Abstract
The influence of geometry of silica nanomaterials on cellular uptake and toxicity on epithelial and phagocytic cells was studied. Three types of amine-terminated silica nanomaterials were prepared and characterized via the modified Stober method, namely spheres (178±27 nm), worms (232±22 nm × 1348±314 nm) and cylinders (214±29 nm × 428±66 nm). The findings of the study suggest that in this size range and for the cell types studied, geometry does not play a dominant role in the modes of toxicity and uptake of these particles. Rather, a concentration threshold and cell type dependent toxicity of all particle types was observed. This correlated with confocal microscopy observations, as all nanomaterials were observed to be taken up in both cell types, with a greater extent in phagocytic cells. It must be noted that there appears to be a concentration threshold at ~100 µg/mL, below which there is limited to no impact of the nanoparticles on membrane integrity, mitochondrial function, phagocytosis or cell death. Analysis of cell morphology by transmission electron microscopy, colocalization experiments with intracellular markers and Western Blot results provide evidence of potential involvement of lysosomal escape, autophagic like activity, compartmental fusion and recycling in response to intracellular nanoparticle accumulation. These processes could be involved in cellular coping or defense mechanisms. The manipulation of physicochemical properties to enhance or reduce toxicity paves the way for the safe design of silica-based nanoparticles for use in nanomedicine.
INTRODUCTION
Silica nanoparticles are an appealing biomedical platform for nanomedicine since ease in physicochemical modification, economic affordability and potential for reasonably simplistic scale up provide the feasibility of rapid translation. This has led to an increased academic and industrial interest in the creation of new silica nanomaterials for therapeutic, diagnostic, prognostic and combinatory applications.
The chemistry and science of nanomaterials has drastically improved over the last several decades, facilitating the development of silica nanoparticles with significantly different physicochemical characteristics such as surface functionalization and alterations in geometry [1–16]. Silica nanoparticles can be engineered to facilitate controlled release rates, targetability, biocompatibility, and protective stability with which one can encapsulate therapeutics or contrast agents [1–2, 8–13]. New mesoporous silica chemistries have allowed for the development of nanoconstructs with variations in nanopore geometry [1,2]. Such variations provide different mechanisms and path lengths for small molecule encapsulation, which is shown to significantly alter their diffusion patterns [1,2]. Additionally, mesoporous silica can have stimuli sensitive capabilities, where encapsulated drug content can remain protected in the nanoparticle until it reaches the desired delivery site where release can take place via changes in the local environment such as pH, reactive species or magnetic fields [3–7]. Investigators have utilized capping agents such as gold nanoparticles, polymeric supports, dendrimers, cadmium sulfide and magnetic nanoparticles to facilitate encapsulation [3–7]. Additionally, the sol-gel chemistries can facilitate the enclosure of a variety of non-releasing molecular agents, such as organic dyes, iron oxide, quantum dots and gold [8–13]. These doped materials can be utilized in optical, magnetic resonance or photonic imaging and evidence suggests an increase in biocompatibility and signal yields [14–16].
With the development of new nanoscale silica-based materials however, comes the necessity to fully understand how they interact with the biological environment to ensure safety. Evidence suggests that both the material’s physicochemical properties and the cell type which the experiments are performed on alter the mode and mechanisms of induced cellular toxicity. For example, epithelial cells treated with silica nanoparticles show very little to no cytotoxic effects [17], while cells with longer population doubling times (i.e., fibroblasts), or phagocytic capabilities (i.e., macrophages and endothelial cell types) have a substantial increase in toxicity [18, 19]. Nanoparticle size, geometry and surface modification have also proven to alter uptake and toxicity patterns. For example, smaller nanoparticles are widely thought of as having an increased uptake and thus toxicity, mostly contributed to their increase in surface area and exposure to cell surfaces [20, 21]. Likewise, tubular silica nanostructures showed a decrease in uptake when compared to their spherical counter parts [22]. Mesenchymal stem cells have been shown to endocytose positively charged silica nanoparticles to a greater extent than their unmodified systems [23].
To provide validity to these existing and future investigations it will be imperative to correlate physicochemical properties with specified induced biological mechanisms in a systematic fashion. This can lead to the development of safer nanoconstructs, and further lead to the potential for effective manipulation of these properties to facilitate better bioengineered materials for use in nanomedicine. In this work, we set out to investigate the safety and biocompatibility of three silica nanoconstructs, namely spherical, worm-like and cylindrical nanoparticles. The induced biological mechanisms following in vitro treatment of these constructs on model epithelial and phagocytic cell lines is further elucidated.
MATERIALS AND METHODS
Silica nanoparticle synthesis and characterization
Spherical silica nanoparticles, nanoworms, and nanocylinders were prepared utilizing previously reported modified Stober methods [24, 25]. Changes in geometry were facilitated by altering the ratios during synthesis of cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), sodium hydroxide (NaOH), aminopropyltrimethoxysilane (APTMS) and water respectively. The following ratios were used respectively to synthesize the worm-like (1.0:8.16:3.85:2.55:4857), cylinder-like (1.0:8.16:1.28:2.55:4857) and sphere-like (1.0:8.16:0:2.55:4857) particles. Following synthesis, CTAB was removed via acid hydrolysis and extensive washing. 3-aminopropyltriethoxysilane (3-APES) was coupled to the surface of silica nanoparticles by procedures described before [16]. All particles were fluorescently labeled with fluorescein isothiocyanate (FITC) to assess cellular uptake [16]. The constructs were sterilized by dry autoclavation. Transmission electron microscopy (TEM) images were taken on a Phillips, TECHAI F2 (Hillsboro, OR) at an accelerating voltage of 80 kV. TEM samples were created by evaporating droplets of particles suspended in deionized water off copper grids. After micrograph collection nanoconstruct size was measured utilizing Adobe Photoshop’s pixilation ruler measurement tool (Adobe, San Jose, CA). At least the sizes of 300 particles of each type were measured. Particle zeta potential of SNPs dispersed in DI water at a concentration of 1.0 mg/ml was measured using a Malvern Instruments Zetasizer Nano ZS (Westborough, MA). SNPs (50 or 25 mg/ml) were sonicated, vortexed and the final particle dispersions were prepared immediately before use from common stock in cultured medium and vortexed before application to the culture cells. With the exception of fluorescent microscopy and flow cytometry studies, all experiments were carried out with unlabeled SNPs. The absence of CTAB and presence of primary amines after acid hydrolysis was ascertained by a Nicolet 740 FT-IR spectrometer (West Palm Beach, FL) and TA Instruments TGA2950 thermogravimetric analyzer (New Castle, DE).
Cell culture
Human adenocarcinoma alveolar basal epithelial A549 cells, and RAW 264.7 murine macrophages were obtained from ATCC (Manassas, Virginia) and maintained in the recommended media supplemented with 10% FBS. Cell cultures were incubated at 37°C in 5% CO2 and 95% humidified air and kept in logarithmic phase of growth throughout all experiments.
Measurement of cell viability and proliferation
Cells were exposed to a range of concentrations (.0001–1000 ug/mL) of silica nanoparticles for 72 hours. They were subsequently washed with phosphate buffer saline (PBS) and relative cell viability was assessed by utilizing a water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt], the key component in the Cell Counting Kit-8 from Dojindo Molecular Technologies, Inc (Rockville, Maryland). IC50 values were calculated utilizing GraphPad Prism software (La Jolla, CA). To assess plasma membrane integrity, cells were exposed to various concentrations of silica constructs for 24 hours and assayed for lactate dehydrogenase (LDH) or leakage with the CytoScan LDH leakage cytotoxicity assay from G-Bioscience (Madison, WI). Maximum LDH was assessed via total control cell lysis induced by 0.1% Triton X-100, and a diluted series of LDH enzymes supplied with the kit were utilized as positive control.
Cellular uptake
The uptake of silica nanoparticles by cultured cells was visualized by confocal microscopy. Cells were grown on 35-mm glass bottom microwell dishes (MatTek, Ashland, MA) and incubated for 24 hours with 50 µg/ml FITC labeled silica nanoconstructs. Cell nuclei were stained with 2.5 µM DRAQ5 (Biostatus Ltd.) according to the manufacturer's protocol. Fluorescent images of live cells were taken under a confocal laser scanning microscope (CLSM) Olympus FluoView® FV1000 (Olympus America Corp., Center Valley, PA). The intensity of the laser beam and the photodetector sensitivity were kept constant in order to compare the relative fluorescence intensities between experiments. Z stacks were collected and used for 3D reconstruction and visualization of intracellular particle localization. All image acquisitions and analyses were performed using FluoView 2.0 software.
Colocalization of nanoconstructs with intracellular compartments
The colocalization of silica nanoparticles with acidic and basic lysosomes by cultured cells was assessed by confocal microscopy. Cells were grown on 35-mm glass bottom microwell dishes (MatTek, Ashland, MA) and incubated for 24 hours with 50 µg/ml FITC labeled silica nanoconstructs. Lysosomes were stained with 2.5 µM LysoSensor Yellow/Blue DND-160 or 50 µg/mL Transferrin Alexa Fluor 546 (Invitrogen Corp., Carlsbad, CA). Fluorescent images of live cells were taken by CLSM as described above.
Annexin V / Propidium Iodine (PI) staining
Cells were grown for 24 hours on 35-mm glass bottom microwell dishes (MatTek, Ashland, MA) and incubated for 24 hours with 50 µg/ml FITC labeled silica nanoconstructs. Annexin V/PI staining was used for detection of the mode of cell death according to the Vybrant Apoptosis Kit #3 (Invitrogen) manufacturer’s instructions.
Transmission Electron Microscope analysis
The uptake of silica constructs by cultured cells was assessed by transmission electron microscopy. Cells were seeded on 6 well plates containing 1×1 cm ACLAR plastic at 2 × 105 cells per well. After an overnight incubation, 50 µg/mL of silica nanoconstructs was added and cells were incubated for 24 hours after which cells were washed with PBS and fixed with a 2.5% glutaraldehyde and 1% formaldehyde in 0.1M sodium cacodylate buffer with sucrose and calcium chloride. Cells were stained with uranyl acetate for 45 minutes at room temperature and TEM images were taken with a Phillips, TECHAI F2 TEM (Hillsboro, OR, USA) at an accelerating voltage of 80 kV.
Phagocytic activity
Relative levels of phagocytic activity were assessed utilizing IgG-FITC coated beads, obtained from Caymen Chemicals (Ann Arbor, Michigan). A 96 well plate was seeded with 10,000 cells per well, 24 hours prior to being incubated for 24 hours with 62.5 µg/mL and 250 mg/mL of silica nanoparticles and a 1:10 dilution in cell culture medium of IgG-FITC coated beads, according to manufactures instructions. The relative bead uptake was assessed utilizing a Molecular Device’s Spectramax M2 (Sunnyvale, CA).
Caspase 3 induction
Relative levels of caspase 3 induction were assessed utilizing Caspase 3 cell lysate kit, obtained from Caymen Chemicals (Ann Arbor, Michigan). RAW 264.7 and A549 cells were plated in a 96 well plate at 10,000 cells per well, 24 hours prior to being incubated with 62.5 µg/mL and 250 µg/mL of each silica nanoparticle for 24 hours. Incubation with adipocyte was utilized as a positive control. Cells were lysed, supernatant removed and incubated with caspase 3 detection protein, according to the manufacturer’s instructions. The relative levels of caspase 3 induction were assessed utilizing a Molecular Device’s Spectramax M2 (Sunnyvale, CA).
Western blot
The relative levels of LC-3 I, LC-3 II, ATG9a, ATG5, and beclin induction were assessed utilizing standard western blot techniques. Cells were plated in a 6 well plate at 500,000 cells per well, incubated for 2.5 hours with particle concentrations of 62.5 µg/mL and 250 mg/mL. Tamoxifen and Rapamycin were utilized as positive controls. Following incubation, cells were washed with phosphate buffered saline (PBS), incubated for 10 minutes with Radio-Immunoprecipitation Assay (RIPA) buffer supplemented with protease inhibitor cocktail (Sigma) and protein was harvested after a 15 minute centrifugation at 14,000× g. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed with a loading of 50 µg of protein per well. Protein immunobloting was performed via gel transfer to a Polyvinylidene Fluoride membrane. The membrane was blocked with a 5% milk, PBS, 0.5% Tween-20 (Sigma) solution and incubated with primary antibodies obtained from Novus Biologicals for 1 hour and then incubated with a peroxidase conjugated secondary stabilized goat anti-rabbit antibody (Pierce) for 1 hour. Proteins were detected with western blotting luminal reagent (Santa Cruz) and imaged utilizing UVP Biospectrum Multispectral Imaging System (Upland, CA).
Statistical analysis
All experiments were performed in triplicate, and the results were presented as mean ± standard deviation. Student’s t-test (two tailed, unpaired) was performed between samples of nanoparticle-treated cells vs controls, unless stated otherwise. The difference between values was considered significant at the level of p<0.05.
RESULTS AND DISCUSSION
Particle synthesis and characterization
Three silica nanoparticle types with various geometries were synthesized; worms, cylinders, and spheres. Each construct had a common dimension of approximately 200 nm, with an average equivalent positive charge density and an approximate 20 percent variation in the size of the nanoparticle (Table 1, Figure 1).
Table 1.
Physicochemical Characteristics of Silica Nanoparticles
| Nanoparticle | Size (TEM) | Zeta Potential |
|---|---|---|
| 232 ± 22 nm | ||
| Worms | × | 87 mV |
| 1348 ± 314 nm | ||
| 214 ± 29nm | ||
| Cylinders | × | 79 mV |
| 428 ± 66nm | ||
| Spheres | 178 ± 27nm | 58 mV |
Figure 1.
Transmission Electron Microscope (TEM) images of aqueous suspensions of silica nanoconstructs. A) Worms; B) Cylinders; C) Spheres.
Variations in nanoparticle geometries were due to alterations in incorporated APTMS and CTAB ratios. CTAB is a surfactant and self associates at a critical concentration, facilitating the formation of micelles that serve as nucleation sites, from which TEOS hydrolyzes to create the solid nanoparticle. Variations in geometry were initiated by altering the critical concentration of the CTAB micelles. In this work APTMS was utilized in various ratios, which altered the degree of association, shape of the micelles and the resulting geometry. This synthetic process can create long micelles that can wiggle in solution and obtain various conformations. When nucleation occurs, these conformations lock, and longer micelles can form additional conformations. Thus, nanoworm constructs had significant variations in length elongation or geometry, while nanocylinders and nanoparticles maintained a relatively uniform geometry. CTAB was removed via acid hydrolysis and confirmation of complete removal was obtained from IR and TGA analysis (data not shown). Following removal of CTAB, APES was added, to ensure total equivalent exposure of the relative level of primary amines across all constructs. This was done so that the surfaces of the particles remained consistent across all geometries. It is important to note that fluorescence labeling did not significantly alter the shape of the constructs (data not shown), and affected zeta potential, minimally.
Cellular Toleration Threshold
Cellular viability, proliferation and function
The materials were designed with a highly positive surface charge to promote cellular uptake [23] and facilitate the assessment and correlation of changes in geometry on uptake and toxicity. RAW 264.7 and A549 cells were chosen as representative in vitro models of cells of the reticulo-endothelial system (RES) and epithelial cell types, in which silica nanoparticles have previously been evaluated and shown a cell type dependent toxicity, with an increased toxicity observed in macrophages [26]. This is not surprising, as one of the primary functions of RES cells is to remove foreign materials from the biological environment. It is important to note, however that while macrophages have an inherent advantage of engulfing nanoconstructs due to their primary phagocytic nature, epithelial cells do occasionally present with phagocytic capabilities [27].
Following synthesis and characterization of silica nanoconstructs, their abilities to interfere with cell proliferation and to compromise plasma membrane integrity were evaluated. All the constructs inhibited cell proliferation in a dose-dependent manner. The toxicity of nanoconstructs was cell-type dependent, showing that macrophages were more susceptible to treatment than epithelial cells (Table 2).
Table 2.
IC50 values (µg/ml) for silica nanoparticles*
| Nanoparticle | A549 | RAW 264.7 |
|---|---|---|
| Cylinders | 141 ± 5 | 82 ± 3 |
| Spheres | N/A | 113 ± 9 |
| Worms | 324 ± 13 | 73 ± 19 |
Concentrations leading to the death of 50% of cell population after 72 h incubation. The values (mean+/−SD, µg/ml) of 3 independent experiments are shown.
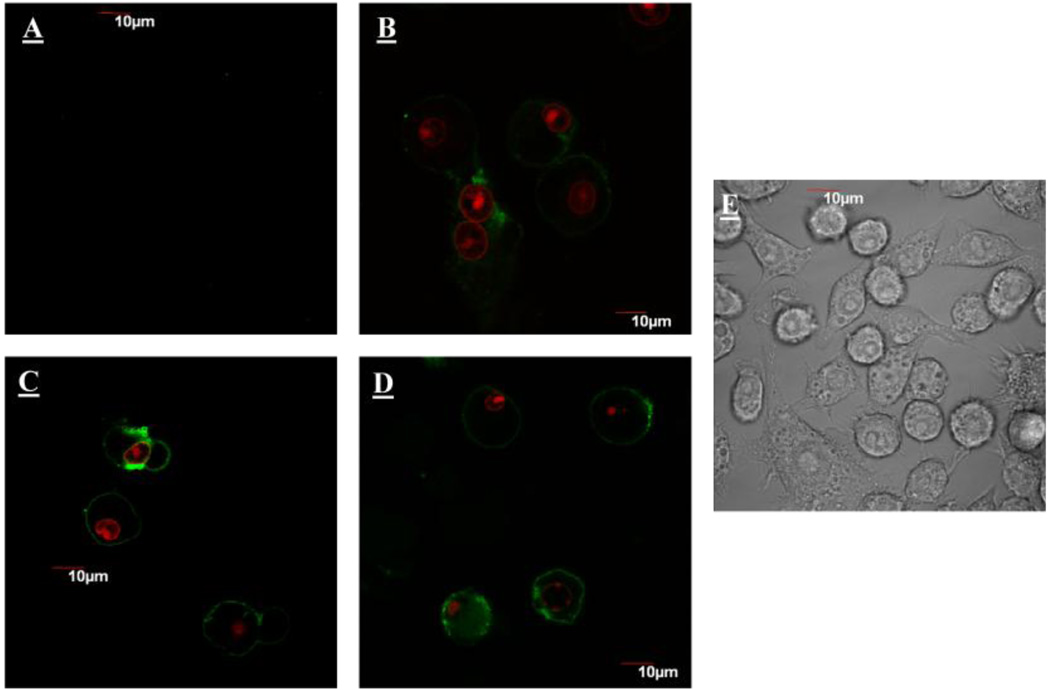
This observation correlated well with cellular uptake results in which particles exhibited a greater accumulation in macrophages when compared to epithelial cells (Figure 2).
Figure 2.
Internalization of 50 µg/mL FITC labeled (green) silica nanoconstructs after 24 hours of incubation with RAW264.7 and A549 cells. Nuclei are stained with DRAQ5 (pink). RAW 264.7 cells incubated with worms (A), cylinders (B), or spheres (C); A549 incubated with worms (D), cylinders (E), or spheres (F).
Proliferative functional damage presented in macrophages at approximately 80 µg/mL while epithelial cells did not present with damage until 200 µg/mL, both with minimal geometric impact (Supplemental Figures 1 and 2). The particles appear to have no affect on cell proliferative function up until a specific concentration at which point cell proliferation drops off rapidly. Additionally, limited plasma membrane damage, as was manifested with minimal LDH release, was observed in both cell lines, with the exception of very high nanoparticle concentrations (Figure 3). Nanoparticle geometry played little to no significant role in the relative release of LDH in both cell lines. These results suggests that the membrane integrity of the particles is maintained up to ~250 µg/mL in both cell lines, however the proliferative function of RAW 264.7 cells initiated a higher release of LDH at those concentrations. While the data presents a cell type dependent toxicity, it does not seem to present with a significant difference when comparing changes in construct geometry within a particular cell type. It is important to note, however, that the design of these constructs was such that they had similar surface characteristics and common dimensionality. It is well known that nanoparticle protein adsorption helps to facilitate cellular internalization and uptake dictating modes and mechanisms of toxicity [28–31]. The highly positive charge associated with these particles could potentially provide an enhanced protein association negating geometric implications. Very slight variations in surface chemistry have proven to present drastic differences in protein absorption on silica nanoconstructs [32]. This could provide a reason for variations, or the lack there of, in results with regards to nanoconstruct geometry. This phenomenon however needs further detailed investigation and can depend on both surface characteristics and cell type.
Figure 3.
Effect of nanoconstructs on plasma membrane integrity measured by LDH release.
Mechanisms of cell death
To assess the mode of cell death induced by the nanoparticles, the phosphatidyl serine translocation and activation of key enzymes of apoptotic cascade were examined.
Annexin V has high affinity to phosphatidylserine which is translocated from the inner layer to the outer layer of the plasma membrane during apoptosis. Propidium Iodide (PI) is an intercalating agent which stains DNA and can only penetrate cells that have compromised membrane integrity. Positive staining with PI is indicative of necrotic cell death. The staining patterns present at higher concentrations (500 µg/mL, Figure 4 and Supplemental Figure 3), are either red or a combination of red and green, which is indicative of late stage apoptotic or necrotic cell death. Little to no Annexin V or PI positive staining was observed when cells were treated with low concentrations (62.5 µg/mL, data not shown) of nanoconstructs. This observation correlates with LDH release, as membrane integrity appears to be intact up to 250 µg/mL. In an attempt to differentiate between apoptotic and necrotic cell death, cell lysates were evaluated for relative levels of active caspase 3. Activation of caspase 3, which present in the cytoplasm as an active proenzyme, plays a central role in the execution phase of apoptosis. No significant activation of the enzyme was observed in treated or control cells, however caspase 3 activation was observed in adipocytes, which were used as the positive control (Figure 5 and Supplemental Figure 4). These results seem to suggest that cells are able to survive and proliferate up to a certain concentration threshold, at which point cells are overwhelmed with the nanoparticles and undergo necrotic cell death due to membrane rupture. It must be noted that nanoparticle geometry played little to no role in caspase activation or observable increases in Annexin V or PI positive staining.
Figure 4.
Mode of cell death induced in RAW264.7 cells after 24 hours of incubation with 500 µg/mL of silica nanoparticles. Annexin V (green) staining provides evidence of apoptotic cell death and PI (red) staining provides evidence of necrotic cell death. A) Control; B) Spheres; C) Worms; D) Cylinders.
Figure 5.
Relative levels of activated caspase 3 by silica nanoparticles treated with RAW 264.7 cells. The star indicates a statisticallyy significant increase in caspase activation when compared to control.
Cellular coping mechanisms
Cellular uptake and internalization
Validation of cellular concentration toleration thresholds claims was attempted utilizing cellular uptake visualization methods. Both cell types did uptake these constructs to a significant extent (Figure 2). However constructs were taken up to a greater extent by RAW 264.7 cells. Particle geometry again, did not appear to have a significant impact on relative nanoparticle uptake, in both cell lines. Increased relative fluorescene in Figure 2 could be due to the depicted focal plane or the fact that the relative fluorescence per particle was not normalized facilitating variations in relative emissions.
Transferrin is a blood plasma glycoprotein utilized in iron transport. Because of well established intracellular transport mechanism, transferrin is often used as a marker of receptor-mediated endocytosis. Co-incubation of transferrin and nanoconstructs, appeared to provide evidence that geometry plays little to no role in the uptake of particles. However, co-localization of transferrin was only observed in RAW 264.7 cells and appeared to be absent in A549 cells (representative image, Supplemental Figure 5). This suggests that receptor mediated endocytosis mechanisms may potentially be responsible for some RAW 264.7 cell particle internalization and cellular trafficking, while such mechanisms are improbable for A549 cells. This phenomenon has previously been confirmed as the most probable mechanistic route of internalization of spherical silica nanoparticles [33–35]. Furthermore, phagocytic activity of Ig-G microparticles was assessed to help rule out interference with phagocytic uptake mechanisms. As expected, all concentrations and particle types did not appear to interfere with Ig-G microparticle uptake (Supplemental Figure 6). This suggests that nanoparticles do not interfere with phagocytic activity of microparticles, and could further indicate the possibility of endocytosis. However other modes of uptake, including phagocytosis, could potentially be involved. Two concentrations of particles were studied; 62.5 µg/mL and 250 µg/mL. Incubation of cells with both concentrations presented with similar results. A549 cells may have internalized particles via another mechanism of endocytosis. Further studies will need to be performed to assess the detailed modes of internalization of silica nanoparticles.
Lysosomal colocalization, escape, and compartmental recycling
Uptaken particles were further analyzed with lysosensor co-localization. Lysosensor dyes accumulate and fluoresce in acidic compartments due to protonation of the basic side chains and are commonly utilized as a colocalization tool to assess relative lysosomal encapsulation. A specific lysosensor that exhibits two different spectral fluorescence peaks dependent on the relative acidity of the compartment was chosen. Particles were incubated for 24 hours and lysosomes were stained with lysosensor to assess the relative lysosomal compartmentalization. Results indicate that constructs were not always co-localized with lysosomes and some appeared to be free within the cytoplasm. However, when co-localized they appear to reside within more highly acidic lysosomal compartments. All particles within A549 and RAW 264.7 cells presented with similar results, suggesting similar cellular sequestering mechanisms despite the variations in geometry (representative results, Supplemental Figure 7). These results suggest lysosomal escape from basic compartments, acidic compartmental fusion or an indication of different stages of lysosome maturation.
Transmission electron microscopy was utilized to validate co-localization via confocal microscopy. Similar to lysosomal co-localization results, particles were observed both in membrane-bound organelles and in cytoplasm (representative image, Figure 6). Cytoplasmic content could help support lysosomal escape mechanisms suggested by delocalized particles in lysosensor colocalization results.
Figure 6.
Representative TEM images of the uptake of spherical particles by A549 cells at 50 µg/mL concentration and over 24 hour incubation. A) and B) Depict numerous vacuoles with particles encapsulated within them. Additionally, numerous lysosomes were observed, while several vacuoles and lysosomes appear to be fusing with one another. Futhermore, a few particles appear to be isolated without vacuole encapsulation within the cytoplasm. C) Depicts an apparent larger vacuole formation after vesicle fusion. No differences were observed across all particle types.
Previous studies have suggested that silica nanoparticles can be trafficked to lysosomal compartments [36], however the lysosomal environment was not sufficient to facilitate degradation of these particles. Thus, it has been suggested that these particles can remain within these compartments through late stage lysosomal digestion and then release into the cytoplasm [36]. Additionally, investigations have provided evidence that these materials remain within these compartments for a sufficient period of time to be involved in compartmental recycling, where the particles are trafficked from the lysosome to the surface of the cell and released extracellularly [37]. These mechanisms could be a process by which cells are attempting to cope with the internalization of a material that they are both unfamiliar with and unable to digest.
Autophagic activity
If compartmental recycling and lysosomal escape are indeed playing a role in these coping mechanisms, it would not be unusual to suggest that other native cell mechanisms that help to remove foreign pathogens or misfolded proteins from cells could be at play. Evidence suggests that other nanoconstructs such as dendrimers, quantum dots and gold nanoparticles also induce autophagic mechanisms [38–40]. Autophagy is the process in which materials that cells cannot digest are trafficked and sequestered into membrane bound vesicles, which isolate them from the rest of the cell and promote degradation (Figure 7). In this work we confirm the autophagic activity by assessing traditional morphological features indicated in TEM images and overexpressed proteins in western blot analysis.
Figure 7.
Illustration of native autophagic vesicle formation. It is hypothesized that the nanoparticles initiate progression and virulence of autophagic processes within the in-vitro cellular environment.
Transmission electron microscopy images of all particle and cell types were evaluated for hallmark autophagic morphological features [41]. Both cell types and all particle treatments, presented with similar morphological features. Particles appear to be co-localized in double membrane vesicles that contain other whole or remnants of cellular organelles (Figure 8).
Figure 8.
Representative TEM images of the uptake of cylindrical and worm-like particles at 50 µg/mL and 24 hour incubation in A549 cells (B), and RAW 264.7 (A and C). Particles appear to be co-localized in double membrane lysosome-like vesicles (A), some of which contain whole (B) or remnants (C) of cellular organelles. Treated cell size is much larger and the mitochondria appear to be swollen, when compared to control (C). No differences were observed as a function of particle geometry.
Additionally, particles appear to be uptaken in small quantities, which are then later sequestered in larger vacuoles containing multiple particles (Figure 6). This is enhanced by apparent increased lysosomal fusion, when compared to control cells. Furthermore, when compared to control cells, the relative size is increased and mitochondria appear to have swollen features.
Autophagic activity was confirmed by assessing the presence of and the expression level of several characteristic marker proteins, namely: LC-3 I, LC-3 II, ATG9a, ATG5, and beclin. LC-3 protein component in the development of autophagic vesicles. LC-3 I is a cytosolic form and is converted to LC-3 II when it is incorporated in the membrane of autophagic vesicles. The upregulation of this conversion can be quantitatively correlated with the number of autophagic vesicles formed [42, 43]. The presence and upregulation of Beclin, ATG9a, and ATG5 correlate but are not specific to autophagic activity [42, 43]. However the presence of these proteins is essential to the development of autophagic vesicles and provides evidence of tracking the autophagic process from initiation to complete development. Beclin is an important regulator of the selective turnover of proteins involved in cell growth and proliferation and participates in the nucleation of the autophagic membrane, ATG-5 is involved in a ubiquitin like conjugated system that facilitates the growth of the membrane, and ATG9a facilitates the last stage of vesicle expansion. The results of protein immunoblot indicate that incubation with nanoconstructs resulted in the upregulation of LC-3 I, LC-3 II, ATG9a, ATG5, ATG16, and beclin, in both cell lines (representative image, Figure 9 and Supplement Figure 8).
Figure 9.
Representative western blot image of LC3-I and LC3-II protein expression. 6 well plates of RAW 264.7 cells were treated with 250 µg/mL of particles and incubated for 2.5 hours before harvesting. Expression was also seen in A549 but to a much lesser extent (data not shown). Control cells did express LC3-I but did not express LC3-II. Tamoxifen was utilized as a positive control to validate methodology. Limited changes in protein expression was seen when cells were treated with 50 µg/mL of particles.
Overall these findings suggest that cells are able to uptake silica nanoparticles and cope with them up to a certain concentration threshold. They utilize native cellular processes including autophagic mechanisms and potentially lysosomal recycling and escape to facilitate this coping. At a certain concentration cells are no longer able to handle nanoparticle internalization, and it appears that the particles consume the extracellular space and facilitate membrane damage and cause cells to undergo necrotic cell death. In the size and shape range studied, geometry in this case, seems to play little to no role in the induced biological toxicity. However surface charge, size and relative curvature might significantly alter uptake and toxicity patterns, which is a subject of further studies. Additionally, it is important to note that geometry might play a significant role in vivo when one accounts for the transport of particles through the blood stream and across endothelial barriers.
CONCLUSION
This work demonstrates cell type dependent toxicity of cationic silica nanoparticles and illustrates limited geometric dependence in biological results across the two cell lines, and in the size range studied. Evidence collected suggests the existence of a cellular toleration concentration threshold and the involvement of autophagic processes in cellular coping mechanisms for silica nanoparticles.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (R01- DE19050), National Science Foundation (NSF-NIRT: 0835342) and the Utah Science Technology and Research (USTAR) Initiative. The authors would also like to thank Nancy Chandler from the Health Sciences Center Research Microscopy Facility at the University of Utah for her help with transmission electron microscopy.
Footnotes
CONFLICT OF INTEREST: No conflicts of interests are present.
REFERENCES
- 1.Stromme M, Brohede U, Atluri R, Garcia-Bennett AE. Mesoporous silica-based nanomaterials for drug delivery: evaluation of structural properties associated with release rate. Wiley interdisciplinary reviews. 2009;1(1):140–148. doi: 10.1002/wnan.13. [DOI] [PubMed] [Google Scholar]
- 2.Brohede U, Atluri R, Garcia-Bennett AE, Stromme M. Sustained release from mesoporous nanoparticles: evaluation of structural properties associated with release rate. Current Drug Delivery. 2008;5(3):177–185. doi: 10.2174/156720108784911686. [DOI] [PubMed] [Google Scholar]
- 3.Radu DR, Lai CY, Wiench JW, Pruski M, Lin VS. Gate keeping layer effect: a poly(lactic acid)-coated mesoporous silica nanosphere-based fluorescence probe for detection of amino-containing neurotransmitters. Journal of the American Chemical Society. 2004;126(6):1640–1641. doi: 10.1021/ja038222v. [DOI] [PubMed] [Google Scholar]
- 4.Aznar E, Marcos MD, Martinez-Manez R, Sancenon F, Soto J, Amoros P, Guillem C. pH- and photo-switched release of guest molecules from mesoporous silica supports. Journal of the American Chemical Society. 2009;131(19):6833–6843. doi: 10.1021/ja810011p. [DOI] [PubMed] [Google Scholar]
- 5.Gruenhagen JA, Lai CY, Radu DR, Lin VS, Yeung ES. Real-time imaging of tunable adenosine 5-triphosphate release from an MCM-41-type mesoporous silica nanosphere-based delivery system. Applied Spectroscopy. 2005;59(4):424–431. doi: 10.1366/0003702053641513. [DOI] [PubMed] [Google Scholar]
- 6.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VS. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. Journal of the American Chemical Society. 2003;125(15):4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 7.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. Journal of the American Chemical Society. 2009;132(2):552–557. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 8.Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano letters. 2009;9(1):442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopp D, Tang D, Niessner R. Review: bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Analytica Chimica Acta. 2009;647(1):14–30. doi: 10.1016/j.aca.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Shantz DF. Ordered mesoporous silica-based inorganic nanocomposites. Journal of Solid State Chemistry. 2008;181:1659–1669. [Google Scholar]
- 11.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2(5):889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Insin N, Tracy JB, Lee H, Zimmer JP, Westervelt RM, Bawendi MG. Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano. 2008;2(2):197–202. doi: 10.1021/nn700344x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Brown S, Walter G, Santra S, Moudgil B. Nanoparticles for bioimaging. Advances in Colloid and Interface Science. 2006;123–126:471–485. doi: 10.1016/j.cis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4(2):699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y, Kannan S, Wu M, Zhao JX. Toxicity of luminescent silica nanoparticles to living cells. Chemical Research in Toxicology. 2007;20(8):1126–1133. doi: 10.1021/tx7001959. [DOI] [PubMed] [Google Scholar]
- 16.Blaaderen AV, Vrij A. Synthesis and characterization of colloidal dispersions of fluorescent silica spheres. Langmuir. 1992;8:2921–2931. [Google Scholar]
- 17.Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle and Fibre Toxicology. 2009;6:14. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JS, Chang KL, Hwang DF, Kong ZL. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environmental Science & Technology. 2007;41(6):2064–2068. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radical Biology & Medicine. 2008;44(7):1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters KM, Masiello LM, Zangar RC, Tarasevich BJ, Karin NJ, Queesenberry RD, Bandyopadhyay S, Teeguarden JG, Pounds JG, Thrall BD. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicological Science. 2009;107(2):553–569. doi: 10.1093/toxsci/kfn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clift MJ, Rothen-Rutishauser B, Brown DM, Duffin R, Donaldson K, Proudfoot L, Guy K, Stone V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicology and Applied Pharmacology. 2008;232(3):418–427. doi: 10.1016/j.taap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Trewyn BG, Nieweg JA, Zhao Y, Lin VS-Y. Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chemical Engineering Journal. 2008;137(1):23–29. [Google Scholar]
- 23.Chung TH, Wu SH, Yao M, Lu CW, Lin YS, Hung Y, Mou CY, Chen YC, Huang DM. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28(19):2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Gu B, Liang L, Hamilton W. Fabrication of two and three-dimensional silica nanocolloidal particle arrays. Journal of Physical Chemistry. 2003;107(15):3400–3404. [Google Scholar]
- 25.Huh S, Wiench JW, Yoo J-C, Pruski M, Lin VS-Y. Organic functionalization and morphology control of mesoporous silicas via a co-condensation synthesis method. Chem. Mater. 2003;15:4247–4256. [Google Scholar]
- 26.Malugin A, Herd H, Ghandehari H. Differential toxicity of amorphous silica nanoparticles toward phagocytic and epithelial cells. Journal of Nanoparticle Research. submitted. [Google Scholar]
- 27.Monks J, Rosner D, Jon Geske F, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12(2):107–114. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced Drug Delivery Reviews. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicological Sciences. 2007;100(1):303–315. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, von Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Experimental Cell Research. 2005;305(1):51–62. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson M, Carlsson U. Protein adsorption orientation in the light of fluorescent probes: mapping of the interaction between site-directly labeled human carbonic anhydrase II and silica nanoparticles. Biophysical Journal. 2005;88(5):3536–3544. doi: 10.1529/biophysj.104.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun W, Fang N, Trewyn BG, Tsunoda M, Slowing II, Lin VS, Yeung ES. Endocytosis of a single mesoporous silica nanoparticle into a human lung cancer cell observed by differential interference contrast microscopy. Analytical and Bioanalytical Chemistry. 2008;391(6):2119–2125. doi: 10.1007/s00216-008-2162-1. [DOI] [PubMed] [Google Scholar]
- 34.Xing X, He X, Peng J, Wang K, Tan W. Uptake of silica-coated nanoparticles by HeLa cells. Journal of Nanoscience and Nanotechnology. 2005;5(10):1688–1693. doi: 10.1166/jnn.2005.199. [DOI] [PubMed] [Google Scholar]
- 35.Muhlfeld C, Gehr P, Rothen-Rutishauser B. Translocation and cellular entering mechanisms of nanoparticles in the respiratory tract. Swiss Med Wkly. 2008;138(27–28):387–391. doi: 10.4414/smw.2008.12153. [DOI] [PubMed] [Google Scholar]
- 36.Huang DM, Hung Y, Ko BS, Hsu SC, Chen WH, Chien CL, Tsai CP, Kuo CT, Kang JC, Yang CS, Mou CY, Chen YC. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19(14):2014–2016. doi: 10.1096/fj.05-4288fje. [DOI] [PubMed] [Google Scholar]
- 37.Tao Z, Toms BB, Goodisman J, Asefa T. Mesoporosity and functional group dependent endocytosis and cytotoxicity of silica nanomaterials. Chemical Research in Toxicology. 2009;22(11):1869–1880. doi: 10.1021/tx900276u. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Liu H, Sun Y, Wang H, Guo F, Rao S, Deng J, Zhang Y, Miao Y, Guo C, Meng J, Chen X, Li L, Li D, Xu H, Wang H, Li B, Jiang C. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. Journal of Molecular Cell Biology. 2009;1(1):37–45. doi: 10.1093/jmcb/mjp002. [DOI] [PubMed] [Google Scholar]
- 39.Seleverstov O, Zabirnyk O, Zscharnack M, Bulavina L, Nowicki M, Heinrich JM, Yezhelyev M, Emmrich F, O'Regan R, Bader A. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano letters. 2006;6(12):2826–2832. doi: 10.1021/nl0619711. [DOI] [PubMed] [Google Scholar]
- 40.Li JJ, Hartono D, Ong CN, Bay BH, Yung LY. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials. 2010;31(23):5996–6003. doi: 10.1016/j.biomaterials.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16(7):956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 43.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.