Abstract
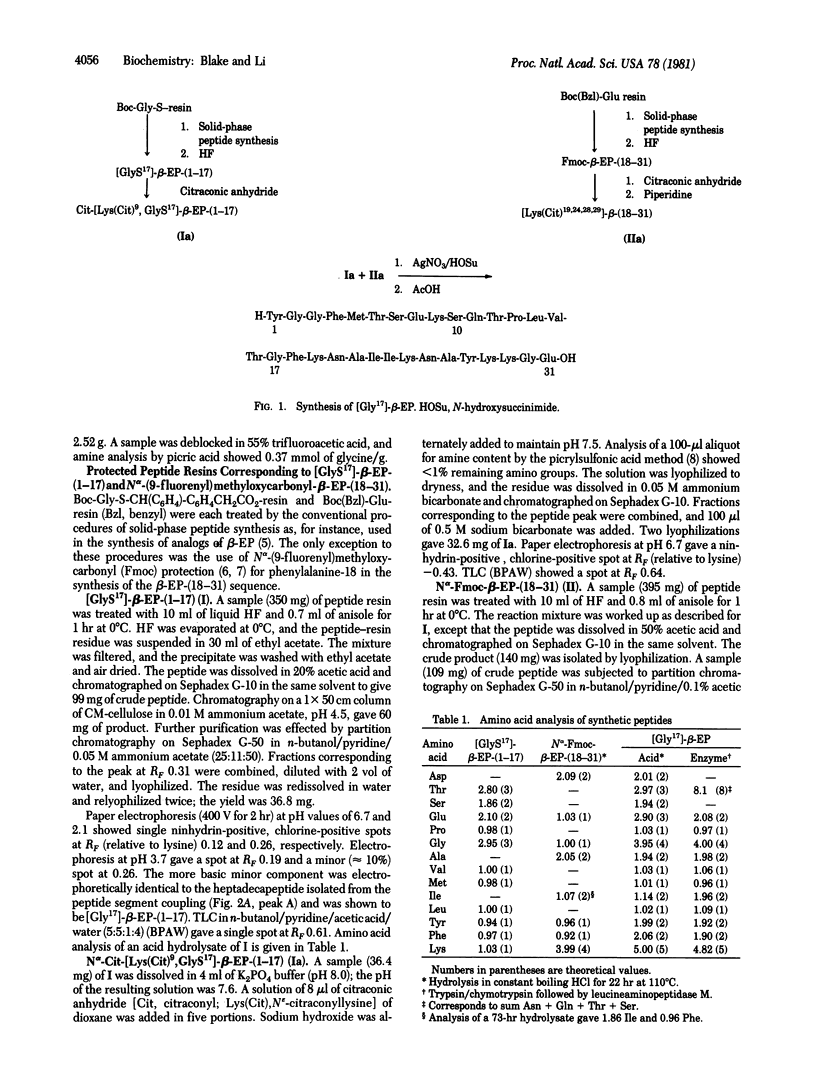
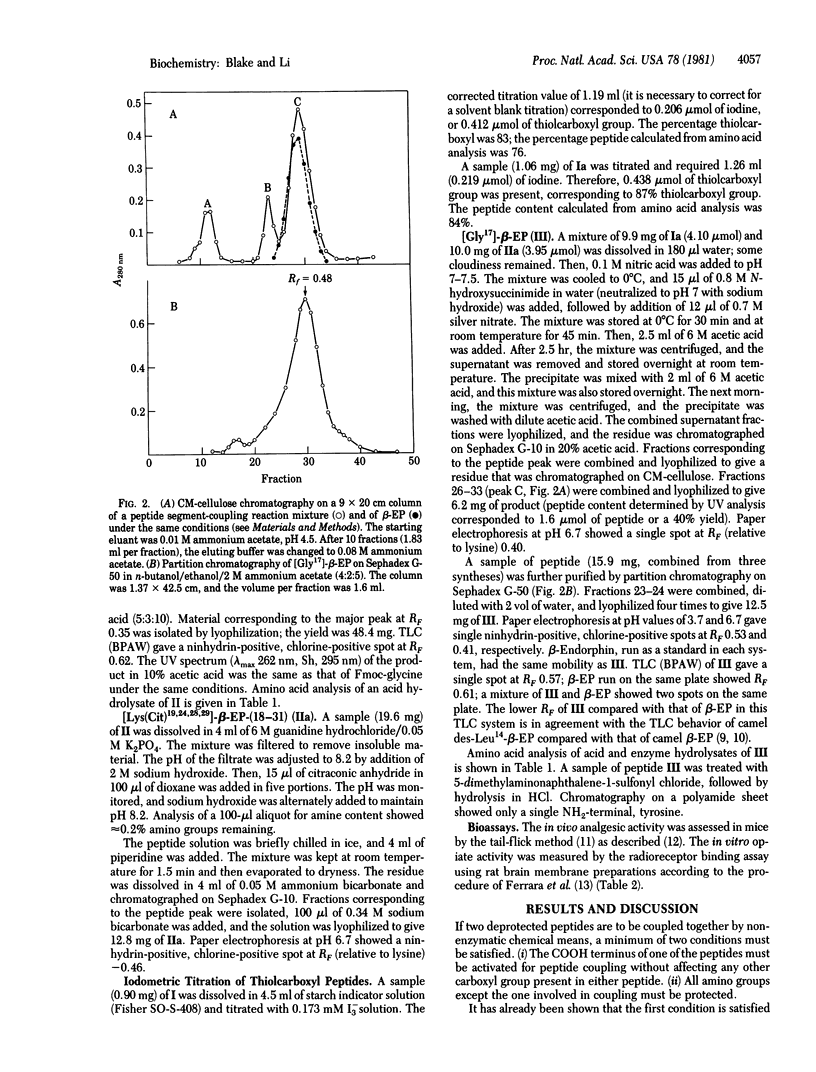
The thiolcarboxyl peptide [17-thiolglycine]-beta-endorphin-(1-17) (I) was synthesized by the solid-phase method. Reaction of peptide I with citraconic anhydride gave citraconyl-[Lys(Cit9,GlyS17]-beta-endorphin-(1-17) (Ia). Peptide Ia was coupled to another synthetic peptide, [Lys(Cit)19,24,28,29]-beta-endorphin-(18-31), by reaction with silver nitrate--N-hydroxysuccinimide in water. All citraconyl groups were removed in aqueous acetic acid, and [Gly17]-beta-endorphin was isolated in 30-40% yield. The synthetic analog had 10% analgesic potency and 59% opiate--receptor binding activity when compared with human beta-endorphin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake J. Peptide segment coupling in aqueous medium: silver ion activation of the thiolcarboxyl group. Int J Pept Protein Res. 1981 Feb;17(2):273–274. doi: 10.1111/j.1399-3011.1981.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Blake J., Tseng L. F., Li C. H. Synthesis and analgesic activity of human beta-endorphin analogs substituted at positions 17, 18, or 19. Int J Pept Protein Res. 1980 Feb;15(2):167–170. doi: 10.1111/j.1399-3011.1980.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Waki M., Ahmad M., Meienhofer J., Lundell E. O., Haug J. D. Preparation and properties of Nalpha-9-fluorenylmethyloxycarbonylamino acids bearing tert.-butyl side chain protection. Int J Pept Protein Res. 1980 Jan;15(1):59–66. doi: 10.1111/j.1399-3011.1980.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara P., Houghten R., Li C. H. beta-Endorphin: characteristics of binding sites in the rat brain. Biochem Biophys Res Commun. 1979 Aug 13;89(3):786–792. doi: 10.1016/0006-291x(79)91847-3. [DOI] [PubMed] [Google Scholar]
- Li C. H., Tseng L. F., Yamashiro D. beta-Endorphin: complete primary structure is required for full analgesic activity. Biochem Biophys Res Commun. 1978 Nov 29;85(2):795–800. doi: 10.1016/0006-291x(78)91232-9. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Moore S., Stein W. H. Activity of bovine pancreatic deoxyribonuclease A with modified amino groups. J Biol Chem. 1971 Feb 25;246(4):939–945. [PubMed] [Google Scholar]
- YAMASHIRO D. PARTITION CHROMATOGRAPHY OF OXYTOCIN ON 'SEPHADEX'. Nature. 1964 Jan 4;201:76–77. doi: 10.1038/201076a0. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. The purification of peptides by partition chromatography based on a hydrophobicity scale. Int J Pept Protein Res. 1979 Jan;13(1):5–11. doi: 10.1111/j.1399-3011.1979.tb01843.x. [DOI] [PubMed] [Google Scholar]


