Abstract
Women are twice as likely as men to suffer from depressive symptoms and disorder. Considerable research has focused on the physiological and psychosocial differences between men and women as sources of depression. An important target of study has been the periods of reproductive changes and events that occur at puberty, postpartum and menopause. A controversy has existed regarding the extent to which, if at all, the menopausal transition or postmenopause increases the risk for elevated depressive symptoms and/or disorders. SWAN provided an opportunity to address the issue with the largest, most representative and diverse cohort currently available for study. The current paper presents the findings from analyses conducted on data collected from the larger core SWAN study and an ancillary study on mental health begun in Pittsburgh in 1995. We found, as did four other recent longitudinal studies, that risk for high depressive symptoms and disorder is greater during and possibly after the menopausal transition. Multiple other factors contribute to risk for depression in our SWAN cohort.
Keywords: menopause, mood, depression, risk factors
Introduction
Depression exacts great emotional, social and economic costs in the form of treatment expenses, lost productivity, and emotional and social impairment. This is particularly consequential for women because the lifetime prevalence of major depression alone is more than 20%.1;2 Women have a two-fold greater risk for depression than men. These sex differences have been found for both major depressive disorder as well as depressive symptoms in a large European study (DEPRES; Depressive Research in European Society3). Considerable research has focused on the physiological and psychosocial differences between men and women as sources of depression. An important target of study has been the periods of reproductive changes and events that occur at puberty, postpartum and menopause.
For decades, a controversy has existed regarding the extent to which, if at all, the menopausal transition or postmenopause increases the risk for elevated depressive symptoms and/or disorders. In 1995, when SWAN began, the state of knowledge about this issue was unclear and confusing, based largely on a cycle of “beliefs” and inconsistent findings from predominantly cross-sectional and small clinical studies that used varied measures of depressive symptoms. At that time, the most recent epidemiological studies of menopause had found no relationship between depressive symptoms and menopausal status or in some cases, higher levels of symptoms during perimenopause (see Reference4 for a review). SWAN provided an opportunity to address the issue with the largest, most representative and diverse cohort until that time.
The current paper presents findings relevant to four important questions regarding depression, menopause, and aging: 1) Does the menopausal transition or postmenopause render women more vulnerable to depression than does the premenopause? 2) Does the risk for depression vary by race/ethnicity or by differing influences on risk by menopausal status among different ethnic groups? 3) If depression is more prevalent during or after the transition, is this due to hormonal alterations, psychological, developmental, and/or somatic changes associated with the transition, midlife (more generally) or genetic vulnerability, and 4) What are the risk factors for depression during midlife and what is the impact of menopausal status on depression relative to these other factors. We also examined these questions for negative mood symptoms including irritability, nervousness, and frequent mood changes (also comprising psychological distress or dysphoric mood).
Depression has been variously defined across a spectrum ranging from a relatively brief negative mood state that includes feeling sad or being “blue” to a medically defined syndrome called Major Depressive Disorder/Minor Depression (a subthreshold depressive disorder that has fewer symptoms than major) or “clinical depression.” The criteria for clinical depression include the duration of symptoms for a minimum of 2 weeks and symptoms that cause significant distress or impairment in functioning. Depressive symptom measures are useful for estimating symptom level or severity, and, in some cases, as a screen for clinical depression. However, the presence of depressive symptoms measured by a variety of scales usually focus on a prescribed period of time (usually, previous 1–2 weeks), does not have a duration or impairment criterion nor does it inquire about past symptoms.
SWAN included a standard measure of depressive symptom levels in its baseline and annual assessments at all sites. With funding from the National Institute of Mental Health, three of the SWAN sites (Chicago, Newark and Pittsburgh) conducted at baseline the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)5;6 a semi-structured psychiatric assessment to ascertain diagnoses of multiple psychiatric disorders, including clinical depression. Pittsburgh has continued to conduct these assessments annually, and now has collected 11 years of annual follow-up data to chart the course of depression as these women have traversed the transition to early postmenopause. This paper presents what we have learned about depression from women participating in Core SWAN as well as the ancillary Mental Health Study during the past 16 years. For comparison purposes, we will review other recent large longitudinal epidemiological studies of menopause and depression conducted during this period.7–10 These recent studies had larger or more diverse samples, longer and more frequent follow-up, more careful definitions of menopausal status and conducted analyses that utilized more fully the longitudinal data than did earlier studies. Published SWAN findings will be presented separately for negative mood and depressive symptoms and clinical disorder (major and minor depressive disorders) outcomes. We will also describe briefly our analyses showing that depression is a risk factor for physical health outcomes.
Depressive symptoms and menopause
How do we measure depression in SWAN at all 7 sites?
All sites have been using the Center for Epidemiological Studies Depression Scale (CES-D)11 to assess depressive symptom levels. The CES-D was designed to be used as a screen for depression in epidemiological studies, not for making diagnoses.12 We chose to use this self-report scale for its minimal cost and burden, its wide use in epidemiological and clinical studies including those of menopause, the evidence that it would provide reliable estimates of depressive symptom levels, and because it has been validated for administration in different ethnic groups.13–17 The CES-D asks about the frequency of being bothered by 20 depressive symptoms during the previous week on a 4-point scale of 0 (rarely) to 3 (most or all of the time).11 A total score of 16 or higher is commonly used to identify potential clinical depression12;18 and was used to indicate clinically relevant depressive symptoms in SWAN.
At baseline, 23% of the sample scored ≥ 16 on the CES-D. Baseline characteristics varied significantly between women with a CES-D ≥ 16 (“high CES-D”) and those with a CES-D < 16. Women with high CES-D were less well educated and more likely to be younger, had a negative attitude toward aging and menopause, and reported greater financial strain. They were also more likely to be African American or Hispanic and less likely to be Chinese or Japanese, and more likely to be early perimenopausal than those with low CES-D (p < 0.0001).
What have we learned about depressive symptoms/negative mood and the menopausal transition in SWAN?
Does the risk for high depressive symptom levels vary by menopausal status and is it independent of known risk factors for depression?
We have published 5 papers on depressive or negative mood symptoms (3 baseline cross-sectional and 2 longitudinal) relevant to the transition to menopause. (See Table 1.) (1) The initial cross-sectional paper was based on data from 10,374 women who completed the screening questionnaire at baseline. Psychologic distress was defined as feeling blue, irritable, and tense. We compared presence of distress in women who were premenopausal with those who were early perimenopausal, late peri, or post.19 (2) In the baseline cohort, we compared the prevalence of persistent mood symptoms and overall dysphoric mood between premenopause and early perimenopause women, adjusted for multiple covariates.20 (3) We examined the race/ethnic differences in the prevalence of depressive symptoms (CES-D ≥ 16) adjusted for multiple covariates including menopausal status at baseline.21 (4) Annual data from baseline through visit 5 were used to examine the longitudinal relationship between menopausal status and risk of elevated depressive symptoms (CES-D ≥ 16).22;23 (5) Annual data from baseline through visit 8 were used to extend the previous analyses to evaluate the relationship between serum hormone levels and high depressive symptoms and whether hormone levels or their changes might explain the association of menopausal status with depressive symptoms.24
Table 1.
SWAN studies examining menopausal status and risk factors for negative mood and depressive symptoms and disorder
| Reference | Design/Sample | Outcome –symptoms |
|---|---|---|
| Bromberger JT, et al. 2001 19 | Cross-sectional/N=10,374 women, aged 40–55 yrs, screened | Psychological distress, feeling blue, irritable and tense |
| Bromberger JT, et al. 2003 20 | Cross-sectional/Baseline cohorta N=3302 | Frequent mood symptoms occurring ≥ 6 days/2 wks (3 above and mood change) |
| Bromberger JT, et al. 2004 21 | Cross-sectional/Baseline cohorta N=3015 | Depressive symptoms, CES-D≥16 |
| Bromberger JT, et al. 2007 22 | Longitudinal/Baseline through V5 N=3302 | CES-D≥16 |
| Bromberger JT, et al. 2010 24 | Longitudinal/Baseline through V8 N=3302 | CES-D≥16 |
| Reference | Design/Sample | Outcome – major depression |
| Bromberger JT, et al. 2009 37 | Longitudinal/Baseline through V7 N= 266 | Major depression |
| Bromberger JT, et al. 2011 23 | Longitudinal/Baseline through V9 N= 221 | Major depression |
Cohort, aged 42–52 years
The first 3 papers reported that prevalences of psychologic distress, dysphoric mood, and CES-D ≥ 16 were lowest in the premenopausal women (20.9%, 9%, 20.9%, respectively); prevalences in the early perimenopausal women were 28.9%, 15%, and 27.8%, respectively. Dysphoric mood consisted of the total score for 4 mood symptoms which ranged from 0–16; we defined dysphoric mood as the top 10% of the distribution, which is why the prevalences are much lower than those for psychologic distress (top 24%) and CES-D ≥16 (top 23%). In the cross-sectional screening study sample,19 prevalences of psychological distress in late perimenopause and postmenopause were 25.6% and 22%, respectively. Compared to premenopause, the adjusted risk of negative mood or depressive symptoms, measured as psychological distress, dysphoric mood, or high CES-D, was 20% – 62% higher in early perimenopause,
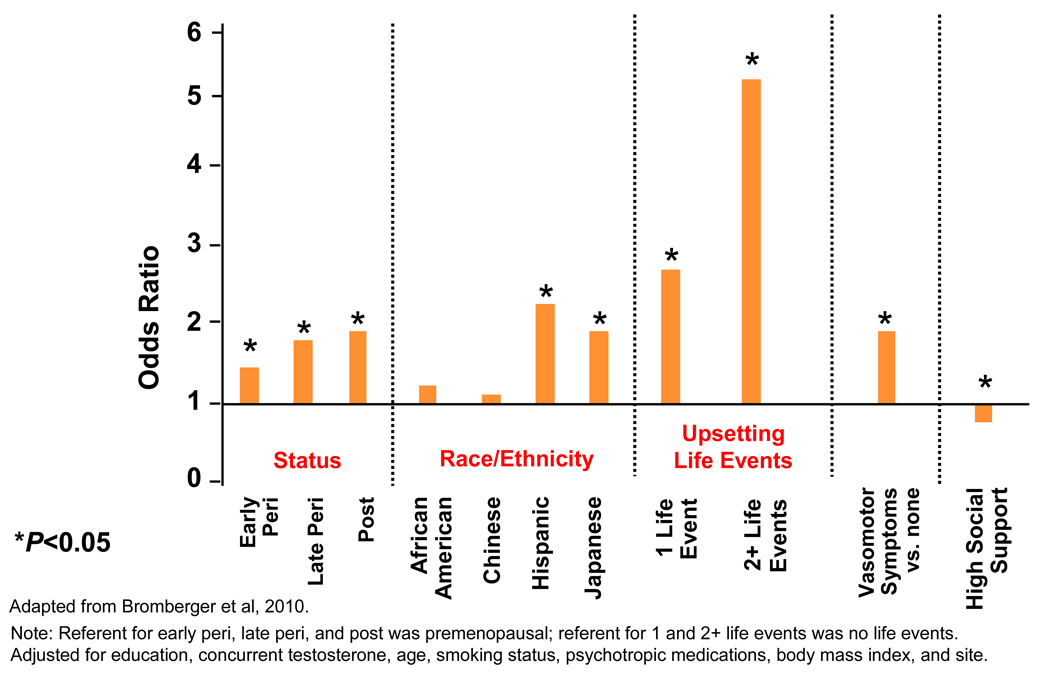
Results of the longitudinal analyses for depressive symptoms were consistent with those from the cross-sectional analyses.22;24 In multivariable random effects logistic regression models using data from 5 years and 8 years of annual assessments, being peri- or postmenopausal compared to being premenopausal remained significantly associated with CES-D≥16 in all analyses. For example, across 5 years, women were significantly more likely to report a high CES-D when early perimenopausal (OR=1.30), late perimenopausal (OR=1.71), and postmenopausal (OR=1.57) relative to when they were premenopausal and when they were late perimenopausal (OR=1.32) compared to when early perimenopausal. In these analyses, we also reported on the risk of depression among women who had ever used exogenous hormones (OR=1.43). These results were independent of education, race/ethnicity, vasomotor symptoms (VMS), stressful life events and low social support at each visit.22 The 8-year findings extended and were consistent with those from the first 5 years.24 (See Figure 1.)
Figure 1.
Fully adjusted random effects logistic regression model examining the odds of high depressive symptoms (CES-D ≥16).
Does the risk for elevated depressive symptoms vary across race/ethnic groups or by differing relationships between symptoms and menopausal status among different race/ethnic groups?
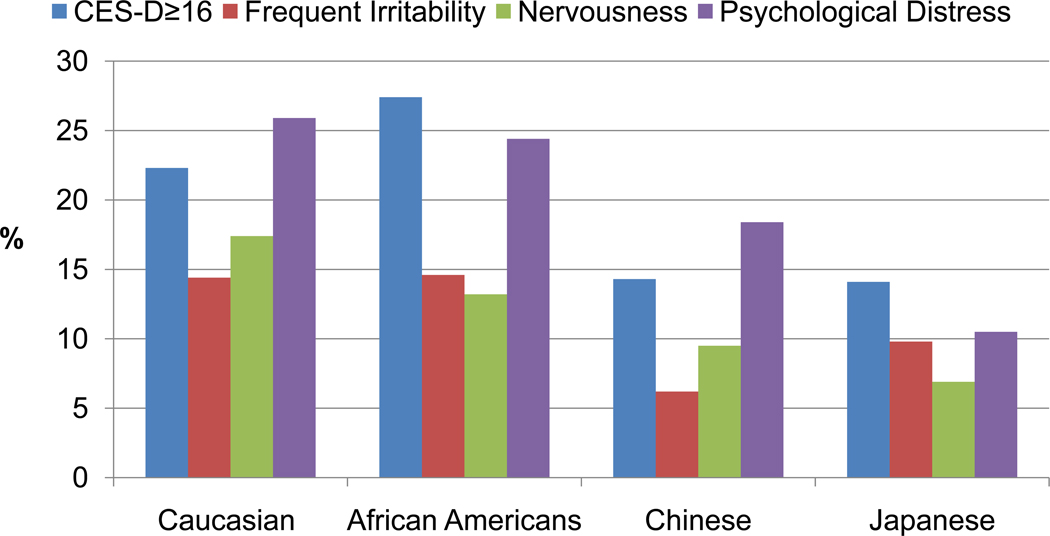
At baseline, there were race/ethnic differences in the prevalence of negative mood symptoms and high CES-D as well as in the relationship of menopausal status with odds of high CES-D. The race/ethnic differences varied depending on the outcomes. In baseline unadjusted analyses, the Caucasian, African American and Hispanic women had the highest rates of CES-D ≥ 16, frequent irritability, nervousness, and psychological distress and Chinese and Japanese the lowest. (See Figure 2.) Adjustments for sociodemographic, psychosocial and health factors attenuated the effects of race/ethnicity. These results suggest that psychosocial and health related factors accounted for higher odds of depressive symptoms in the African American and Hispanic women, but not in the Chinese and Japanese.21
Figure 2.
Prevalence of CES-D ≥16, frequent irritability, nervousness and psychological distress at baseline by race/ethnicity.
The multivariable longitudinal analyses for race/ethnicity were not entirely consistent with the cross-sectional analyses. Over the first eight years of the study the odds of high depressive symptoms significantly increased in the Hispanic and Japanese compared to the Caucasians. The odds of the African American and Chinese women were not significantly different than those of the Caucasian women.24 (See Figure 1.) The two sets of analyses differed somewhat in the covariates included, which may account for the different findings. It is also possible that over time, the risk for high depressive symptoms among the race/ethnic groups relative to Caucasians changed. However, the elevated risk for the Japanese women over time was unexpected and not readily explained. Associations between menopausal status and high depressive symptoms were similar among four of the five race/ethnic groups. Among Hispanic women, perimenopause was associated with more than a two-fold risk of high depressive symptoms (OR=2.45).21
What factors contribute to risk for high depressive symptoms among midlife women, what is the relative importance of menopausal status, and do hormone levels or changes or VMS account for association of menopausal status with depression?
Numerous factors were independently associated with high CES-D over the first five and eight years of SWAN. The models for the two sets of analyses varied somewhat, but the results were similar. The significant predictors included VMS, being a current smoker, low social support, very stressful events,22;24 financial strain,22 having less than a college education and higher body mass index (BMI).24 For example, having less than a college education was associated with an increased odds ratios of 1.5 to 2 times compared to having a college education or more; vasomotor symptoms increased the odds by 62% – 77% and reporting two or more life events increase the odds by more than 5 times. For every 1 unit increase in BMI, the odds of high depressive symptoms increased by 1%. High social support was protective and reduced the odds by nearly 20%.24 (See Figure 1.)
A key question is the role of reproductive hormones in the development of depression and negative mood during the menopausal transition or shortly after. Neurobiologic data have indicated that gonadal steroids affect a wide range of neuromodulator processes, including the neuromodulators serotonin and norepinephrine, which are implicated in the development of depression.25;26 However, epidemiological and clinical studies that have examined associations between reproductive hormones and depression have yielded inconsistent results.10;27 Using eight years of follow-up data, we assessed whether reproductive hormones were related to risk of high depressive symptoms (CES-D ≥ 16).24 Specifically, we examined the association between serum levels and changes in follicle stimulating hormone (FSH), estradiol (E2), and testosterone (T) and odds of high depressive symptoms. Multivariable random effects logistic regression models showed that log transformed testosterone (logT) was significantly positively associated with higher odds of CES-D≥16 (OR=1.15) across 8 years and a larger increase in logT from baseline to each annual visit was significantly associated with increased odds of CES-D≥16 (OR=1.23). 24
Figure 1 shows results of the fully adjusted multivariable random effects model examining predictors of CES-D ≥ 16.24 The multiple risk factors for high depressive symptoms ranged in magnitude from an odds ratio of 5.13 for two or more stressful life events in the past year to 1.42 for being a current smoker. As noted above, the independent effect of status on odds of high CES-D was substantial, showing a graded increase in the odds of reporting high depression symptoms across the transition from early perimenopause (OR=1.35) to late perimenopause (OR=1.68) to postmenopause (OR=1.83). Furthermore, reporting VMS was also significantly independently associated with high CES-D in these analyses. Thus, each menopause related factor (menopausal status, T, VMS) made an independent contribution to the risk for high CES-D. Nevertheless, it is noteworthy that experiencing 2 or more upsetting life events in the previous year was the strongest predictor of risk, increasing it by more than 5 times.
Finally, being peri- or postmenopause remained significantly associated with risk for CES-D≥16 in all analyses. These results suggest that neither hormones nor VMS accounted for the association of menopausal status defined by bleeding patterns with elevated depressive symptoms.
Major depressive disorder and menopause
How is major depression defined and how was it assessed in the Chicago, Newark and Pittsburgh SWAN?
Major depression (MD) is defined as the presence of at least 5 depressive symptoms with impaired functioning for at least most of two weeks.28 (See Table 2.) It is a highly prevalent major health problem and the leading cause of health-related disability in women.29 About 22% of women in midlife have a history of MD1 and 5% have current MD.30 Similar findings were reported in the NCS Replication study.2 Of individuals who have a first episode, 50–80% experience another.31 MD is associated with a worse course and outcome of physical illness and can complicate its treatment, and has a major impact on a woman’s functioning in her various roles.32;33
Table 2.
Criteria for Major Depressive Episode (MDE)
|
| Criteria for Minor Depression |
| 2–4 symptoms lasting for at least 2 weeks |
| Criteria B–E for major depression above |
Adapted from: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. 1994 American Psychiatric Association
As noted above, diagnoses of lifetime and current major depressive disorders were determined from interviews conducted by trained clinicians utilizing the Structured Clinical Interview for the Diagnosis of DSM-IV Axis I Disorders (SCID).6 The SCID has been used with many different ethnic groups and extensive field-testing has demonstrated its suitability for research purposes; adequate reliability has been demonstrated in numerous studies,5 including SWAN’s Mental Health Study. The SCID was administered at baseline by all 3 sites and annually by Pittsburgh only.
At baseline, 35% of the cohort had a lifetime history of MD; 17% had a history of recurrent MD. Our prevalences of current MD (past month) was 3%. SWAN lifetime prevalence was higher than those reported in epidemiological studies such as the National Comorbidity Survey (NCS),1 and its replication (NCS-R).1;2 On the other hand, MD rates in the Virginia Twin Study 34;35 were considerably higher than the NCS, 30%–35% for women based on same-sex pairs of twins. The methods used in the latter study involved extensive training procedures and clinically trained interviewers and are similar to those we used. It is also possible that women who participate in a longitudinal study of menopause may be more likely to have had previous emotional problems or more willing to discuss these. Baseline characteristics between women with and without a history of MD varied significantly. Women with a history were less well educated and were more likely to report greater financial strain, be unemployed, smoke currently, and report low social support. Prevalence of history of MD did not vary by race/ethnicity. Among the middle-aged overall36 and women specifically aged 45–54 years,30 prevalence rates for 30-day, 12-month, and lifetime Major Depressive Episodes (MDEs) are similar between or higher in Caucasians than African Americans. However, in the National Comorbidity Survey, there were few women in this age group.30 Importantly, to our knowledge, other than the Penn Ovarian Aging Study (POAS),27 no studies have examined the occurrence of MDEs during and soon after the menopausal transition in substantial numbers of African Americans.
What have we learned about major depression and the menopausal transition in SWAN?
Does the risk for major depression vary by menopausal status and is it independent of known risk factors for depression?
We have published 2 papers on MD relevant to menopause. (Table 1.) Both used the longitudinal data and therefore included only the data from Pittsburgh. To examine whether women were more likely to experience a major depressive episode during perimenopause or postmenopause compared to when they were premenopausal, we analyzed data from the baseline and the first eight annual assessments of 221 women who were premenopausal at study entry.23 Using repeated measures logistic regression, we found that women were two to four times more likely to experience a major depressive episode when they were perimenopausal (OR=2.27) or postmenopausal (OR=3.57), even after controlling for a variety of factors associated with depression, including a history of major depression at baseline, annual psychotropic medication use, higher BMI, very upsetting life events and frequent VMS. These data also were analyzed separately for African American and Caucasian women, and both had similar odds for onset of a depressive episode during the transition.
The second paper reported on the determinants of a first onset of MD during midlife.37 We were particularly interested in the role of menopausal factors in first onsets. The subcohort included in this analysis was comprised of the 266 women without past or current major depression at baseline. Cox Proportional Hazards analyses of data from 8 annual assessments identified predictors of first onsets. Forty-two (15.8%) of the 266 women met criteria for a first onset major depressive episode during the transition. In contrast to the results of the analyses in the full sample described above, menopausal status was not significant in univariate or multivariate analyses.
What factors contribute to risk for major depression among midlife women, what is the relative importance of menopausal status, and do hormone levels or changes or VMS account for the association of menopausal status with risk for major depression?
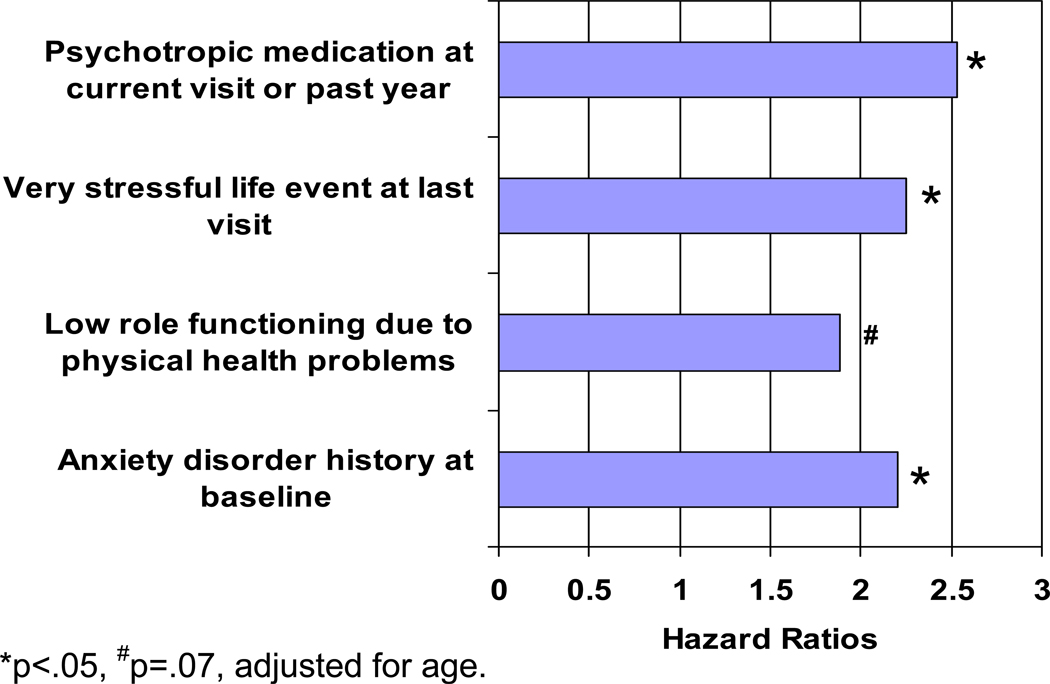
As noted above, MD is multifactorially determined. It is well-known that the strongest predictor of an episode of MD is having a history of MD,38 which tripled the odds in our analysis. Higher BMI was also marginally significantly associated with MD over the study. In both sets of analyses described above, we found that experiencing very upsetting life events was a consistently strong predictor of MD. In the case of predictors of first onset MD, whereas menopausal status and frequent VMS were non-significant, very upsetting life events doubled the odds of experiencing a new major depressive episode. VMS were significant in the univariate analysis only, suggesting that upsetting life events were more important for first onset MD than frequent VMS. In addition to menopausal status and upsetting life events, we examined health-related risk factors as predictors of first MD because of their salience for midlife women. While higher baseline social and role functioning and previous annual BMI and frequent VMS were each significantly associated with odds of first onset MD in univariate analyses, they did not retain their significance in the multivariate analyses. Indeed, as shown in Figure 3, the results of Cox regression multivariable analyses indicated that a history of an anxiety disorder (hazard ratio [HR] =2.20) at baseline, and psychotropic medication use (HR=2.53) and at least one very stressful event (HR = 2.25) at the last visit prior to meeting criteria for a depressive episode currently or in the year since prior visit, were significant independent predictors of first MD onset. Low role functioning due to physical health was marginally significant.
Figure 3.
Results of multivariable model predicting first depression onset.
Neither baseline reproductive hormone levels nor changes over time were significantly associated with MD in any of the analyses. Similar to findings for high depressive symptoms, we observed that neither endogenous hormones nor VMS attenuated the menopausal status-MD associations, suggesting that they did not account for the association of menopausal status defined by bleeding patterns with MD.
What about genetics?
The SWAN data showed that selected estrogen-related single nucleotide polymorphisms (SNPs) from 3 genes were associated with the CES-D score ≥ 16 in women who were premenopausal or perimenopausal and there were ethnic differences in these associations.39 Caucasian women with the CYP1A1 rs2606345 CC genotype had significantly higher odds of reporting a high CES-D than did those with either the AA (OR=2.49) genotype or the AC (OR=1.98) genotype. In African American women, the magnitude of the odds was substantially and significantly greater (OR = 10.17) only for those with CC compared with the AA genotype. Neither the Chinese nor the Japanese women had the AA genotype. Japanese women with the CYP 19 rs936306 TT genotype had almost a 5-fold higher odds of reporting high depressive symptoms than did women with the CC genotype and greater than 9.6 fold higher odds than did women with the CT genotype. Chinese women with the 17HSD rs615942 TT genotype had higher odds of reporting high depressive symptoms than did Chinese women with the GT genotype (OR=10.87) or the GG (OR=7.65) genotype. Whereas both cytochrome P450 aromatase (encoded by CYP 19) and 17beta-hydroxysteroid dehydrogenase (17HSD) are enzymes that synthesize estrone (CYP 19) or androgens (17HSD) to estradiol, CYP1A1 is an enzyme responsible for hydroxylating both estradiol and estrone.
Although these are findings are only preliminary and observed in relatively small samples, they do suggest that variation in these 3 estrogen-related genes may be associated with depressive symptoms in women. Moreover, for each of these three polymorphisms, the results remained significant after controlling for psychosocial factors that we have shown to be associated with depressive symptoms in different ethnic groups.21 Our findings lend support to notions that genes together with psychosocial factors may influence risk for depressive symptoms and that estrogen may contribute to the development of these symptoms in midlife women.
Other longitudinal epidemiological studies of menopause and depression
Four recent longitudinal studies have examined the relationship between menopausal status and depressive symptoms7–10 and/or clinical or severe depression (see Tables 3a and 3b)7;8;27. Despite using different sampling frames, sizes, and compositions and varying designs and analyses, the results were consistent – they all reported that risk for depression (symptoms or disorder) increased during the perimenopause. Moreover, with the exception of Maartens, et al,9 in which 2,103 women provided data 3 years apart via mailed questionnaires, these studies did not find an increased risk for depressive symptoms in the postmenopause compared to premenopause. The null findings may have been due to insufficient power to detect a difference because of relatively small numbers of women followed through postmenopause. For example, the POAS8 reported that depressive symptoms but not disorder increased in the perimenopause and subsequently declined in the postmenopause over 4 years of follow-up. However, in this study 73% of the 332 participants remained premenopausal over the 4 years of follow-up and only 3% completed the transition, making the numbers for postmenopause and MD too small to determine statistical significance.
Table 3.
| a. Depression by Menopause Transition: Recent Longitudinal Studies of Midlife Women | |||||
|---|---|---|---|---|---|
| Study | Design | Sample | Covariates/predictors | Outcome(s) | Results |
| University of Tilburg, the Netherlands - Maartens LW et al. 2002 9 | Postal Quest. 2 waves of data 3.5 years apart |
N=2,103, aged 47–53 years | Demographics, life events ↑depressive symptoms at T1. |
Edinburgh Depression Scale (EDS) – EDS symptom score > 12 | Peri- and post sig. Financial prob sig. |
| The Penn Ovarian Aging Study(POAS) - Freeman EW et al. 2004 8 | In-home visits. 6 assessments over 4 years |
N=436, aged 35–47 years | Center for Epidemiologic Studies of Depression Scale (CES-D) – CES-D symptom score ≥ 16 in past week. Primary Care Evaluation of Mental Disorders in past month |
Peri-, African American, VMS, PMS, poor sleep significant. | |
| Seattle Midlife Women's Health Study - Woods NF et al. 2008 10 | 9 yrs. apart | N=508, aged 35–55 | CES-D symptom score ≥ 16 | Late peri-, greater BMI, postpartum blues, no children, stressor significant. | |
| b. First Onset Depression by Menopause Transition: Longitudinal Studies | |||||
|---|---|---|---|---|---|
| Study | Design | Sample | Covariates | Outcome | Results |
| The Harvard Study of Moods and Cycles - Cohen LS et al. 2006 7 | 8 yrs. apart | N=460, no history of depression | First onset high depressive symptoms - CES-D, CES-D+ (severe symptoms) | Perimenopause marginally significant overall. | |
| The Penn Ovarian Aging Study - Freeman EW et al. 2006 27 | 8 yrs. apart | N=231 women, no history of depression | First onset high depressive symptoms CES-D and depressive disorder | Perimenopause significant for symptoms, not for depressive disorder. Greater BMI, E2, and greater E2 SD significant. | |
Also in the POAS, Freeman and colleagues27 found a significantly increased adjusted odds ratio (AOR=5.44) for the first onset of high depressive symptoms during perimenopause compared to premenopause. In the Harvard Study of Moods and Cycles, Cohen et al7 reported that women who became perimenopausal had only a marginally significant higher odds of first onset high depressive symptoms (AOR=1.9) after adjustment for age and stressful life events than did those who remained premenopausal across 8 years. For depressive disorder, the POAS also found that, in unadjusted analysis, perimenopause doubled the odds compared with premenopause, but perimenopause was no longer a significant predictor in multivariable analyses. Importantly, neither study included women who were postmenopausal and each only examined a subset of women.
The longitudinal studies examined multiple and varying potential risk factors for depression in addition to menopausal status. Freeman and colleagues8 reported that African Americans had nearly twice the odds of high depressive symptoms relative to Caucasians. However, their analyses did not adjust for socioeconomic and psychosocial factors that have been shown in other studies21;40 to account for the higher risk for depression in African Americans. Other significant predictors included a history of depression at study entry, lack of employment, severe PMS, poor sleep, and hot flashes. Although postpartum depression was significant in bivariate analyses, it did not remain significant in the multivariable analysis possibly because of the high correlation between severe PMS and postpartum depression. Woods and colleagues10 reported that number of undesirable events in the previous year, self-reported BMI, postpartum blues, and no live births each increased the odds of high depressive symptoms by 1.5 to 2 times. Hot flashes were significant in bivariate analyses, but not in multivariable ones. Maartens and colleagues9 found that being unemployed, prior depression (at T1, Edinburgh Postnatal Depression Scale (EPDS≥12), financial problems and death of partner were significant predictors of an increase in depressive symptoms over 3.5 years. This study did not include hot flashes.
The factors associated with first onset high depressive symptoms in the POAS included BMI, hot flashes, severe PMS, current smoking, and estradiol. Higher BMI, presence of hot flashes and greater variability of estradiol at 2 consecutive follicular phase blood draws increased the odds whereas current PMS and smoking significantly decreased them.27 Cohen and colleagues7 did not present the odds of VMS or adverse life events in the full model but their data suggest that only the perimenopausal women with VMS or adverse life events had a significantly increased risk of first onset depression. Freeman and colleagues 27 reported results that contrasted with those from SWAN.24 They found that only BMI and variability of estradiol measured in two consecutive follicular phase blood draws were associated with significant odds of depressive disorders. Hot flashes, premenstrual symptoms (PMS), and smoking were non-significant. In their analysis, perimenopause was not a significant predictor, which may have been due to the confounding of estradiol variability and hot flashes with perimenopause or the small number of women with a depressive disorder.
Clinical implications
Thus far, we have presented SWAN data about the magnitude of negative mood and depressive symptoms and disorder during the perimenopause and early postmenopause and associated risk factors for depression during this stage of a woman’s life. However, depression can affect a woman’s psychological well-being and pose a risk for her health. Depressive symptoms and disorder are associated with multiple medical conditions and symptoms, including cardiovascular disease (CVD), diabetes, and pain,41–45 and can be both a risk factor for and a consequence of illness.46;47 For example, depression has been associated with morbidity and subsequent coronary events in patients with cardiovascular disease.45 Further, concurrent physical illness can have synergistic effects on worsening physical functioning.48 We have shown in SWAN that depressive symptoms are associated longitudinally with a 3-year increase in diabetes risk in 2,662 women with baseline glucose < 126 mg/dL,49 inflammatory and hemostatic factors,50 and progression of coronary calcification.51 Further, independent of standard CVD risk factors, past recurrent major depression doubled the odds of early predictors of clinical disease, including carotid plaque,52 coronary and aortic calcification53 and incident metabolic syndrome over 2–5 years.54 Recurrent major depression was also associated with progression of coronary calcification.55 Past recurrent MD increased the odds at baseline of bodily pain (odds ratio, OR=2.3), treatment for back pain (OR=4.2), and low social functioning (OR=2.1) in women without current depression.56
SWAN is not a treatment study. Nevertheless, the findings from SWAN are important and contribute to our knowledge about depression in women during the menopausal transition. The increased risk of depression during the transition and its implications for women’s health and functioning suggest that women may benefit from close monitoring of mood and functioning as well as an assessment of their situational and environmental circumstances. Such monitoring could lead to earlier interventions designed to interrupt the progression from dysphoric mood to minor or major depression. These could include interventions indicated at other times in the life cycle, such as behavioral, antidepressants and psychotherapy.
Early interventions can include brief counseling on coping with changes in mood and symptoms associated with perimenopause. Behavioral interventions, such as regular exercise or relaxation, can reduce depression.57;58 Treatment approaches might also target symptoms that may exacerbate or be associated with depression and are unique to this period in a woman’s life, such as vasomotor and genitourinary symptoms and sleep difficulties. 59 Because the potential consequences of under-recognized and untreated depression are considerable, clinicians need to be cognizant that depression can be present with physical as well as mood symptoms in women during this transition. Frey and Soares60 have reviewed the potential therapeutic benefits of pharmacotherapies, both hormonal and non-hormonal, for perimenopausal depression.
Summary
Data from SWAN as well as from other prospective epidemiological studies described above and those reviewed by Freeman et al61 and Soares and Frey62 clearly indicate that perimenopause is a period of heightened risk for depressive symptoms as well as clinical and subclinical depressive disorders. As we and others62;63 have described, vulnerability to depression during the perimenopause can be attributed to a number of factors, including discomfort from somatic symptoms associated with the transition (particularly vasomotor symptoms), psychosocial stressors, inadequate social support, health behaviors, lifestyle, sociodemographic characteristics and history of clinical depression. Given the changes in reproductive hormone dynamics during the menopausal transition, it is likely that the altered estrogen and progesterone milieu contribute to the risk for depression at this time although the data supporting this are largely indirect and sparse. Our findings are similar to those of the recent large longitudinal studies7–10 as described above, although, except for Maartens and colleagues, these studies did not find an increased risk postmenopause possibly due to insufficient postmenopausal data. In our sample, the increased vulnerability to depression during or after the menopausal transition was not accounted for by frequent VMS or by levels of or changes in reproductive hormones. Thus, while we have evidence that the period of transition from the late reproductive years to postmenopause is a time of increased risk for depression, we still do not fully understand why this is the case. In our future work, we plan to evaluate further the characteristics of women at risk for depression during the menopausal transition and postmenopause and whether there are subgroups of women at greater or lesser risk. We also hope to continue the work we have begun to look at depression as a risk factor for other health outcomes as women age.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Supplemental funding from The National Institute of Mental Health is also gratefully acknowledged. University of Pittsburgh, Pittsburgh, PA — Joyce T. Bromberger, PI (R01 MH59689); Rush University, Medical Center, Chicago, IL — Howard M. Kravitz, PI (R01 MH59770); New Jersey Medical School, Newark, NJ — Adriana Cordal, PI (R01 MH59688).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
Reference List
- 1.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Angst J, Gamma A, Gastpar M, et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252:201–209. doi: 10.1007/s00406-002-0381-6. [DOI] [PubMed] [Google Scholar]
- 4.Matthews KA, Bromberger JT, Egland G. Behavioral antecedents and consequences of the menopause. In: Korenman SG, editor. The Menopause. Norwell, MA: Serono Symposia; 1990. [Google Scholar]
- 5.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer RL, Williams JB, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 7.Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD, Liu L, et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;42:195–200. doi: 10.1016/s0378-5122(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 10.Woods NF, Smith-DiJulio K, Percival DB, et al. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2008;15:223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 11.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 12.Boyd JH, Weissman MM, Thompson WD, et al. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic sales. Arch Gen Psychiatry. 1982;39:1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- 13.Jones-Webb RJ, Snowden LR. Symptoms of depression among blacks and whites. Am J Public Health. 1993;83:240–244. doi: 10.2105/ajph.83.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter LB, Rogler LH, Moscicki EK. Depression among Puerto Ricans in New York City: the Hispanic Health and Nutrition Examination Survey. Soc Psychiatry Psychiatr Epidemiol. 1995;30:185–193. doi: 10.1007/BF00790657. [DOI] [PubMed] [Google Scholar]
- 15.Roberts RE. Reliability of the CES-D Scale in different ethnic contexts. Psychiatry Res. 1980;2:125–134. doi: 10.1016/0165-1781(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 16.Salgado-de Snyder VN, Maldonado M. The psychometric characteristics of the Depression Scale of the Centro de Estudios Epidemiologicos in adult Mexican women from rural areas. Salud Publica Mex. 1994;36:200–209. [PubMed] [Google Scholar]
- 17.Ying YW. Depressive symptomatology among Chinese-Americans as measured by the CES-D. J Clin Psychol. 1988;44:739–746. doi: 10.1002/1097-4679(198809)44:5<739::aid-jclp2270440512>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Comstock GW, Helsing KJ. Symptoms of depression in two communities. Psychol Med. 1976;6:551–563. doi: 10.1017/s0033291700018171. [DOI] [PubMed] [Google Scholar]
- 19.Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435–1442. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberger JT, Assmann SF, Avis NE, et al. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158:347–356. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 21.Bromberger JT, Harlow S, Avis N, et al. Racial/ethnic differences in the prevalence of depressive symptoms among middle-aged women: The Study of Women's Health Across the Nation (SWAN) Am J Public Health. 2004;94:1378–1385. doi: 10.2105/ajph.94.8.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN) Psychol Med. 2011:1–10. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden R, Gilmore J. Serotonin and mood disorders. Psychiatric Annals. 1990;20:580–586. [Google Scholar]
- 26.Janowsky H, Halbreich U, Rausch J. Association among ovarian hormones, other hormones, emotional disorders, and neurotransmitters. In: Jensvold M, Halbreich U, Hamilton J, editors. Psychopharmacology and Women: Sex, Gender, and Hormones. Washington, DC: American Psychiatric Press; 1996. pp. 85–106. [Google Scholar]
- 27.Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 28.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Murray C, Lopez A. The Global Burden of Disease. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 30.Blazer DG, Kessler RC, McGonagle KA, et al. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 31.Kessler RC, Zhao S, Blazer DG, et al. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- 32.Beekman AT, Deeg DJ, Geerlings SW, et al. Emergence and persistence of late life depression: a 3-year follow-up of the Longitudinal Aging Study Amsterdam. J Affect Disord. 2001;65:131–138. doi: 10.1016/s0165-0327(00)00243-3. [DOI] [PubMed] [Google Scholar]
- 33.Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19:663–696. doi: 10.1016/s0749-0690(03)00042-9. v. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: The Guilford Press; 2006. [Google Scholar]
- 36.Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945–1952. doi: 10.2105/ajph.93.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberger JT, Kravitz HM, Matthews K, et al. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39:55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- 39.Kravitz HM, Janssen I, Lotrich FE, et al. Sex steroid hormone gene polymorphisms and depressive symptoms in women at midlife. Am J Med. 2006;119:S87–S93. doi: 10.1016/j.amjmed.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Holzer CE, Swanson JW, Shea BM. Ethnicity, social status, and psychiatric disorder in the Epidemiologic Catchment Area Survey. In: Price RK, Shea BM, Mookherjee HN, editors. Social Psychiatry Across Cultures: Studies from North America, Asia, Europe, and Africa. New York, NY: Plenum Press; 1995. pp. 93–104. [Google Scholar]
- 41.Dew M. Disorder in the cotext of physical illness. In: Dohrenwend B, editor. Adversity, stress, and psychopathology. New York: Oxford University Press; 1998. pp. 219–232. [Google Scholar]
- 42.Hotopf M, Mayou R, Wadsworth M, et al. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. Br J Psychiatry. 1998;173:255–261. doi: 10.1192/bjp.173.3.255. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan KR, Delong M, Kraemer H, et al. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52:559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- 44.Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34:623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 45.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 46.Bruce ML. Psychosocial risk factors for depressive disorders in late life. Biol Psychiatry. 2002;52:175–184. doi: 10.1016/s0006-3223(02)01410-5. [DOI] [PubMed] [Google Scholar]
- 47.Matthews KA, Schott LL, Bromberger JT, et al. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun. 2010;24:96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz N, Wang J, Malla A, et al. Joint effect of depression and chronic conditions on disability: results from a population-based study. Psychosom Med. 2007;69:332–338. doi: 10.1097/PSY.0b013e31804259e0. [DOI] [PubMed] [Google Scholar]
- 49.Everson-Rose SA, Meyer PM, Powell LH, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–2862. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 50.Matthews KA, Schott LL, Bromberger J, et al. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom Med. 2007;69:124–130. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- 51.Janssen I, Powell LH, Matthews KA, et al. Depressive symptoms are related to progression of coronary calcium in midlife women: The Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. doi: 10.1016/j.ahj.2011.03.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones DJ, Bromberger JT, Sutton-Tyrrell K, et al. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch Gen Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- 53.Agatisa PK, Matthews KA, Bromberger JT, et al. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165:1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 54.Goldbacher EM, Matthews KA. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Ann Behav Med. 2007;34:240–252. doi: 10.1007/BF02874549. [DOI] [PubMed] [Google Scholar]
- 55.Matthews KA, Chang YF, Sutton-Tyrrell K, et al. Recurrent major depression predicts progression of coronary calcification in healthy women: Study of Women's Health Across the Nation. Psychosom Med. 2010;72:742–747. doi: 10.1097/PSY.0b013e3181eeeb17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bromberger JT, Kravitz HM, Wei HL, et al. History of depression and women's current health and functioning during midlife. Gen Hosp Psychiatry. 2005;27:200–208. doi: 10.1016/j.genhosppsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 58.Ernst E, Rand JI, Stevinson C. Complementary therapies for depression: an overview. Arch Gen Psychiatry. 1998;55:1026–1032. doi: 10.1001/archpsyc.55.11.1026. [DOI] [PubMed] [Google Scholar]
- 59.Stewart DE, Khalid MJ. Menopause and mental health. In: Romans SE, Seeman MV, editors. Women's mental health: a life-cycle approach. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 297–309. [Google Scholar]
- 60.Frey BN, Lord C, Soares CN. Depression during menopausal transition: a review of treatment strategies and pathophysiological correlates. Menopause Int. 2008;14:123–128. doi: 10.1258/mi.2008.008019. [DOI] [PubMed] [Google Scholar]
- 61.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17:823–827. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 62.Soares CN, Frey BN. Is there a role for estrogen in treating depression during menopause? J Psychiatry Neurosci. 2010;35:E6–E7. doi: 10.1503/jpn.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]