.1% ovalbumin, 0.2 ng / mL triiodothyroxine and 0.4 ng / mL corticosterone supplemented with 20% conditioned media. Neurons were allowed to mature and all experiments were performed 14–18 days following plating of neurons to allow formation of networks, adequate neuronal maturation and development of NMDARs.
Whole-cell current clamp electrophysiology
Whole-cell current clamp electrophysiology was performed according to previously described procedures (Sombati & Delorenzo, 1995; Deshpande et al., 2008b). In short, the culture plates were transferred to the temperature-controlled stages of either a Nikon Diaphot inverted microscope (Garden City, NY, USA) or Olympus IX-70 (Olympus, Center Valley, PA, USA) in order to perform electrophysiological recordings. A Flaming-Brown P-80C electrode puller (Sutter Instruments, Sarasota, FL, USA) was utilized to pull patch microelectrodes with resistances of 3–7 MΩ from thin borosilicate glass capillaries (World Precision Instruments, Inc., Sarasota, FL, USA). These microelectrodes were filled with an internal solution consisting of (in mm): 140 K+ gluconate, 1 MgCl2, 10 HEPES and 1.1 ethylene glycol tetraacetic acid (EGTA), adjusted to pH 7.2, and osmolarity of 310 mOsm.
An Axopatch 200B amplifier (Molecular Devices, Foster City, CA, USA) in current clamp mode was used to obtain recordings from phase-bright pyramidal-shaped neurons. Together, the Neurocorder DR-890 (Neurodata Instruments Corp., New York, NY, USA) and Sony Videocassette Recorder allowed for digitization and storage of data on videotapes. Acquired data were then analyzed by playing recordings back through a DC-500 Hz chart recorder (Astro-Med Inc., Warwick, RI, USA) or to a computer via a Digidata 1322A (Molecular Devices). All the recordings were performed in the current-clamp mode at I = 0 setting. No current was injected at any stage of recording in the presence or absence of drugs.
Calcium microfluorometry
To determine [Ca2+]i in hippocampal neuronal cultures, cells were loaded with 1 µm Fura-2-acetoxymethyl ester (AM) (Molecular Probes, Eugene, OR, USA) for 30 min at 37°C in 5% CO2 / 95% O2, as described previously (Nagarkatti et al., 2008). The plates were then transferred to a temperature-controlled stage (Harvard Apparatus, Holliston, MA, USA) on an Olympus IX-70 inverted microscope. Cultures were visualized using a 20 ×, 0.7 numerical aperture fluorite water-immersion objective (Olympus America). Fura-2 ratios were obtained from populations of neurons within a single field. We routinely imaged 5–10 neurons within a certain field. Neurons were easily distinguishable from glia in our culture preparation. They appeared phase-bright, had distinct pyramidal shape and were present just above the focal plane of the glial cell layer. In addition, fluorescence in neurons was more intense than in the glial cells, allowing clear resolution between glia and neurons. Only such clearly identifiable neurons were selected for our experiments. The region of interest within each neuron was restricted to neuronal soma and for the purpose of this paper dendritic signal was not analysed. Ratio images were acquired using alternating excitation wavelengths (340 and 380 nm) with a filter wheel (Sutter Instruments, Novato, CA, USA) and fura filter cube at 510 / 540 nm emissions with a dichroic mirror at 400 nm. Thus, the resulting 340 / 380 ratio recorded corresponds directly to the total concentration of Ca2+ inside the cell and can be used to determine changes in [Ca2+]i. The utilization of the 340 / 380 ratio is important in that it accounts for potential confounders such as unequal loading for Fura-2AM or variable cell thickness from interfering with the measurements. This system is connected to the fluorescent imaging software, MetaFluor (Olympus), which allows for image acquisition and analysis. Images were acquired from any given field at various time intervals depending on the specific experiment and background fluorescence was subtracted from all acquired data by imaging hippocampal neuronal cultures that were not loaded with Fura-2AM.
The AM ester form of Fura-2 is widely used by investigators to monitor Ca2+ in neurons. Although the AM ester form has the distinct advantage of enabling loading of multiple neurons simultaneously while avoiding delicate microelectrode loading, it also has certain disadvantages such as incomplete hydrolysis and sequestration in intracellular organelles (Roe et al., 1990). To overcome these issues, Fura-2 is loaded in a solution free of primary and secondary amines (in pBRS – see composition below). The washout of indicator is extensive using the pBRS solution and the final imaging is also performed in the same solution. Neuronal preparations are routinely checked for any fluorescence discrepancies by incorporating Pluronic® F-127 and probenecid (2.5 mm) during the loading process and also monitoring loading temperature (34–37°C). We have not observed any significant differences in our fluorescence ratios under our experimental conditions and expect minimal contributions of ester dye anomaly. Intracellular pH was not monitored in this study, but we have previously reported that the changes observed in [Ca2+]i due to spontaneous, recurrent epileptiform discharges (SREDs) were not due to changes in intracellular pH affecting the fluorescence ratio (Sun et al., 2001). Furthermore, recent studies have shown that redox transformations of the Fura-2 forms do not affect the binding ability for Ca2+ ions and thus do not interfere with [Ca2+]i measurements (Gulaboski et al., 2008).
In vitro SE and SREDs in hippocampal neuronal cultures
In vitro SE was generated using a low Mg2+-containing solution (Sombati & Delorenzo, 1995; McNamara et al., 2006). Hippocampal neuronal culture media was replaced with physiological basal recording solution (pBRS) containing (in mm): 145 NaCl, 2.5 KCl, 10 HEPES, 2 CaCl2, 10 glucose, 1 MgCl2 and 0.002 glycine, pH 7.3, and osmolarity adjusted to 325 ± 5 mOsm with sucrose or pBRS without any added MgCl2 (referred to hereafter as low Mg2+). The cells were then incubated at 37°C under 5% CO2 / 95% O2 atmosphere for 3 h. During this time, neurons in low Mg2+ demonstrate high-frequency spiking, characterized as in vitro SE. Neurons treated with pBRS for 3 h served as sham-controls.
After 3 h of low Mg2+ or pBRS treatment, the cells were returned to normal, Mg2+-containing maintenance media and were evaluated using whole-cell current clamp electrophysiology at least 12 h after in vitro SE to determine expression of SREDs, the in vitro correlate to epilepsy. Each episode of SREDs is characterized by paroxysmal depolarization shifts and, generally, 5–9 SREDs are observed per hour. These SREDs last for the lifetime of the culture. We did not observe any significant differences in neuronal densities between cultures from different weeks. In addition, as reported by us previously, although in vitro SE is associated with cell death, the majority of cell death occurs immediately following SE and ongoing SREDs do not contribute to increased cell death (Deshpande et al., 2007b, 2008a).
Cell death assay
Neuronal death was assessed using propidium iodide (PI) as well as Annexin V conjugated to fluorescein isothiocyanate (FITC) (Deshpande et al., 2007b). PI binds DNA in membrane-compromised neurons, whereas Annexin V binds phosphotidylserines that translocate to the outer cell membrane in the early stages of apoptosis. Several randomly selected fields from each plate were marked and both phase and fluorescent images were captured using MetaMorph (Olympus) at various time points after in vitro SE. Fraction of neuronal death was calculated as the number of neurons labeled with only Annexin V plus the number of neurons labeled with Annexin V and PI, divided by the total number of neurons. The data were subjected to an outlier test wherein all values outside of two standard deviations from the mean were considered outliers.
Data analyses
Data are expressed as mean ± SEM. To determine significance between treatment groups for Ca2+ imaging, SREDs and neuronal death assays, Student’s t-test or one-way anova tests were employed, followed by Fisher’s post-hoc test when appropriate. Calcium decay curves were constructed by normalizing data to peak calcium ration at time t = 0. The decay curves were fitted with an exponential decay function ([y = a*exp(b / (x+c))]). Corresponding parameter estimates and 95% confidence intervals were also calculated. For the Ca2+ imaging and cell death experiments, at least six plates were used per treatment group. For electrophysiology experiments, 2–3 neurons are patch-clamped from each plate with a minimum of 10 plates used for each treatment group. All experiments were performed over the period of several weeks so that the results were representative of multiple cultures. A P-value < 0.05 was considered significant. Statistical analysis was performed using StatView 5.0.1 and Statistical Analysis Software 9.1 (SAS Institute, Cary, NC, USA). Graphs were drawn using SigmaPlot 11 (Systat Software, San Jose, CA, USA).
Results
In vitro SE causes significant, long-lasting elevations in [Ca2+]i (Ca2+ plateau)
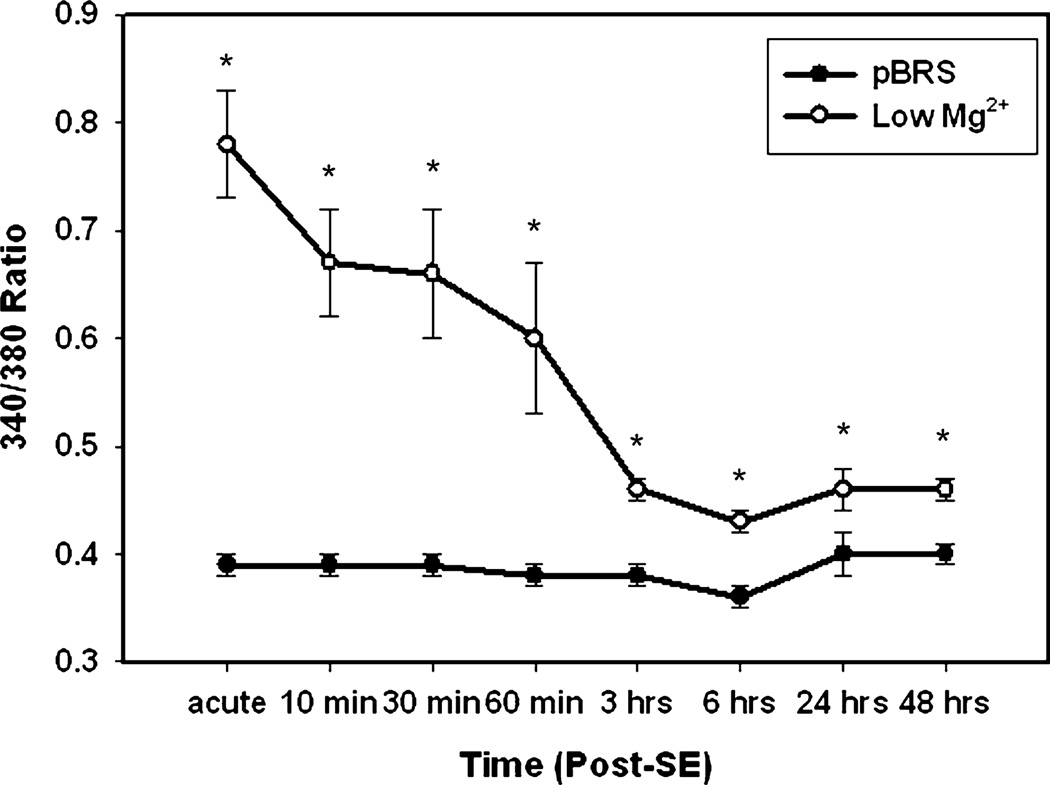
Neurons were treated for 3 h with either low Mg2+ to simulate in vitro SE or pBRS as a sham control, in accordance with previously described methodology (Sombati & Delorenzo, 1995). Sham-control neurons displayed 340 / 380 ratio values of 0.33 ± 0.02, whereas neurons treated with low Mg2+ displayed ratio values of 0.78 ± 0.05 acutely at the end of this 3 h treatment (Fig. 1). In order to end in vitro SE, MgCl2 was restored to these cultures by washing them with pBRS. The same neurons were imaged for the first hour after restoration of MgCl2. Within 10 min of restoring the MgCl2 to the low Mg2+ -treated plates, the ratio dropped from 0.78 ± 0.01 to 0.67 ± 0.05. At 30 min after in vitro SE, the ratios fell slightly to 0.66 ± 0.06 and at 60 min to 0.60 ± 0.07. At the 3- and 6-h time points, the ratios had dropped further to 0.46 ± 0.01 and 0.43 ± 0.01, respectively. Finally, at 24 and 48 h after in vitro SE, the low Mg2+-treated cells exhibited ratios of 0.46 ± 0.02 and 0.46 ± 0.01, respectively. There was no significant difference in ratios between the sham control neurons that were treated with pBRS at any of the time points tested. The recorded ratios in the low Mg2+ -treated group were significantly elevated when compared with the controls at each of the time points tested (Fig. 1, *P < 0.05; Student’s t-test, n ≥ 6 plates at each time point tested with ~60 neurons imaged per treatment group). This suggests that low Mg2+ -induced in vitro SE triggers long-lasting elevations in [Ca2+]i, as evidenced by maintenance of increased ratios for at least 48 h.
Fig. 1.
In vitro SE causes significant, long-lasting elevations in [Ca2+]i (Ca2+ plateau). Time course comparing sham controls treated with pBRS (●) vs. neurons treated with low Mg2+ (○) at the end of in vitro SE (acute) and at various time points following in vitro SE (10, 30, 60 min, 3, 6, 24, 48 h). All data represented as average 340 / 380 ratio ± SEM. *P < 0.05, Student’s t-test, n = 6 or more plates with at least 60 neurons imaged per condition at each time point studied.
Dantrolene lowers [Ca2+]i to baseline levels following in vitro SE
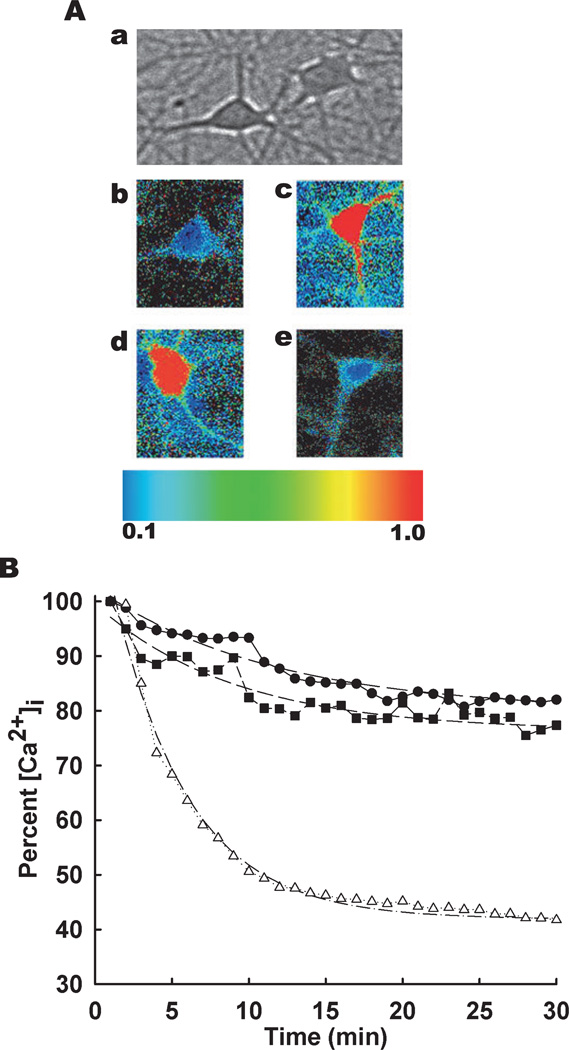
We next tested whether the persistent elevations in [Ca2+]i following in vitro SE were mediated by Ca2+ entry from NMDA channel or release of Ca2+ from intracellular stores. MK-801, an inhibitor of NMDAR, and dantrolene, an inhibitor of RyR, were utilized in separate experiments immediately after in vitro SE to determine whether their inhibition could lower the elevated [Ca2+]i. To test this, hippocampal neuronal cultures were treated for 3 h with low Mg2+ and were then loaded with Fura-2AM. Following 3 h of in vitro SE (time 0 on x-axis, Fig. 2), neurons showed elevations in 340 / 380 ratios that were normalized to the peak ratio. These neurons were then treated with either vehicle (0.1% DMSO) or MK-801 (10 µm) or dantrolene (50 µm). At 5 min post-treatment, vehicle-treated cells showed a slight decrease in 340 / 380 ratio; in contrast, dantrolene-treated cells exhibited 340 / 380 ratios that were 47% of the peak observed at the end of in vitro SE. Ten minutes after treatment, the 340 / 380 ratio values were 83 and 43% of the post-SE peak in the vehicle- and dantrolene-treated groups, respectively. At 15 min post-treatment, the 340 / 380 ratio values were 82 and 40% of the post-SE peak in the vehicle- and dantrolene-treated groups, respectively. In the cells that were exposed to dantrolene after the low Mg2+ treatment, [Ca2+]i returned to the baseline levels observed in the sham controls within 30 min. In contrast, the MK-801-treated group showed elevated [Ca2+]i similar to the vehicle-treated group at all time points tested. The 340 / 380 ratios fell by only 18% over the first 20 min following in vitro SE, consistent with the data reported in Fig. 1. There was a significant drop in the ratios between the dantrolene- and vehicle-treated groups (Table 1) with the dantrolene-treated group showing marked enhancement in the ability to rapidly lower the [Ca2+]i (n = 6 plates were used per treatment group with ~60 neurons imaged in total per condition).
Fig. 2.
Dantrolene lowers [Ca2+]i to baseline levels following in vitro SE. (A) Pseudocolor ratiometric images of representative neurons in culture. (a) Phase bright reference image depicting typical pyramidal-shaped hippocampal neurons that lay above the focal plane of glia. (b–e) Representative ratiometric (340 / 380) images of neurons from control (b), low Mg2+ (c), MK-801 (d) and dantrolene (e) treated cultures. All the ratiometric images are at the 30-min time point following respective treatments. (B) Following 3 h of in vitro SE (time = 0), cells were treated with vehicle (0.1% DMSO, ●), MK 801 (10 µm, ■) or dantrolene (50 µm, Δ). 340 / 380 ratios were recorded every 30 s for 30 min and normalized to percentage of the peak ratio observed at time = 0. Dotted lines represent data fitted using exponential decay function [y = a*exp(b / (x+c))] using parameter estimates reported in Table 1. n = 6 plates per treatment group with ~60 neurons imaged per condition.
Table 1.
Parameter estimates and corresponding confidence intervals for in vitro status epilepticus (SE) alone and MK-801 and dantrolene treatments
| Parameter* | Estimate | 95% confidenceinterval limits |
|---|---|---|
| Low Mg2+ SE | ||
| a | 22.583 ± 0.9369 | 20.683–24.483 |
| b | 0.0885 ± 0.0102 | 0.0677–0.1092 |
| c | 79.979 ± 0.6863 | 78.587–81.37 |
| SE + MK 801 | ||
| a | 23.233 ± 1.5574 | 20.074–26.391 |
| b | 0.111 ± 0.0169 | 0.0768–0.1453 |
| c | 76.323 ± 0.7593 | 74.783–77.863 |
| SE + dantrolene | ||
| a | 76.257 ± 2.0577 | 72.084–80.43 |
| b | 0.2046 ± 0.0086 | 0.1872–0.2219 |
| c | 41.896 ± 0.412 | 41.06–42.732 |
Estimates are expressed as mean ± SEM.
Calcium decay curves were constructed by normalizing data to peak calcium ration at time t = 0 and fitted with an exponential decay function ([y = a*exp(b / (x+c))]).
We then used first-order decay kinetics to fit the data for each treatment condition. The predicted functions are plotted as solid lines in Fig. 2. Parameter estimates and corresponding confidence intervals were obtained. The 95% confidence intervals did not overlap between the SE alone and dantrolene treatment groups but did overlap between the SE alone and MK-801 treatment groups. This suggested that dantrolene treatment but not MK-801 treatment was significantly different from the SE alone condition (Table 1). This rigorous statistical analysis confirms our results that intervention with dantrolene alone and not MK-801 rapidly lowers the elevated [Ca2+]i levels following SE, thus suggesting that RyR and not NMDA receptors play a role in mediating the post-SE elevations in [Ca2+]i.
In vitro SE is not inhibited by dantrolene
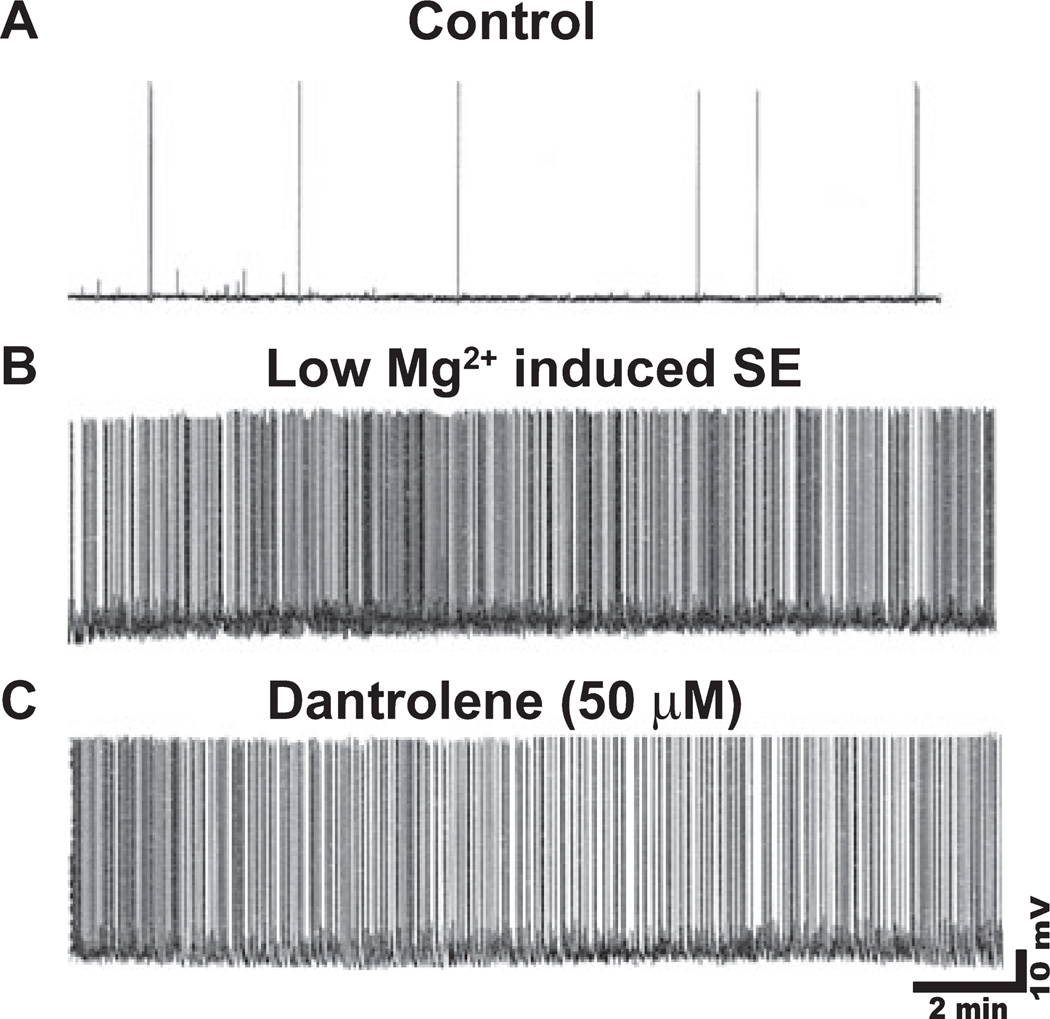
We next investigated if the ability of dantrolene to lower post-SE elevations in [Ca2+]i was due to its effect on SE-like activity. Using whole-cell current clamp electrophysiology, we observed that naïve, cultured hippocampal neurons exhibited infrequent spontaneous action potentials while in pBRS (Fig. 3A, n = 15 neurons). Upon replacing the culture media with low Mg2+, neurons demonstrated continuous, high-frequency epileptiform discharges (Fig. 3B, n = 15 neurons). The spike frequency observed in cultures treated with low Mg2+ was greater than 3 Hz. We observed that treatment with dantrolene (50 µm) failed to slow down the low Mg2+ -induced high-frequency spiking (Fig. 3C, n = 15 neurons). There was no significant difference in spike frequency between neurons treated with low Mg2 + plus dantrolene vs. low Mg2+ alone; this suggests that dantrolene is unable to inhibit low Mg2+ -induced in vitro SE. In addition, no difference in resting membrane potential or mean input resistance was observed between treatment groups. Neurons treated with low Mg2+ and low Mg2+ plus dantrolene had mean membrane potentials of −63.4 ± 1.6 and −62.8 ± 1.4 mV and mean input resistances of 140.5 ± 7.3 and 138.8 ± 6.5 MΩ, respectively. These values were not different from each other (n = 10 neurons, Student’s t-test, P = 0.7 and 0.6, respectively). Thus, the observation that dantrolene lowered post-SE elevations in [Ca2+]i does not appear to be attributable to its effect on SE spike frequency.
Fig. 3.
In vitro SE is not inhibited by dantrolene. Representative current-clamp trace from control neuron showing occasional action potentials (A), neuron in low Mg2+ or in vitro SE showing high-frequency spiking (B), and neuron in low Mg2+ plus dantrolene (50 µm) showing no effects of dantrolene on high-frequency spiking (C). n = 15 culture plates.
Dantrolene maintains baseline Ca2+ levels 24 and 48 h after SE
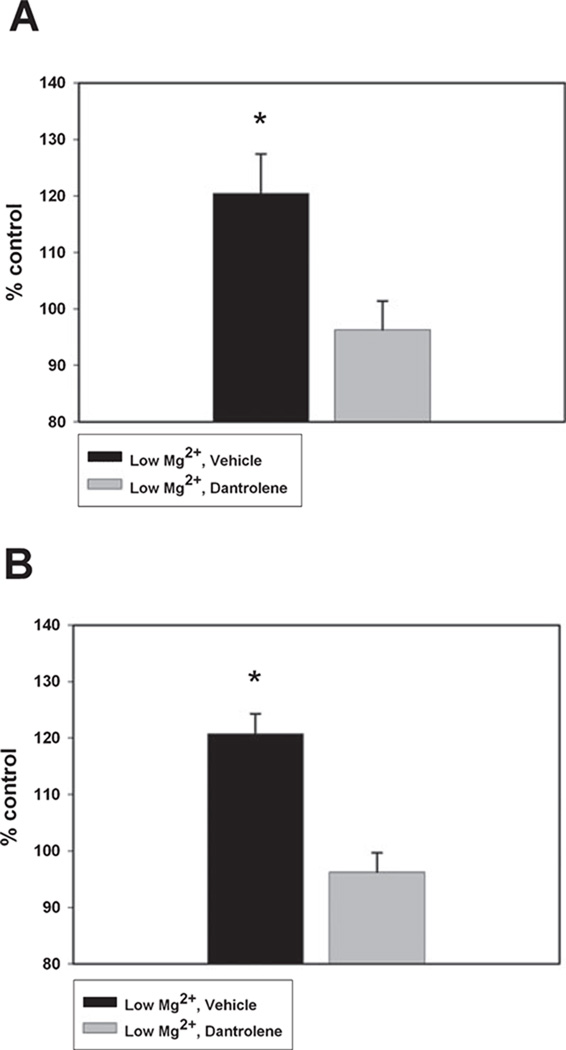
In order to evaluate whether the dantrolene-mediated changes in [Ca2+]i observed acutely were long-lasting, we exposed neurons to 3 h of low Mg2+ followed by dantrolene (50 µm) or vehicle (0.1% DMSO). Dantrolene was removed after 12 h and the cells were analysed 24 and 48 h later. As shown in Fig. 4, neurons treated with low Mg2+ and then vehicle exhibited significantly elevated 340 / 380 ratios when compared with the control, and these values were consistent with data collected in Fig. 1. In neurons treated with low Mg2+ and then dantrolene, 340 / 380 ratios were significantly lower than vehicle-treated neurons and not significantly different from those observed in the sham control. Thus, as shown in Fig. 4A, 24 h after SE, dantrolene was able to maintain [Ca2+]i at baseline levels (96.25 ± 5.13% of the control). By contrast, hippocampal neuronal cultures treated with low Mg2+ and then vehicle demonstrated significant elevations in [Ca2+]i (120.47 ± 6.95% of the control; *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 10 or more plates with ~100 cells imaged per treatment group).
Fig. 4.
Dantrolene maintains baseline Ca2+ levels 24 and 48 h after SE. Following in vitro SE, the effect of dantrolene (50 µm) (gray bar) vs. vehicle (black bar) on 340 / 380 ratios 24 h after treatment (A) and 48 h after treatment (B). Data represented as percentage control ± SEM. *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 10 or more plates with ~100 cells imaged per treatment group for 24-h time point; n = 6 plates with ~60 cells imaged per treatment group at 48-h time point.
At 48 h post-treatment (Fig. 4B), [Ca2+]i in the dantrolene-treated neurons was comparable with concentrations observed in controls (96.23 ± 3.45% of the control). In contrast, neurons treated with low Mg2+ and then vehicle showed significant elevations in 340 / 380 ratios compared with both the drug-treated and the control neurons (120.74 ± 3.58% of the control; *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 6 plates with ~60 cells imaged per treatment group). Therefore, dantrolene appears to be able to maintain [Ca2+]i for at least 48 h after in vitro SE at the baseline concentrations observed in control neurons.
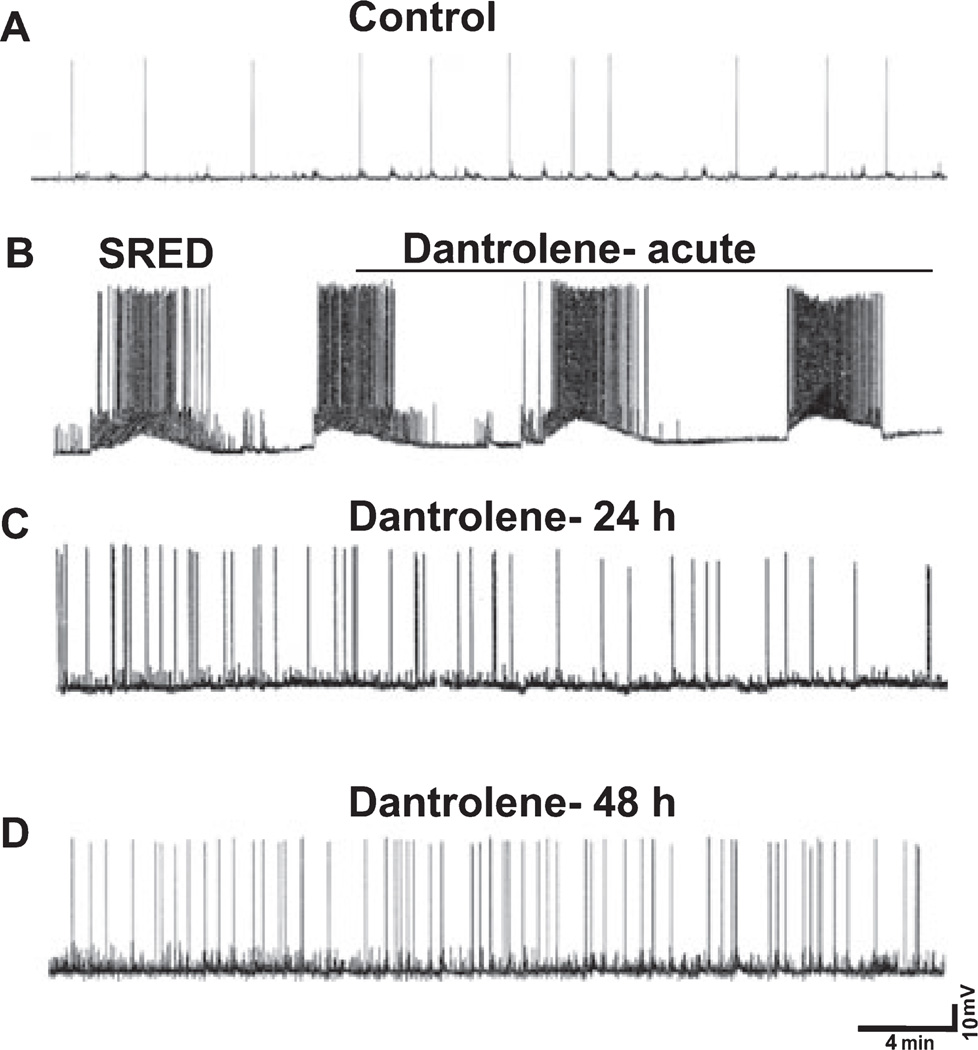
Dantrolene prevents the development of SREDs 24 and 48 h after SE but does not inhibit SREDs acutely
Also at 24 and 48 h after in vitro SE, neurons were patched using current-clamp electrophysiology to evaluate the expression of SREDs. Control neurons demonstrated ‘normal’ activity consisting of sporadic firing of action potentials (Fig. 5A). In contrast, 84 and 100% of neurons treated with low Mg2+ showed SREDs at 24 and 48 h after in vitro SE, respectively. A representative trace is shown that highlights the characteristics of the observed SREDs (Fig. 5B). These episodes consisted of paroxysmal depolarization shifts and occurred at a frequency of 5–8 episodes per hour with each SRED lasting 1–3 min. Neurons from the plates that were treated with low Mg2+ followed by dantrolene showed a marked inhibition in the expression of SREDs 24 h (Fig. 5C) and 48 h (Fig. 5D) after treatment. Although some hyperexcitability is noted in dantrolene-treated neurons, this excitability does not resemble epileptiform discharges that are characterized by repetitive burst discharges with each burst comprising multiple spikes overlying a depolarization shift. Only 10 and 20% of neurons showed SREDs at 24 and 48 h after SE, respectively. Of this small percentage, the SRED frequency was 1.7 /h at the 24-h time point and 1.9 /h at the 48-h time point, a marked reduction when compared with the SRED frequency observed in cells treated with low Mg2+ but no drug (n = 12–15 culture plates with a minimum of three neurons recorded from each plate).
Fig. 5.
Dantrolene prevents the development of SREDs 24 and 48 h after SE but does not inhibit SREDs acutely. Representative current-clamp trace from control neuron (A), low Mg2+ -treated neuron displaying characteristic SREDs (B), and neurons treated with low Mg2+ then dantrolene (50 µm) at both 24 h (C) and 48 h (D) after treatment and were devoid of SREDs. Dantrolene did not inhibit SRED activity when added acutely to neurons displaying SREDs (n = 12–15 culture plates with a minimum of threee neurons recorded from each plate).
To test for potential anti-convulsant properties, we studied whether acutely adding dantrolene (50 µm) to neurons already demonstrating SREDs could inhibit these episodes. Dantrolene did not inhibit SREDs when administered acutely (line over trace in Fig. 5B); in fact, there was no change in the frequency or duration of the SREDs (n = 10 culture plates). Neurons displaying SREDs and dantrolene-treated neurons had mean membrane potentials of −61.2 ± 2.1 and −60.9 ± 1.8 mV and mean input resistances of 139.6 ± 8.2 and 137.7 ± 8.6 MΩ, respectively. These values were not different from each other (n = 6 neurons, Student’s t-test, P = 0.8 and 0.7, respectively).
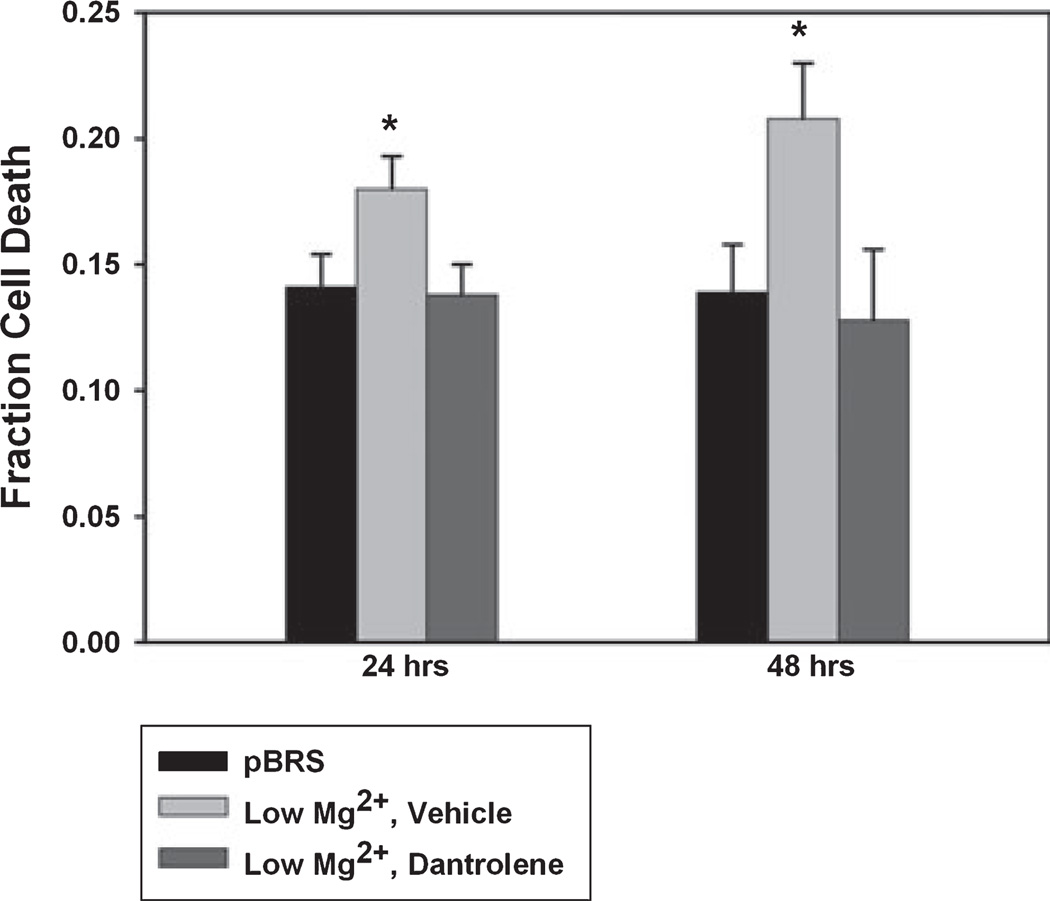
Dantrolene is neuroprotective
Although several studies have demonstrated in other models of epilepsy that dantrolene demonstrates some neuroprotective properties (Niebauer & Gruenthal, 1999; Hernandez-Fonseca & Massieu, 2005; Mori et al., 2005; Muehlschlegel & Sims, 2009), we wanted to evaluate whether it could be neuroprotective when given after the SE-related injury in the hippocampal culture model. We treated cells with low Mg2+ for 3 h followed by dantrolene or vehicle and determined cell viability 24 and 48 h later. Cell viability was ascertained using PI and Annexin V-FITC. At the 24-h time point, sham control cultures that had been subjected to 3 h of pBRS instead of low Mg2+ had a fraction of cell death of 0.141 ± 0.013. Cultures exposed to 3 h of low Mg2+ and then vehicle had an average cell death fraction of 0.180 ± 0.013, whereas neurons treated with low Mg2+ and then dantrolene (50 µm) had an average cell death fraction of 0.138 ± 0.012 (Fig. 6, *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 7 or more plates per treatment group). This demonstrated that at 24 h post-treatment with low Mg2+ or pBRS, dantrolene was able to reduce cell death to levels observed in the sham controls. In contrast, the low Mg2+ –vehicle neurons showed significantly more cell death than the sham or drug-treated group.
Fig. 6.
Dantrolene is neuroprotective. Cell death was assessed at 24 (A) and 48 (B) h after treatment with pBRS (sham, black bars), low Mg2+ then vehicle (0.1% DMSO) (low Mg2+, gray bars), and low Mg2+ then dantrolene (50 µm) (dantrolene, white bars). No significant difference was observed between the sham and drug-treated group at either of the time points. Low Mg2+ neurons demonstrated significantly more cell death than the other two groups at both 24 and 48 h. Data represented as mean fraction cell death ± SEM. *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 7 or more plates per treatment and time point.
Data were also collected 48 h after SE. There was no significant difference in cell death fraction between the sham and low Mg2+ plus dantrolene groups; however, low Mg2+ plus vehicle neurons showed a significant increase from the other two groups. Fraction of cell death was 0.139 ± 0.019, 0.208 ± 0.022 and 0.128 ± 0.028 in the sham, low Mg2+ plus vehicle, and low Mg2+ plus dantrolene neurons, respectively (Fig. 6, *P < 0.05, one-way anova, Fisher’s post-hoc test, n = 7 or more plates per treatment group). Thus, these results showed that dantrolene-treated neurons exhibited decreased fraction cell death, which suggests that dantrolene may be neuroprotective when administered after in vitro SE.
Discussion
This study demonstrated that neuronal injuries such as SE can cause an increase in [Ca2+]i that is sustained well past the duration of the injury itself in the hippocampal neuronal culture model of SE-induced AE. Entry of Ca2+ through the NMDAR channel does not underlie maintenance of this Ca2+ plateau. However, dantrolene, an RyR inhibitor, is able both to lower [Ca2+]i to levels observed in control neurons and to prevent the development of SREDs when used after in vitro SE. The observation that an injury-triggered, sustained elevation in [Ca2+]i (the Ca2+ plateau) exists in vitro correlates closely to findings reported in Raza et al. (2004). This group demonstrated in the rat pilocarpine model of SE-induced AE that hippocampal neuronal [Ca2+]i increases during injury and remains elevated only in those animals that later develop epilepsy (Raza et al., 2004). This study suggests that changes in [Ca2+]i and Ca2+ homeostatic mechanisms lead to a Ca2+ plateau, which may be a pathophysiological characteristic of neuronal injury and epileptogenesis. Understanding various cellular and molecular changes that occur either during or following neuronal injuries is important in developing new therapeutics to prevent the ultimate development of AE. Therefore, the finding that the changes in [Ca2+]i that were observed in vivo are also seen in vitro suggests that this culture model may provide a useful tool in screening various pharmacological agents and performing new molecular modulations in order to prevent epileptogenesis.
Much of what is currently known regarding the role of RyR in epilepsy is based on studies using dantrolene as a prophylactic neuroprotective agent before injury. In agreement with these studies, our results have shown that intervention with dantrolene after in vitro SE affords neuroprotection. High concentrations of dantrolene are reported to block NMDAR (Obenaus et al., 1989; Salinska et al., 2008). Thus, there is a possibility of NMDAR antagonism contributing to the neuroprotective effects of dantrolene observed at 24 and 48-h after in vitro SE. However, such an effect is not significant at the acute time point as intervention with NMDAR blocker MK-801 failed to lower elevated [Ca2+]i immediately following 3 h of in vitro SE. Our data suggest that RyR, a regulator of CICR, may contribute to the maintenance of the Ca2+ plateau. It has been demonstrated that animals pre-treated with dantrolene require significantly greater stimuli to generate seizures (Yoshida & Sakai, 2006). Moreover, after the animals are kindled, during the chronic phase of epilepsy, dantrolene has no anti-convulsant effects (Kulak et al., 2004; Yoshida & Sakai, 2006). In agreement with this, our studies have shown that dantrolene does not block SREDs when administered after in vitro SE. Thus, although such studies have suggested that treatment with dantrolene could provide tremendous benefits when administered before the inciting injury, there is still a need for studies to examine its effects when administered after the injury, as this would be more clinically relevant.
The in vitro model utilizing low Mg2+ to elicit SE-like activity and ultimately SREDs is a commonly used model to study various characteristics of both SE and epilepsy such as pharmacoresistance, plasticity changes, mechanisms of epileptogenesis and cell death (McNamara et al., 2006; Deshpande et al., 2007a, 2007b). The model exhibits several features consistent with the development of AE in both animal models and the clinical condition, which make it a useful tool in elucidating the underlying mechanisms of epileptogenesis and seizures (Sombati & Delorenzo, 1995; Deshpande et al., 2007b). Moreover, the cell injury observed in the in vitro and in vivo models correspond to that seen in clinical studies documenting the relationship between SE and neuronal injury (DeGiorgio et al., 1992; Sloviter, 1999; Tsuchida et al., 2007). It should be noted that the hippocampal neuronal cultures do not have the true anatomical connections and normal neuronal circuitry maintained in slice preparations or the intact brain. In addition, there are clear differences between the in vitro model of epileptogenesis and the human condition, including the duration of latency between SE and development of epilepsy, percentage of SE cases that develop epilepsy and co-morbidities associated with the pathology (Pitkanen, 2004). However, the cultures are an essential tool in the development of new drugs and the study of molecular mechanisms because they provide a controlled environment to do high-throughput screenings and are more easily manipulated for molecular studies.
Although currently there are several AEDs, there are no anti-epileptogenic drugs. Such therapeutic agents that can be administered immediately following injury so as to prevent long-term sequelae, namely the development of AE, would be extremely important clinically. In this study, we demonstrated that dantrolene, when given after SE, inhibited the Ca2+ plateau and prevented the development of SREDs in the in vitro low Mg2+ model. Our results also highlighted that dantrolene did not stop SE or serve as an anti-convulsant but is neuroprotective in the low Mg2+ model even when used following the neuronal injury. The ability to offer neuroprotection following a neuronal injury is an important strategy to prevent the development of injury-related chronic neurological conditions (Acharya et al., 2008; Dykstra et al., 2009). The results from this study may have tremendous clinical implications and studies have been initiated to determine if the drug lowers [Ca2+]i and demonstrates anti-epileptogenic properties in vivo. The pharmacological modulation of Ca2+ in vivo is a challenge in that the ubiquitous nature of this ion suggests that systemic inhibition would potentially have numerous side effects (Nagarkatti et al., 2009). However, a recent clinical trial has utilized a Ca2+ chelating agent in patients following stroke and the results have indicated that not only are stroke outcome scores improved, but the patients experienced no significant negative cardiovascular or neurological side effects (Rosenberg et al., 2005; Diener et al., 2008).
Thus, this study is the first to demonstrate the novel finding that in vitro SE causes long-lasting elevations in [Ca2+]i, which can be lowered after injury by treatment with dantrolene. More studies are needed to better understand and characterize the post-SE Ca2+ plateau, as this may be a useful tool in the development of anti-epileptogenic targets. Lowering the post-SE Ca2+ plateau may prevent the second messenger effects that lead to epileptogenesis and the development of chronic epilepsy.
Acknowledgements
We thank Elisa Attkisson for her help with preparation and maintenance of neuronal cultures. We also thank Dr Viswanathan Ramakrishnan for his help with the statistics. This study was supported by the National Institute of Neurological Disorders and Stroke Grants RO1NS051505 and RO1NS052529, and award UO1NS058213 from the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke to R.J.D. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. In addition, the Milton L. Markel Alzheimer’s Disease Research Fund and the Sophie and Nathan Gumenick Neuroscience Research Fund also funded this work. In addition, a pre-doctoral fellowship from the American Heart Association to N.N. funded this research.
Abbreviations
- AE
acquired epilepsy
- AED
anti-epileptic drug
- [Ca2+]i
intracellular calcium concentration
- CICR
calcium-induced calcium release
- Fura-2 AM
Fura-2 acetoxymethyl ester
- NMDA
N-methyl-d-aspartic acid
- pBRS
physiological basal recording solution
- RyR
ryanodine receptor
- SE
status epilepticus
- SRED
spontaneous recurrent epileptiform discharge
References
- Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog. Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol. Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Nagarkatti N, Sombati S, Martin BR, DeLorenzo RJ. Development of pharmacoresistance to benzodiazepines but not cannabinoids in the hippocampal neuronal culture model of status epilepticus. Exp. Neurol. 2007a;204:705–713. doi: 10.1016/j.expneurol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Lou JK, Mian A, Blair RE, Sombati S, DeLorenzo RJ. In vitro status epilepticus but not spontaneous recurrent seizures cause cell death in cultured hippocampal neurons. Epilepsy Res. 2007b;75:171–179. doi: 10.1016/j.eplepsyres.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Lou JK, Mian A, Blair RE, Sombati S, Attkisson E, DeLorenzo RJ. Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: role of NMDA receptor activation and NMDA dependent calcium entry. Eur. J. Pharmacol. 2008a;583:73–83. doi: 10.1016/j.ejphar.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Nagarkatti N, Ziobro JM, Sombati S, Delorenzo RJ. Carisbamate prevents the development and expression of spontaneous recurrent epileptiform discharges and is neuroprotective in cultured hippocampal neurons. Epilepsia. 2008b;49:1795–1802. doi: 10.1111/j.1528-1167.2008.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Schneider D, Lampl Y, Bornstein NM, Kozak A, Rosenberg G. DP-b99, a membrane-activated metal ion chelator, as neuroprotective therapy in ischemic stroke. Stroke. 2008;39:1774–1778. doi: 10.1161/STROKEAHA.107.506378. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Dykstra CM, Ratnam M, Gurd JW. Neuroprotection after status epilepticus by targeting protein interactions with postsynaptic density protein 95. J. Neuropathol. Exp. Neurol. 2009;68:823–831. doi: 10.1097/NEN.0b013e3181ac6b70. [DOI] [PubMed] [Google Scholar]
- Friel D. Interplay between ER Ca2+ uptake and release fluxes in neurons and its impact on [Ca2+] dynamics. Biol. Res. 2004;37:665–674. doi: 10.4067/s0716-97602004000400024. [DOI] [PubMed] [Google Scholar]
- Gulaboski R, Pereira CM, Cordeiro MN, Silva AF, Hoth M, Bogeski I. Redox properties of the calcium chelator Fura-2 in mimetic biomembranes. Cell Calcium. 2008;43:615–621. doi: 10.1016/j.ceca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. New York: Demos; 1990. [Google Scholar]
- Hernandez-Fonseca K, Massieu L. Disruption of endoplasmic reticulum calcium stores is involved in neuronal death induced by glycolysis inhibition in cultured hippocampal neurons. J. Neurosci. Res. 2005;82:196–205. doi: 10.1002/jnr.20631. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Wojtal K, Czuczwar SJ. Calcium modulation in epilepsy. Pol. J. Pharmacol. 2004;56:29–41. [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- Mori F, Okada M, Tomiyama M, Kaneko S, Wakabayashi K. Effects of ryanodine receptor activation on neurotransmitter release and neuronal cell death following kainic acid-induced status epilepticus. Epilepsy Res. 2005;65:59–70. doi: 10.1016/j.eplepsyres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit. Care. 2009;10:103–115. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti N, Deshpande LS, DeLorenzo RJ. Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci. Lett. 2008;436:289–293. doi: 10.1016/j.neulet.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti N, Deshpande LS, DeLorenzo RJ. Development of the calcium plateau following status epilepticus: role of calcium in epileptogenesis. Expert Rev Neurother. 2009;9:813–824. doi: 10.1586/ern.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebauer M, Gruenthal M. Neuroprotective effects of early vs. late administration of dantrolene in experimental status epilepticus. Neuropharmacology. 1999;38:1343–1348. doi: 10.1016/s0028-3908(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Mody I, Baimbridge KG. Dantrolene-Na (Dantrium) blocks induction of long-term potentiation in hippocampal slices. Neurosci. Lett. 1989;98:172–178. doi: 10.1016/0304-3940(89)90505-3. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. On the way to cure epilepsy. Expert Rev Neurother. 2004;4:917–920. doi: 10.1586/14737175.4.6.917. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48 Suppl 2:13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl Acad. Sci. USA. 2004;101:17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe MW, Lemasters JJ, Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990;11:63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg G, Angel I, Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: first administration to humans. Br. J. Clin. Pharmacol. 2005;60:7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinska E, Sobczuk A, Lazarewicz JW. Dantrolene antagonizes the glycineB site of the NMDA receptor. Neurosci. Lett. 2008;432:137–140. doi: 10.1016/j.neulet.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Status epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999;40 Suppl 1:S34–S39. doi: 10.1111/j.1528-1157.1999.tb00876.x. discussion S40–31. [DOI] [PubMed] [Google Scholar]
- Sombati S, Delorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J. Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, DeLorenzo RJ. Glutamate injury-induced epileptogenesis in hippocampal neurons: an in vitro model of stroke-induced ‘epilepsy’. Stroke. 2001;32:2344–2350. doi: 10.1161/hs1001.097242. [DOI] [PubMed] [Google Scholar]
- Tsuchida TN, Barkovich AJ, Bollen AW, Hart AP, Ferriero DM. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr. Neurol. 2007;36:253–257. doi: 10.1016/j.pediatrneurol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Willmore LJ. Post-traumatic epilepsy: cellular mechanisms and implications for treatment. Epilepsia. 1990;31 Suppl 3:S67–S73. doi: 10.1111/j.1528-1157.1990.tb05861.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Sakai T. Dantrolene, a calcium-induced calcium release inhibitor, prevents the acquisition of amygdaloid kindling in rats, a model of experimental epilepsy. Tohoku J. Exp. Med. 2006;209:303–310. doi: 10.1620/tjem.209.303. [DOI] [PubMed] [Google Scholar]