Abstract
Melanocortin-1 receptor (MC1-R) and melanin are two attractive melanoma-specific targets for peptide-targeted radionuclide therapy for melanoma. Radiolabeled peptides targeting MC1-R/melanin can selectively and specifically target cytotoxic radiation generated from therapeutic radionuclides to melanoma cells for cell killing, while sparing the normal tissues and organs. This review highlights the recent advances of peptide-targeted radionuclide therapy of melanoma targeting MC1R and melanin. The promising therapeutic efficacies of 188Re-(Arg11)CCMSH (188Re-[Cys3,4,10, d-Phe7, Arg11]-α-MSH3-13), 177Lu- and 212Pb-labeled DOTA-Re(Arg11)CCMSH (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-[ReO-(Cys3,4,10, d-Phe7, Arg11)]-α-MSH3-13) and 188Re-HYNIC-4B4 (188Re-hydrazinonicotinamide-Tyr-Glu-Arg-Lys-Phe-Trp-His-Gly-Arg-His) in preclinical melanoma-bearing models demonstrate an optimistic outlook for peptide-targeted radionuclide therapy for melanoma. Peptide-targeted radionuclide therapy for melanoma will likely contribute in an adjuvant setting, once the primary tumor has been surgically removed, to treat metastatic deposits and for treatment of end-stage disease. The lack of effective treatments for metastatic melanoma and end stage disease underscores the necessity to develop and implement new treatment strategies, such as peptide-targeted radionuclide therapy.
Keywords: Peptide-targeted, radionuclide therapy, melanoma
2. INTRODUCTION
Malignant melanoma is the most lethal form of skin cancer and is sixth most commonly diagnosed cancer with an increasing incidence in the United States. It is predicted that 59,940 new cases will be diagnosed and 8,110 fatalities will occur in the year 2007 (1). At the present time, more than 1.3% of Americans will develop malignant melanoma during their lifetime (2). Among young adults, melanoma is the most commonly diagnosed malignancy (3). Early diagnosis and prompt surgical removal of primary melanoma lesions provide the best opportunities for cures or prolonged survival to melanoma patients (4-6). Melanoma metastases are very aggressive and the survival time for patients with metastatic melanoma averages 3-15 months (7). Current available treatments for melanoma patients are limited and mainly include surgery, chemotherapy, immunotherapy and external beam radiation therapy. Unfortunately, there is no satisfactory treatment for metastatic melanoma due to its resistance to current chemotherapy and immunotherapy regimens (8). Hence, novel and more effective therapeutic approaches are urgently needed for melanoma treatment.
Significant advances in the use of radiolabeled peptides for cancer therapy make peptide-targeted radionuclide therapy a very attractive treatment modality for cancer. Receptor-avid peptides are employed as effective delivery vehicles to selectively and specifically target cytotoxic radiation generated from radionuclides to tumor cells, resulting in tumor cell death (9). In comparison with external beam radiation therapy and chemotherapy, peptide-targeted radionuclide therapy can specifically deliver the radiation dose to tumor cells, while sparing the normal tissues and organs. Beta-particle-emitting, α-particle-emitting and Auger-electron-emitting radioisotopes may be used to radiolabel receptor-targeting peptides for targeted radionuclide therapy (10). High-energy β-emitters such as 188Re and 90Y appear appropriate for the treatment of larger tumors or large tumor burdens. Medium- and lower-energy β-emitters, such as 177Lu, may be more suitable for treating smaller tumors or metastatic deposits (11-16). Alpha-emitters are attractive for treating small tumor and metastases due to their short path-length and high linear energy transfer (LET). Auger-emitters appear to have favorable properties for treating metastases and disseminated tumor cells due to their highly localized energy deposition and short path-length. The selection criteria for therapeutic radionuclides will be further discussed in this review.
Currently, two melanoma-associated molecular targets, melanocortin-1 receptor (MC1-R) and melanin, have been used as targets for melanoma imaging and therapy. MC1-R belongs to the superfamily of G protein-coupled receptors (GPCRs) and is over-expressed in both melanotic and amelanotic melanomas (17-20). Greater than 80% of human metastatic melanoma samples have been identified to display MC1 receptors (17). The MC1-R density of human melanoma cells are presented in Table 1. Radiolabeled alpha-melanocyte stimulating hormone (α-MSH) peptide analogues exhibit nanomolar MC1-R binding affinities, making them very promising melanoma-specific molecular agents for melanoma detection and therapy. The radiolabeled α-MSH peptides targeting the MC1-Rs for melanoma imaging has been reviewed elsewhere (21). Pigment melanin is another attractive molecular target for melanoma imaging and therapy due to its existence in most melanomas and neoplastic melanocytes that ultimately develop to melanoma. Two major types of melanin, namely eumelanin and pheomelanin, are found in melanomas. Eumelanin is the predominant pigment in primary tumors, whereas pheomelanin is associated with the progression of the disease (22). Melanin is a negatively-charged dark pigment that is produced by melanocytes (23, 24). Normally, melanin is sequestered within melanocytes and melanoma tumor cells, however, programmed cell death or necrosis lead to melanin release into the surrounding environment (25). Melanin’s insolubility in aqueous environments and inert chemical properties limit redistribution once released from cells.
Table 1.
MC1 receptor density on human melanoma cells
| Cell line | MC1 receptor density (receptors/cell) |
|---|---|
| TXM13 | 5,700 |
| 3M | 5,000 |
| UACC257 | 2,800 |
| M14 | 1,500 |
| UACC62 | 1,000 |
| LOX | 1,000 |
| SKMEL5 | 1,000 |
| SKMEL28 | 900 |
Miao Y, et al. (20).
This review highlights the recent advances of peptide-targeted radionuclide therapy for melanoma targeting MC1-Rs and melanin. While radiolabeled peptides that target the somatostatin-2 G protein-linked receptor have been examined for melanoma therapeutic efficacy, recent clinical results with the radiolabeled somatostatin-2 receptor-targeting peptide, [177Lu-DOTA0,Tyr3-octreotate] (26), demonstrated no therapeutic benefits for patients with melanoma. Hence, this review will focus on radiolabeled peptides targeting MC1-Rs and melanin for melanoma therapy. Since there are few published preclinical peptide-targeted radionuclide therapy studies and an even smaller number of clinical case studies on melanoma, this review will focus on published preclinical studies.
3. SELECTION CRITERIA FOR THERAPEUTIC RADIONUCLIDES
Preclinical peptide-targeted radionuclide therapy studies for melanoma have primarily focused on MC1-R and melanin. Peptides that target the MC1-R and melanin were labeled with beta-particle-emitting radionuclides 188Re (27-32), 90Y (33), 177Lu (33, 34), 64Cu (35-37), alpha-particle-emitters 212Pb/212Bi (38) and Auger-emitter 125I (39-43). While the therapeutic properties of 111In-labeled somatostatin peptides have been examined in preclinical (44, 45) and clinical (46, 47) studies, it is likely that nuclear localization and trapping may be necessary for optimal effectiveness in cancer treatment (48). Numerous 111In- and 18F-labeled MC1-R-targeting peptides have been developed (49-57), however, they have been primarily characterized as imaging agents and not examined for therapeutic applications. Table 2 lists the physical characteristics of the therapeutic radionuclides examined in preclinical melanoma biodistribution and therapy studies. Selection of the therapeutic radionuclide was dependent on its physical decay properties and the biological half-life of the targeting peptide (58). The radionuclides examined for peptide-targeted melanoma therapy had relatively short half-lives from several hours to several days, with the exception of 125I (T1/2=60.1 days). For optimal targeted radiation dose deposition, the half-life of the radionuclide should be similar to the in vivo biological half-life of the peptide in the tumor. Radionuclides with very short half-lives present formulation and administration hurdles that may limit their abilities to target an optimal radiation dose to the tumor, while radionuclides with long half-lives may not achieve a high enough dose rate over a long enough period of time to insure tumor cell death. The use of targeted radionuclide nanogenerators was employed to significantly improve the targeted dose of the short half-life alpha-emitters 213Bi (59) and 212Bi (38). By targeting parent radionuclides with longer half-life, high tumor doses of the alpha-emitting radionuclides where achieved, resulting in high therapeutic efficacies. Long-lived radionuclides (i.e. 177Lu) would benefit from the strategies of improving tumor retention such as cellular internalization of the radiolabeled peptide or enhancing tumor binding affinities by using tumor targeting vectors with multiple targeting peptide display.
Table 2.
Physical properties of radionuclides examined for peptide-targeted melanoma therapy.
The energies and ranges of particles emitted by therapeutic radionuclides are also important considerations in peptide-targeted radionuclide therapy. High-energy β-particle-emitters like 90Y and 188Re have long maximum path-lengths, which permit the irradiation of several layers of tumor cells (58). Particles that traverse multiple layers of cells yield cross-fire effects, in which tumor cells are irradiated from radionuclides targeted to adjacent or nearby cells. Cross-fire effects establish a homogeneous radiation field in the tumor that can overcome targeting antigen expression heterogeneity. The high energies and longer particle path-lengths of 90Y and 188Re appear to be best suited for larger tumors or large tumor burdens, enabling deposition of the particle’s ionizing energy within the confines of the tumor volume (60-62). Lower-energy β-emitters such as 177Lu, appear suited for targeting smaller tumors and metastases. Shorter β-particle path-length of 177Lu should result in more energy being deposited within a smaller target volume. The importance of matching radionuclide particle path-length with tumor size was demonstrated in preclinical pancreatic cancer therapy studies with radiolabeled somatostatin-2 targeting [DOTA, Tyr3]-octreotate (63). Significant differences were found in the radiotherapeutic effects of 90Y-[DOTA, Tyr3]-octreotide and 177Lu-[DOTA, Tyr3]-octreotate in tumors of different sizes (61, 62). Yttrium-90-labeled [DOTA, Tyr3]-octreotide was more effective in treating larger tumors than smaller tumor, while the converse was true for 177Lu-labeled [DOTA, Tyr3]-octreotate.
Beta-particle-emitting radionuclides are classified as producing low linear energy transfer (LET) radiations, since their ionizing energies are deposited over a long particle path-length (~1-12 mm). Conversely, alpha-particle-emitting radionuclides are classified as producing high LET radiations since they deposit their ionizing energies over a very short distance. The path-lengths of alpha-particles are ~50-70 μm, which corresponds to 3-5 cell diameters. Alpha-particle-emitters have been under increased investigation at both the pre-clinical and clinical stages due to their beneficial decay properties. First, cytotoxicity is independent of dose rate (64). This is important in radioimmunotherapy where the initial dose rate is relatively low to begin with and decreases over time. Second, the toxicity of α-particles is independent of tissue oxygen levels so tumors with hypoxic regions can still be effectively irradiated (65). Third, the path-lengths of α-particles are short, therefore, the cytotoxic properties of α-particles are highly focused, which minimizes collateral radiation damage of healthy tissues. Finally, only a few α-particle-emitting radionuclei per cell are necessary to cause irreversible damage resulting in cell death (66). The short path-lengths of alpha-emitters make them attractive for treating small tumor and metastases. Likewise, Auger-emitters appear to have favorable properties for treating metastases and disseminated tumor cells due to their highly localized energy deposition. Auger and conversion electrons have very short path-lengths (~0.06-17 μm). However, if they are localized close to chromosomal DNA, their biological effectiveness is close to high LET radionuclides (67). Recently, Maecke et al reported in vitro results for a trifunctional peptide that targeted the Auger-emitter 111In to the nuclei of pancreatic AR4-2J cells (48). These results highlighted the potential of peptides that encode multiple targeting addresses to deliver short path-length high LET therapeutic radionuclides to the nuclei of tumor cells.
Preclinical therapy study results were reported for radiolabeled peptides that targeted the MC1-R (31, 34, 38) and melanin (32). Peptide-targeted radionuclide therapy for melanoma via the MC1-R has been the most extensively investigated. The MC1-R is attractive as a therapeutic target because it has high affinity for cognate ligands and the peptide-receptor complex is internalized with the cytoplasm of the cell upon peptide agonist binding (68-70). Internalization, particularly for charged radiometal complexes, contributed to improved cellular retention and greater proximity to the cell nucleus resulting in more effective tumor cell irradiation. Melanoma therapy studies targeting the MC1-R were performed with the CCMSH family of peptides cyclized by direct 188Re incorporation into the peptide to yield 188Re-(Arg11)CCMSH (31) (Fig. 1) or the Re-cyclized DOTA-Re(Arg11)CCMSH peptide (Fig. 1) radiolabeled with 212Pb/212Bi (38) and 177Lu (34) via an N-terminal DOTA chelator. Peptides selected from phage display libraries that bound melanin-like compounds from C. neoformans (71) were employed to target melanin released in melanoma tumors. Preliminary immunofluorescence studies demonstrated that the melanin binding peptide 4B4 (Fig. 1) bound only non-viable melanoma cells and did not bind intact healthy melanoma cells. It was postulated that non-viable cells released their melanin and short melanin-targeting radiolabeled peptides should be able to rapidly diffuse into areas of melanin release, targeting therapeutic radionuclides to the tumor area.
Figure 1.
Schematic structures of 188Re-(Arg11)CCMSH, DOTA-Re(Arg11)CCMSH and HYNIC-4B4.
4. MC1-R-TARGETING RADIOLABELED PEPTIDES
4.1. 188Re-(Arg11)CCMSH
The first radiolabeled peptide therapy study targeting the MC1-R was performed with 188Re-(Arg11)CCMSH (30). The radionuclide 188Re was selected for its high-energy beta-particle emission, coordination chemistry, imageable gamma-emission and because its half-life was consistent with the biological half-life of the peptide targeting vector in the tumor. The linear peptide (Arg11)CCMSH was labeled with 188Re, obtained from 188W/188Re radionuclide generator (Oak Ridge National Laboratory), via a glucoheptonate transchelation reaction (20, 30). Site-specific coordination of 188Re via the three cysteine thiols and cysteine4 amide nitrogen resulted in the formation of the radiolabeled metal-cyclized peptide (27). The 188Re-(Arg11)CCMSH peptide was purified to single species on a C-18 analytical column by reverse-phase high performance liquid chromatography (RP-HPLC). Biodistribution studies in B16/F1 murine melanoma-bearing C57 mice demonstrated high tumor uptake and extended tumor retention of 188Re-(Arg11)CCMSH, coupled with rapid whole-body clearance (30). Tumor uptake values at 1 h, 4 h and 24 h were 20.44±1.91 %ID/g, 16.37±3.27 %ID/g and 3.50±2.32 %ID/g, respectively. Co-injection of the MC1-R agonist NDP-MSH with 188Re-(Arg11)CCMSH reduced greater than 90% of the tumor uptake value, demonstrating the tumor uptake was receptor-mediated. The clearance kinetics was rapid, with 90% of the injected radioactivity was in the urine 4 h post-injection. Radioactivity in the normal organs and tissues was low except for the kidney, which exhibited non-specific uptake values of 11.79±1.29 %ID/g, 3.67±0.51 %ID/g and 0.37±0.11 %ID/g at 1 h, 4 h and 24 h post-injection. Gamma scintigraphy was performed on melanoma-bearing mice 4 h and 18 h post-injection of 188Re-(Arg11)CCMSH (31). Accumulation and retention of the peptide-targeted 188Re was clearly visualized in flank tumors, highlighting the feasibility of utilizing gamma or SPECT imaging to monitor 188Re-(Arg11)CCMSH biodistribution and to calculate individual patient-specific dosimetry in vivo.
Therapy studies were performed in C57 mice bearing B16/F1 syngeneic murine melanoma tumors (31). Palpable dark-colored melanoma tumors were identifiable three days post tumor cell implantation. Groups of 10 tumor-bearing mice were treated with a saline placebo, 7.4 MBq, 22.2 MBq or 2×14.8 MBq of 188Re-(Arg11)CCMSH on day four and seven (2nd dose of 2×14.8 MBq). In contrast to the saline placebo control group, all 188Re-(Arg11)CCMSH treatment groups showed substantial tumor growth inhibition during the period of therapy studies. Three days post treatment, the average tumor volumes of mice receiving 7.4 MBq, 22.2 MBq and 2×14.8 MBq of 188Re-(Arg11)CCMSH were 77.4%, 35.8% and 54.7% of the tumor volumes of mice in the control group, respectively. The average tumor volumes of mice receiving 7.4 MBq, 22.2 MBq and 2×14.8 MBq of 188Re-(Arg11)CCMSH were 83.2%, 72.5% and 59.2% of that of mice in the control group eight days post treatment, respectively (Fig. 2). The therapeutic effect of 188Re-(Arg11)CCMSH treatment on B16/F1 melanoma tumor growth was dose-dependent. A single dose administration of 22.2 MBq of 188Re-(Arg11)CCMSH resulted in a more pronounced delay in tumor growth than a single 7.4 MBq dose. Mice receiving 2×14.8 MBq doses of 188Re-(Arg11)CCMSH exhibited the best overall tumor growth inhibition. The mean survival times for the groups of mice treated with 7.4 MBq, 22.2 MBq and 2×14.8 MBq of 188Re-(Arg11)CCMSH were 10.2±1.0 days, 10.3±1.3 days and 13.3±1.9 days, respectively, compared to a mean survival time of 9.4±1.1 days for the control group (Fig. 2). Although single dose administration of 188Re-(Arg11)CCMSH resulted in tumor growth rate reduction, it did not significantly extend the mean survival times of the treatment group over the control mice. Only the multi-dose regimen resulted in a significant (P<0.05) improvement in mean survival time over the control group (Fig. 2).
Figure 2.
Effect of 188Re-(Arg11)CCMSH treatment on the growth rate of B16/F1 murine melanoma tumors (A). Mice were implanted with B16/F1 tumor cells on day 0 and treated with 188Re-(Arg11)CCMSH on day 4. Tumor measurements were performed daily starting 24 h post administration of a saline placebo (▲), 7.4 MBq (●), 22.2 MBq (◆) or 2×14.8 MBq (■) of 188Re-(Arg11)CCMSH. The second 14.8 MBq dose was given on day 7. Kaplan-Meier survival curves (B) for B16/F1 murine melanoma-bearing mice treated with a saline placebo (□), 7.4 MBq (▼), 22.2 MBq (▲) or 2×14.8 MBq (■) of 188Re-(Arg11)CCMSH. Mean survival time was 9.4±1.1 days for untreated control group, 10.2±1.0 days (p>0.05) for 7.4 MBq treated group, 10.3±1.3 days (p>0.05) for 22.2 MBq treated group and 13.3±1.9 days (p<0.05) for 2×14.8 MBq treated group. Reproduced by permission from the Society of Nuclear Medicine (31).
The melanoma targeting properties of 188Re-(Arg11)CCMSH were also examined in human amelatonic tumor (TXM-13) xenografted severe combined immunodeficiency (SCID) mice (20). Biodistribution studies of 188Re-(Arg11)CCMSH in TXM-13 xenografted SCID mice demonstrated high tumor uptake and extended tumor retention, coupled with rapid whole-body clearance. Tumor uptake values at 1 h, 4 h and 24 h were 3.06±0.68 %ID/g, 2.02±0.27 %ID/g and 0.93±0.49 %ID/g, respectively. Tumor uptake was significantly lower in the TXM-13 tumors than in the B16/F1 tumors. Eighty-two percent of the injected activity was in the urine 4 h post-injection, highlighting the rapid clearance kinetics. Radioactivity in the normal organs and tissues was low except for the kidney, which exhibited non-specific tumor uptake values of 9.70±3.69 %ID/g, 6.24±1.14 %ID/g and 0.27±0.06 %ID/g at 1 h, 4 h and 24 h post-injection. The biodistribution pattern for 188Re-(Arg11)CCMSH in TXM-13 melanoma-bearing mice was similar to B16/F1 melanoma-bearing mice except for the tumor uptake. The differences in the tumor uptake of 188Re-(Arg11)CCMSH between TXM-13 and B16/F1 tumors were likely due to several factors such as receptor density, cellular metabolic activity and tumor morphology/architecture. First, TXM-13 tumor cells have ~5,700 MC1-Rs per cell versus ~7,000 MC1-Rs per cell for B16/F1 tumors. Second, TXM-13 tumors take 7 weeks to grow to a measurable size while the B16/F1 tumors take less than a week. The differences in cellular metabolic activities were also likely to contribute to the differences in 188Re-(Arg11)CCMSH tumor uptake. Another factor contributing to the differences in 188Re-(Arg11)CCMSH uptake between the TXM-13 and B16/F1 tumors is their tumor morphology/architecture. Histopathological examination of the tumors revealed that the B16/F1 tumors consist of highly vascularized dense gelatinous masses, whereas the TXM-13 tumors formed solid masses with viable tumor cells surrounding a necrotic center. There appeared to be less viable cells per tumor mass in the TXM-13 tumors versus the B16/F1 tumors.
The therapeutic efficacy of 188Re-(Arg11)CCMSH was examined in TXM-13 human melanoma xengrafted SCID mice (31). Measurable TXM-13 tumors appeared 7 weeks post tumor cell implantation. TXM-13 tumors are amelatonic and grew much slower than B16/F1 tumors. Groups of 10 TXM-13 tumor-bearing mice were treated with a saline placebo, 22.2 MBq, 2×14.8 MBq or 37.0 MBq of 188Re-(Arg11)CCMSH. A second 14.8 MBq dose was administered to the 2×14.8 MBq group 14 days after the initial dose. 188Re-(Arg11)CCMSH treatment resulted in reduction in tumor growth rates compared to the saline placebo controls. Eight days after the initial 188Re-(Arg11)CCMSH treatment, the average tumor volumes of the groups treated with 37.0 MBq, 22.2 MBq and 2×14.8 MBq were 11.4%, 20.0% and 55.6% of that of the untreated control group (Fig. 3). The pattern of the reduction in tumor growth rates remained the same at 22 days post treatment, with the average tumor volumes of the groups treated with 37.0 MBq, 22.2 MBq and 2×14.8 MBq were 6.5%, 21.5% and 47.0% of the average tumor size in the control group. A single 37.0 MBq dose of 188Re-(Arg11)CCMSH was more effective in reducing tumor growth than a single 22.2 MBq dose or a 2×14.8 MBq fractionated dose. 188Re-(Arg11)CCMSH treatment resulted in mean survival times of 72.7±18.3 days, 57.6±24.2 days and 41.4±10.9 days for groups of mice receiving 37.0 MBq, 22.2 MBq and 2×14.8 MBq doses, compared to a mean survival time of 39.6±15.0 days for the placebo control group (Fig. 3). The mean survival times between the control group and the 37.0 MBq and 22.2 MBq treatment groups were significant (P<0.05), but not statistically significant for the 2×14.8 MBq treatment group (P>0.05). It appeared that the 14 day period between the two dose administrations in the 2×14.8 MBq treatment group was too large for synergistic or additive therapeutic effects. The treatment of 2×14.8 MBq instead acted as two single 14.8 MBq doses. This interpretation would be consistent with the reduction in tumor growth rates and increase in mean survival times improving as the doses increased from 14.8 MBq to 37.0 MBq. Weight gain, appearance and hematology profiles for mice treated with 37.0 MBq of 188Re-(Arg11)CCMSH were examined. The average weight gain of the 37.0 MBq treatment group reach a nadir (81% of control group) 8 days post dose administration, rebounding to the average weight of the control group by day 15. Hematology profiles revealed that there was a significant (P<0.05) decrease in white blood cells and red blood cells 1-2 weeks after injection of 37.0 MBq of 188Re-(Arg11)CCMSH, while platelet counts reached a nadir after 1 week. The hematology profile of the 37.0 MBq treatment group was completely recovered by week 3. Post mortem histological examination of the major organs, including the kidneys, showed no evidence for radiation-related toxicity.
Figure 3.
Effect of 188Re-(Arg11)CCMSH treatment on the growth rate of TXM-13 human melanoma tumors (A). Measurable TXM-13 tumors were obtained on day 0 and treated with 188Re-(Arg11)CCMSH on day 4. Tumor measurements were performed twice a week starting 4 days post administration of a saline placebo (◆), 22.2 MBq (▲), 2×14.8 MBq (●) or 37.0 MBq (■) of 188Re-(Arg11)CCMSH. The second 14.8 MBq dose was given on day 18. Kaplan-Meier survival curves (B) for TXM-13 human melanoma-bearing mice treated with a saline placebo (□), 22.2 MBq (▲), 2×14.8 MBq (■) or 37.0 MBq (▼) of 188Re-(Arg11)CCMSH. Mean survival time was 39.6±15.0 days for untreated control group, 41.4±10.9 days (p>0.05) for 2×14.8 MBq treated group, 57.6±24.2 days (p<0.05) for 22.2 MBq treated group and 72.7±18.3 days (p<0.05) for 37.0 MBq treated group. Reproduced by permission from the Society of Nuclear Medicine (31).
4.2. 177Lu-DOTA-Re(Arg11)CCMSH
The biodistribution and therapeutic efficacy of 177Lu-DOTA-Re(Arg11)CCMSH for MC1-R-targeted melanoma radionuclide therapy examined in B16/F1 tumor-bearing mice (34). The MC1-R-targeting peptide DOTA-Re(Arg11)CCMSH was very similar to 188Re-(Arg11)CCMSH, except that it was cyclized by non-radioactive Re and contains an N-terminal DOTA chelator for radionuclide coordination (50). DOTA-Re(Arg11)CCMSH was developed to allow one to target a large number of diagnostic and therapeutic radionuclides to melanoma tumor via the specific interaction between MC1-R and DOTA-Re(Arg11)CCMSH. DOTA-Re(Arg11)CCMSH was radiolabeled with the beta-particle-emitter 177Lu (Missouri University Research Reactor, MURR) and purified to homogeneity (33, 34). Biodistribution studies of 177Lu-DOTA-Re(Arg11)CCMSH showed high and prolonged tumor uptake and rapid whole-body clearance of radioactivity (33). Tumor uptake values at 2 h, 4 h, and 24 h were 14.48±0.85 %ID/g, 17.68±3.32 %ID/g and 9.05±4.31 %ID/g, respectively. Radioactivity clearance from the normal organs and tissues was rapid with the exception of the kidney. Greater than 90% of the injected radioactivity was cleared into the urine by 2 h. The kidney uptakes of 177Lu-DOTA-Re(Arg11)CCMSH were 17.99±2.47 %ID/g, 19.09±2.38 %ID/g and 13.75±3.72 %ID/g at 2 h, 4 h, and 24 h post-injection. Co-injection of non-radioactive NDP-MSH peptide reduced 93% of the tumor uptake but did not affect the kidney uptake of 177Lu-DOTA-Re(Arg11)CCMSH, demonstrating that the radioactivity in the tumor was MC1-R-mediated, while radioactivity in the kidney was non-specifically retained. In vivo localization of 177Lu-DOTA-Re(Arg11)CCMSH was visualized in a B16/F1 tumor-bearing mouse 2 h post-injection (34). Lutetium-177 emits a 208 keV gamma ray that was directly imaged. Radioactivity was clearly visible in the tumor as well as in the kidneys, with the remainder of the mouse body showing background levels of radioactivity (Fig. 4). The imaging results highlighted the potential to employ gamma scintigraphy or SPECT imaging of 177Lu-DOTA-Re(Arg11)CCMSH in patients to calculate individual dosimetry and determine optimal individual therapeutic dose administration.
Figure 4.

Whole body (A) and transaxial (B) images of 177Lu-DOTA-Re(Arg11)CCMSH in a B16/F1 flank melanoma-bearing C57 mouse at 2 h post-injection. Arrows indicate the locations of the melanoma lesions. Reproduced by permission from Mary Ann Liebert, Inc. (34).
The therapeutic efficacy of 177Lu-DOTA-Re(Arg11)CCMSH was examined in B16/F1 melanoma-bearing mice (34). Dark palpable tumors were identifiable 3 days post tumor cell implantation. Treatment groups of 10 mice were administered a saline placebo, 37.0 MBq, 2×18.5 MBq of 177Lu-DOTA-Re(Arg11)CCMSH via tail vein injection. The second 18.5 MBq dose of 177Lu-DOTA-Re(Arg11)CCMSH was given 4 days after the initial dose. Tumor measurements and body weights were recorded daily. The mean tumor growth rates and mean body weights for each treatment group are presented in Figure 5. In contrast to the placebo control group, the tumor growth rates of mice treated with 37.0 MBq and 2×18.5 MBq of 177Lu-DOTA-Re(Arg11)CCMSH were substantially reduced. For instance, the mean tumor volumes of mice treated with 37.0 MBq and 2×18.5 MBq of 177Lu-DOTA-Re(Arg11)CCMSH were 65.4% and 82.7% of the mean tumor volume of the control group 3 days post-injection, respectively. Eleven days post-injection, the mean tumor volumes of the groups receiving 37.0 MBq and 2×18.5 MBq of 177Lu-DOTA-Re(Arg11)CCMSH were 29.4% and 25.9% less than that of the saline placebo control group. The placebo control group of mice exhibited a 13.3±2.3 day mean survival time compared to 16.2±3.6 days and 15.1±1.8 days for the 37.0 MBq and 2×18.5 MBq treatment groups (Fig. 6). Improvements in mean survival time for the treatment groups compared to the saline placebo controls was significant (P<0.05). The mean survival results also demonstrated that the fractionated 2×18.5 MBq dose administration was as effective as a single 37.0 MBq dose.
Figure 5.
Growth rates of B16/F1 murine melanoma tumors (A) and body weight ratios of mice post 177Lu-DOTA-Re(Arg11)CCMSH treatment (B). Mice were implanted with B16/F1 tumor cells on day 0 and treated with a saline placebo (◆), 2×18.5 MBq (▲) or 37.0 MBq (■) of 177Lu-DOTA-Re(Arg11)CCMSH on day 3. The second 18.5 MBq dose was given on day 7. Tumor measurements and mouse weights were obtained daily. Reproduced by permission from Mary Ann Liebert, Inc. (34).
Figure 6.
Kaplan-Meier survival curves for B16/F1 murine melanoma-bearing mice treated with a saline placebo (◆), 2×18.5 MBq (□) or 37.0 MBq (■) of 177Lu-DOTA-Re(Arg11)CCMSH. Mean survival times were 13.3±2.3 days for the untreated tumor control group, 15.1±1.8 days (p=0.038) for the 2×18.5 MBq treated group and 16.2±3.6 days (p=0.029) for the 37.0 MBq treated group. Reproduced by permission from Mary Ann Liebert, Inc. (34).
The average weights of the treated and placebo control groups increased at similar rates, except for a brief decrease after the second injection of 177Lu-DOTA-Re(Arg11)CCMSH in the 2×18.5 MBq group (Fig. 5). Hematology profiles of the 37.0 MBq treated group and control group showed a significant decrease in red blood cell and white blood cell counts 2 weeks after treatment. Platelet counts reached a nadir 1 week post treatment. The treatment group receiving 2×18.5 MBq of 177Lu-DOTA-Re(Arg11)CCMSH showed only a drop in white blood cells 2 weeks post-injection. There were no significant changes in red blood cell and platelet levels. Serum creatinine levels were monitored during the study at a measure of kidney function. No increases in creatinine levels were observed in either treatment group. Histopathological examinations of the kidneys from the treatment and placebo groups were performed post mortem. The histopathology results showed no evidence of radiation-related damage to the kidneys of the treatment groups.
4.3. 212Pb/212Bi-DOTA-Re(Arg11)CCMSH
Targeted radionuclide therapy in the treatment of malignant melanoma will be focused on metastatic disease, since primary melanoma tumors are surgically removed. Metastases are likely to consist of disseminated small tumors. Radionuclides that yield short path-length high LET radiations appear to be well suited to target metastases (64, 65). DOTA-Re(Arg11)CCMSH peptide was labeled with 212Pb (38), the parent radionuclide of 212Bi, which yields an alpha particle and a beta particle upon its decay (72). The major advantage of targeting 212Pb to the tumor instead of 212Bi is that 212Pb delivers greater than 10 times the dose per unit of administered activity compared to 212Bi alone or the alpha-emitter 213Bi (73). Another benefit of administering 212Pb-DOTA-Re(Arg11)CCMSH is that the radiolabeled peptide will circulate, target melanoma tumor cells and be cleared from the body as the 212Pb-labeled peptide and not the alpha-emitting 212Bi compound, minimizing normal tissue exposures. Peptide-targeted 212Pb, internalized and retained by tumor cells will decay to the alpha-particle emitting 212Bi, localizing the highly toxic short-ranged alpha-radiation within the tumor. Finally, the 10.6 h half-life of 212Pb makes dose preparation and administration easier and more efficient than the short half-life (T1/2=60.6 min) 212Bi.
The biodistribution and tumor targeting properties of 212Pb-DOTA-Re(Arg11)CCMSH were examined in B16/F1 tumor-bearing mice (38). DOTA-Re(Arg11)CCMSH was labeled with 212Pb eluted from a 224Ra/212Pb radionuclide generator (74) supplied by AlphaMed Inc. 212Pb-DOTA-Re(Arg11)CCMSH was purified to homogeneity by RP-HPLC. Biodistribution studies demonstrated that 212Pb-DOTA-Re(Arg11)CCMSH exhibited rapid tumor accumulation and prolonged retention in B16/F1 melanoma tumors. Tumor uptake values were 11.25±1.52 %ID/g, 12.84±2.53 %ID/g and 4.59±1.45 %ID/g at 2 h, 4 h and 24 h post-injection. Whole-body clearance of radioactivity from normal organs and tissues was rapid with the exception of the kidney. Approximately 90% of the injected radioactivity was in the urine by 2 h post-injection. Non-specific uptakes of radioactivity in the kidneys were 7.31±1.26 %ID/g, 4.56±1.27 %ID/g and 2.93±0.53 %ID/g at 2 h, 4 h, and 24 h post-injection. Co-injection of the NDP-MSH blocked 81% of tumor uptake of 212Pb-DOTA-Re(Arg11)CCMSH but did not effect kidney uptake, demonstrating that radioactivity in the tumor cells was receptor-mediated and that radioactivity in the kidneys was not MC1-R-specific.
As reported in the publications of ours and others, approximately one-third of the radioactivity escaped the DOTA chelator due to super-ionization associated with the decay of 212Pb to 212Bi (38, 75). Redistribution was not a concern for 212Pb internalized in tumor cells since diffusion of a charged metal across the cell membrane would be very slow. However, loss of 212Bi from circulating 212Pb-DOTA-Re(Arg11)CCMSH could allow 212Bi to redistribute and irradiate normal organs. To determine if appreciable amounts of 212Bi were being redistributed during circulation, biodistribution time points from 5 min to 48 h post-injection were monitored in 212Pb and 212Bi energy windows. No difference was detected in the biodistribution of 212Pb and 212Bi during the 48 h study, demonstrating that no significant amount of 212Bi was escaping the 212Pb-DOTA-Re(Arg11)CCMSH molecule and redistributing in vivo. In a separate study, mice were administered with the metal chelator 2,3-dimercapto-1-propanesulfonic acid (DMPS) (76) prior to and during 212Pb-DOTA-Re(Arg11)CCMSH administration for intra-vital chelator of any freely circulating 212Bi. Dual window counting revealed that there was no difference in the biodistribution of 212Pb and 212Bi in mice treated with DMPS and without DMPS. It is likely that the rapid clearance of 212Pb-DOTA-Re(Arg11)CCMSH prevented measurable amounts of 212Bi from being released. These results indicated that no detectable amounts of 212Bi were freed during circulation and that radioactivity in the kidneys was due to non-specific retention of the radiolabeled peptide. Co-administration of positively charged amino acids in conjunction with a diuretic should further decrease non-specific radioactivity in the kidneys.
The therapeutic efficacy of 212Pb-DOTA-Re(Arg11)CCMSH was determined in B16/F1 melanoma-bearing C57 mice (38). Palpable dark tumors were detected 3 days post tumor cell inoculation. On the fourth day post tumor cell implantation, groups of 10 mice were treated with 1.85 MBq, 3.7 MBq or 7.4 MBq of 212Pb-DOTA-Re(Arg11)CCMSH via tail vein injection, respectively. A control group of mice received a saline placebo injection. Tumor measurements and body weights were recorded daily. Mice receiving 212Pb-DOTA-Re(Arg11)CCMSH treatment displayed dramatic reductions in tumor growth rates at all doses compared to the saline placebo control group. The mean tumor growth rates and mean body weights for each treatment group are presented in Figure 7. A comparison between the 1.85 MBq, 3.7 MBq and 7.4 MBq treatment groups and the placebo group showed that tumor volume was 19.0%, 4.8 and 4.8% of the control group 16 days post the treatment, respectively. The decrease in tumor growth was 212Pb-DOTA-Re(Arg11)CCMSH dose-dependent. Twenty percent of the mice in the 3.7 MBq treatment groups and 45% of the mice in the 7.4 MBq group survived the entire 120-day therapy study. Post mortem histopathological examination of the tumor site and other major organs showed no sign of primary or metastatic melanoma or melanoma associated S100 antigen, allowing the mice to be classified as complete remissions or cures. All treatment groups displayed a significant improvement in mean survival time (P<0.05) compared to the saline placebo control group (Fig. 8). The mean survival times for groups of mice receiving 1.85 MBq, 3.7 MBq and 7.4 MBq of 212Pb-DOTA-Re(Arg11)CCMSH that did not survive the 120-days study were 22.0±5.5 days, 28.0±8.8 days and 49.8±27.3 days, respectively, compared to 14.6±4.4 days for the control group. Histopathological examination of the kidneys showed moderate toxicity at the 7.4 MBq dose as evidenced by damage to tubule and glomerular structures and a thin renal cortex. Kidney damage at the 1.85 MBq and 3.7 MBq doses was classified as minor. Despite the evidence of moderate kidney damage in the 7.4 MBq treatment group, no behavior effects (diarrhea and scruff coat) from high-dose 212Pb-DOTA-Re(Arg11)CCMSH treatment were observed.
Figure 7.
Body weight ratios of B16/F1 murine melanoma-bearing C57 mice (A) and growth rates of B16/F1 murine melanoma tumors post 212Pb-DOTA-Re(Arg11)CCMSH treatment (B). Mice were weighted and implanted with B16/F1 tumor cells on day 0 and treated with 212Pb-DOTA-Re(Arg11)CCMSH on day 4. Body weights and tumor sizes were determined daily starting 24 h post administration of a saline placebo (◆), 1.85 MBq (■), 3.7 MBq (▲) or 7.4 MBq (●) of 212Pb-DOTA-Re(Arg11)CCMSH. Reproduced by permission from American Association of Cancer Research (38).
Figure 8.
Kaplan-Meier survival curves for B16/F1 murine melanoma-bearing C57 mice treated with a saline placebo (□), 1.85 MBq (▲), 3.7 MBq (▼) or 7.4 MBq (■) of 212Pb-DOTA-Re(Arg11)CCMSH. Mean survival time was 14.6±4.4 days for untreated tumor control group, 22.0±5.5 days (p=0.004) for 1.85 MBq treated group, 28.0±8.8 days (p=0.002) for 3.7 MBq treated group and 49.8±27.3 days (p=0.02) for 7.4 MBq treated group. Two out of ten mice in 3.7 MBq treated group and four out of nine mice in 7.4 MBq treated group were free of tumor and survived 120-day studies. Reproduced by permission from American Association of Cancer Research (38).
5. MELANIN-TARGETING RADIOLABELED PEPTIDE
5.1. 188Re-HYNIC-4B4
The melanin-binding 4B4 peptide (YERKFWHGRH) was synthesized with D-amino acids to improve resistance to proteolytic degradation (32). The HYNIC (hydrazinonicotinamide) ligand was conjugated to the amino terminus of the 4B4 peptide during the final step of peptide synthesis. HYNIC-4B4 (Fig. 1) was radiolabeled with 188Re eluted from a 188W/188Re radionuclide generator (Oak Ridge National Laboratory) via incubation with 188Re-gluconate (32). 188Re-HYNIC-4B4 was purified on a Sep-Pak18 as needed. Melanoma cell binding studies with 188Re-HYNIC-4B4 demonstrated that it bound both the highly pigmented MNT1 as well as the lightly pigmented SK-MEL-28 human melanoma cells.
Biodistribution studies (Fig. 9) of 188Re-HYNIC-4B4 were performed in MNT1 tumor-bearing nude mice (32). Tumor uptake of 188Re-HYNIC-4B4 was highest (~4.5 %ID/g) at the early time points (30 min and 1 h post-injection), decreasing over time to 0.5 %ID/g at 24 h post-injection. It was estimated that a dose of 3 Gy/37 MBq was delivered to the MNT1 tumors by 188Re-HYNIC-4B4. The clearance of 188Re-HYNIC-4B4 from the blood was rapid with only 0.5% ID/g of radioactivity remaining at 24 h post-injection. Liver and spleen uptakes were 7 %ID/g and ~4 %ID/g at 1 h post-injection, respectively. There was ~7-8 %ID/g of the radioactivity accumulated in the stomach at 1 h post-injection. Radioactivity in the kidneys was ~30% ID/g at 0.5-1 h post-injection, decreasing to 10 %ID/g at 24 h post-injection. Low amounts of radioactivity were found in normal melanized tissues including the skin and eyes. No difference in uptakes of radioactivity in the skin and eyes was observed between light and dark pigmented mice (Fig. 9).
Figure 9.
Tissue distribution of 188Re-HYNIC-4B4: A. MNT1 tumor-bearing nude mice. Four (4) mice per group were used. B. White BALB/c and black C57BL6 mice. Five (5) mice per group were used. In both experiments, mice were injected intravenously 2 μg 188Re-HYNIC-4B4 (50 μCi). Reproduced by permission from Mary Ann Liebert, Inc. (32).
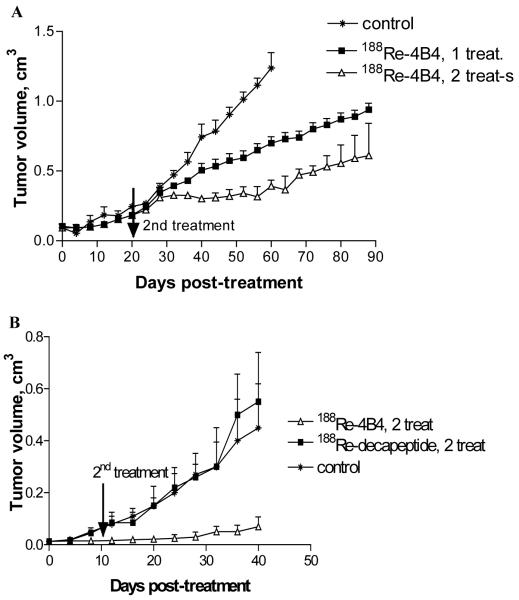
Melanoma therapy studies (Fig. 10) were performed with MNT1 human melanoma-bearing nude mice (32). Two therapy studies were performed on mice with starting tumors sizes of 0.5-0.7 cm in diameter and 0.3-0.4 cm in diameter, respectively. In the first therapy study, groups of 10 mice received 37.0 MBq or 2×37.0 MBq of 188Re-HYNIC-4B4 intraperitoneally, in addition to an untreated control group. The second dose of 188Re-HYNIC-4B4 was administered 20 days after the initial dose in the multi-dose treatment group. In the second therapy study, groups of 10 mice received 2×37.0 MBq of 188Re-HYNIC-4B4 or 2×37.0 MBq of 188Re-HYNIC-PA1, a non-melanin-binding peptide, in addition to the untreated control group. Administration of the second dose occurred 10 days after the initial dose. In contrast to the untreated group of mice, slower tumor growth was observed in the 37.0 MBq treated group, while significantly (P=0.01) slower growth was recorded for the 2×37.0 MBq treatment group (Fig. 10). In the second therapy study, the effect of tumor size and non-targeted radiation were examined. Tumor growth in mice receiving 2×37.0 MBq of 188Re-HYNIC-4B4 was arrested for the first 20 days of the study. No melanoma cells were identifiable in mice that showed no tumor growth after the 2×37.0 MBq treatments. In addition, no therapeutic effect was observed in mice administrated with equal doses of 188Re-HYNIC-PA1 control peptide. Post mortem analyses of the kidneys of the 188Re-HYNIC-4B4 treated mice revealed normal glomeruli and tubules with no signs of radiation induced toxicity. Additional behavioral and histological studies were performed in C57 mice treated with 2×37.0 MBq of 188Re-HYNIC-4B4. Treated mice showed no difference in behavioral assessment according to the SHIRPA protocol and exhibited equal weight gain to the untreated control group.
Figure 10.
Therapy of MNT1 pigmented melanoma tumors in nude mice with 188Re-HYNIC-4B4 peptide. Points represent means of tumor size of 10 mice. The bars represent standard deviation. A. First study in mice with 0.5-0.7 cm tumors. B. Second study in mice with 0.3-0.4 cm tumors. “188Re-decapeptide” is 188Re-labeled irrelevant decapeptide HYNIC-PA1. Reproduced by permission from Mary Ann Liebert, Inc. (32).
6. PROSPECTS AND CHALLENGES OF PEPTIDE-TARGETED MELANOMA THERAPY
Preclinical reports clearly demonstrate the treatment potential of peptide-targeted radionuclide therapy for melanoma. Radiolabeled peptides that bind two melanocyte/melanoma-associated molecular targets, MC1-R and melanin, have been examined for their therapeutic efficacies for melanoma. MC1-R-targeting radiolabeled peptides have been studied for melanoma imaging and therapy for many years due to the selective over-expression of MC1-R on melanoma tumor cells and its nanomolar ligand affinity (20, 68-70). An additional advantage of MC1-R-targeting is peptide internalization, which particularly important for radiotherapy, since it sequesters the radionuclide inside the tumor cell close to the nucleus. However, low receptor density limit total tumor uptake per administration and require the use of high specific activity radiolabeled peptide preparations (20, 77). The MC1-R-avid peptides, (Arg11)CCMSH and DOTA-Re(Arg11)CCMSH were labeled with the high-energy beta-emitter 188Re (31), the low-energy beta-emitter 177Lu (34) and the alpha-emitter 212Pb/212Bi (38) and examined for their therapeutic efficacies in B16/F1 melanoma-bearing C57 mice. Calculated doses delivered to the tumor and selected organs are shown in Table 3. A comparison of therapeutic efficacy with the calculated dose to the tumor shows an expected correlation in the radiolabeled (Arg11)CCMSH studies. The alpha-particle-emitting 212Pb-DOTA-Re(Arg11)CCMSH peptide delivered ~20× and ~40× the dose to the tumor as the high-energy beta-emitter 188Re- and low-energy beta-emitter 177Lu-labeled peptides, respectively, resulting in greater reduction in tumor growth, longer mean survival times and in many cases complete remission of disease. MC1-R-mediated internalization of the 212Pb-DOTA-Re(Arg11)CCMSH complex into the tumor cells coupled with the short path-length of the alpha-particles focused the radiation within the volume of the tumor. Alpha-radiation causes direct cellular and chromosome damage, independent of oxygen effects, destroying both hypoxic and norm-oxic tumor cells.
Table 3.
Calculated peptide-targeted radionuclide doses to tumor and selected normal organs.
Melanoma treatment with the high-energy beta-emitting 188Re-(Arg11)CCMSH complex resulted in significant improvements in mean survival times for B16/F1 tumor-bearing mice. A comparison of the tumor size with the path-length of the 188Re beta-particle suggests that a portion of the targeted radiation fell outside the volume of the tumor. As a result the dose to the tumor is less. It is likely that cross-fire effects contributed to high enough radiation levels within the tumor to yield positive therapeutic results. Likewise, 177Lu-DOTA-Re(Arg11)CCMSH treatment displayed therapeutic efficacy in B16/F1 tumor-bearing mice. Despite having a lower energy beta-particle and shorter path-length than 188Re, treatment with 177Lu-DOTA-Re(Arg11)CCMSH resulted in improved mean survival. The calculated radiation dose delivered by 177Lu-DOTA-Re(Arg11)CCMSH to the tumor was approximately half that of 188Re-labeled peptide even though the 177Lu beta-particle energy was approximately one-quarter of the 188Re beta-particle. These results suggest that the shorter path-length of 177Lu allowed more of the radiation to be deposited within the tumor volume. 177Lu-DOTA-Re(Arg11)CCMSH exhibited longer tumor retention properties than 188Re(Arg11)CCMSH, which also contributed to it effectiveness.
Targeted melanoma radionuclide therapy using the melanin-targeting peptide 188Re-HYNIC-4B4 peptide exhibited therapeutic efficacy in MNT1 human melanoma tumor-bearing nude mice (32). The authors successfully demonstrated that a peptide targeting the negatively-charged pigment melanin was effective in delivering the therapeutic radionuclide. However, melanin available for peptide targeting is only released from dead or dying tumor cells. Presumably, the melanin-targeting peptides will diffuse and localize to regions of necrotic or apoptotic tumor cells. Therefore the melanin-targeting peptide should be labeled with a radionuclide that emits particles with long enough path-lengths to affect surrounding viable tumor cells. Tumor localization of the high-energy beta-particle emitter 188Re yielded a dose of 3 Gy/37 MBq of 188Re-HYNIC-4B4 peptide. The authors postulate that despite the modest dose to the tumor, cross-fire irradiation and some as yet uncharacterized biological effect of the peptide were responsible for the observed therapeutic efficacy (32). It is also possible that the observed therapeutic effect was due to low-dose hyper-radiosensitivity (78). Multi-dose administration (2×37.0 MBq) of 188Re-HYNIC-4B4 was found to yield greater therapeutic efficacy than a single dose administration resulting in a higher cumulative tumor dose no observable normal organ toxicities. The authors go on to state that a dose of 3.3 Gy from 2-[18F]fluoro-2-deoxy-D-glucose (18F-FDG) was effective therapeutically in mice xenografted with human breast tumors (79). Unfortunately, no normal organ doses were reported in the 188Re-HYNIC-4B4 peptide and 18F-FDG studies, making it difficult to predict potential toxicities associated with the treatments. It is difficult to extrapolate if a treatment dose proportional to the 3 Gy or 3.3 Gy doses effective in mice will be effective for human therapy, however, the preclinical results with 188Re-HYNIC-4B4 are encouraging.
Small tumors, <0.5 cm in diameter, were susceptible to treatment with the high-energy beta-emitters (i.e 90Y and 188Re) despite the fact that a portion of the beta-radiation was deposited outside the tumor volume (30, 32, 60, 61). It could be argued that the improvements in survival were due to non-targeted radiation due to the long path-length of the 188Re beta-particle. Dadachova and co-workers demonstrated that the therapeutic gains were not from indirect radiation (32). A 188Re-labeled peptide that did not target melanin showed no therapeutic efficacy, demonstrating that it was tumor-targeted deposition of radiation that resulted in extending survival. 188Re-(Arg11)CCMSH was also effective in treating small melanoma tumors. Perhaps the developing vasculature of smaller tumors is more susceptible to radiation effects or smaller tumors are less likely to have necrotic regions, which are resistant to the treatment with beta-emitters. It would be interesting to compare the low-dose tumoricidal effects of a 177Lu-DOTA-4B4 construct with 188Re-HYNIC-4B4. The deposition of more radiation within the tumor volume may yield greater therapeutic results. 212Pb- and 177Lu-labeled DOTA-Re(Arg11)CCMSH treatments of small tumors were also effective. Both radionuclides have short path-length emissions resulting in more of the decay energy being localized within the tumor volume. The high LET alpha-particle-emitter 212Pb was the most effective. A correlation between tumor size, radionuclide decay properties and treatment outcome was also reported with radiolabeled Octreotide (60-62). Peptide-targeted radionclide therapy with radiolabeled Octreotide showed that the high-energy beta-emitter 90Y (60-62) was more effective in treating medium-sized tumors, while the short path-length beta-emitter 177Lu was effective in treating small tumors (63).
While the dose delivered to the tumor is critical for effective treatment outcomes, equally important is minimizing the dose to normal organs and tissues. The dose-limiting normal organ is the kidney for most radiolabeled peptides (80-86). Non-specific retention of radiolabeled peptides dramatically limits the administered treatment dose. In preclinical therapy studies with 188Re-(Arg11)CCMSH, 177Lu-DOTA-Re(Arg11)CCMSH and 188Re-HYNIC-4B4, no evidence of kidney damage was observed in mice over a 3-4 month period post treatment (31, 34). Post mortem histological examination of kidneys from mice receiving 74 MBq of 188Re showed no signs of radiation related toxicity. While these results appear reassuring, kidney toxicity was a dose-limiting issue in radiolabeled Octreotide clinical trials with the high-energy beta-emitter 90Y and low-energy beta-emitter 177Lu, despite low kidney uptake values in preclinical studies. Delayed onset renal failure, 12 months or more post treatment, is a serious problem that is not accurately modeled in mouse studies (81, 82). Kidney damage is more acute in mice treated with alpha-emitters. Mice treated with 212Pb-DOTA-Re(Arg11)CCMSH showed histological evidence of mild or moderate kidney damage at 3.7 MBq or 7.4 MBq doses, respectively (38). Strategies to reduce renal toxicity associated with targeted alpha-particle therapy were reported for 213Bi- and 225Ac-labeled antibodies (85). In addition to the proven reduction in non-specific renal retention of radiolabeled peptides by amino acid co-infusion, the use of circulating chelators for intravital coordination of un-complexed radionuclides from the blood and the use of diuretics to enhance radionuclide clearance were shown to reduce radioactivity accumulation in the kidneys. Renal toxicity issues have curtailed peptide-targeted radionuclide therapy from becoming a mainline treatment modality. Implementation of kidney protective strategies should improve the tumor to kidney dose ratio resulting in greater therapeutic efficacy and lower normal organ toxicity.
The availability of therapeutic radionuclides is a major consideration in the development of peptide-targeted radiotherapy. Rhenium-188 is attractive since it is produced from a 188W/188Re generator that yields high specific activity 188Re over a several-month period. Tungston-188/188Re generators can be located on-site in hospital nuclear pharmacies. Lutetium-177 is also readily available from high-neutron flux reactors. With a 6.7-day half-life, 177Lu can be shipped to hospitals for on-site radiopharmaceutical formulation or to a regional radiopharmacy for drug formulation and subsequent shipment to the hospitals. The high-energy beta-emitter, 90Y is also readily available and has been used extensively in clinical trials with 90Y-DOTATOC. From a regulatory point of view, 177Lu, 111In and 90Y have been used in patient clinical trials (14, 44-47), which may simplify the approval of future peptide-targeted radiotherapeutic agents. The therapeutic radionuclides 212Bi and 213Bi are both produced from high specific activity 224Ra/212Bi and 225Ac/213Bi radionuclide generators. Therapeutic application of 212Bi and 213Bi is hampered by short half-lives of 60.6 and 45 min, respectively. Limitations imposed by the short half-lives of 212Bi and 213Bi can be overcome by labeling the peptides with the longer half-lives parent radionuclides 212Pb (T1/2=10.6 h) (38) and 225Ac (T1/2=10.0 d) (94). Antibodies radiolabeled with 213Bi have been used in phase-1 targeted alpha-therapy trials to treat patients with myeloid leukemia (95), while targeted 225Ac therapy studies are underway.
The preclinical results reported for peptide-targeted radionuclide therapy of melanoma highlight the potential for clinical translation. Despite the differences in receptor density, tumor morphology and tumor uptake between TXM13 human melanoma and B16/F1 murine melanoma, 188Re-(Arg11)CCMSH administration resulted in therapeutic efficacies in TXM13 human melanoma greater than or equal to that in B16/F1 murine melanoma (31). A key aspect of using peptide-targeted radionuclide therapy in the clinic is to tailor patient-specific doses to maximize tumor irradiation, while minimizing normal organ toxicity. The most effective method of estimating a safe therapy dose is to determine patient-specific dosimetry for the treatment agent. The advantage of labeling the melanoma-targeting peptides with the therapeutic radionuclides 188Re, 177Lu and 212Pb is that the melanoma tumors can be imaged directly with the therapeutic radionuclides or with a matched-pair imaging radioisotope. Direct tumor imaging with a therapeutic radionuclide or a matched-pair imaging radionuclide has a big advantage over employing 111In as a surrogate imaging radionuclide for the therapeutic radionuclide 90Y, since there are differences in biodistribution results of the same peptide labeled with one or the other radionuclide (33, 50). Clinical studies with 177Lu-DOTATOC have shown that imaging studies can be successfully used to determine patient-specific therapy doses. Preclincial imaging studies of 188Re-(Arg11)CCMSH (31) and 177Lu-DOTA-Re(Arg11)CCMSH (Fig. 4) in B16/F1 tumor-bearing mice highlight their potential to be used at lower doses for patient-specific dosimetry to support melanoma peptide-targeted radionuclide therapy. Likewise, 203Pb-DOTA-Re(Arg11)CCMSH was shown to yield high quality SPECT images in B16/F1 tumor-bearing mice (87), demonstrating its ability as a matched-pair imaging agent for 212Pb-DOTA-Re(Arg11)CCMSH. While no imaging data was reported for 188Re-HYNIC-4B4, it is likely that lower doses of the 188Re-labeled peptide will serve as an imaging agent in support of therapy.
The application of diagnostic imaging in melanoma management has been limited to the use imaging agents such as 99mTc-sulfur colloid, 99mTc-human serum albumin (99mTc-HSA), 99mTc-2-methoxy-isobutyl-isonitrile (99mTc-MIBI) and 18F-FDG. Both 99mTc-sulfur colloid and 99mTc-HSA are used to map lymphatic drainage of primary melanoma tumors and would not be applicable in monitoring melanoma treatment. Minimally invasive imaging using 99mTc-MIBI and 18F-FDG have been investigated for detection of nodal and systemic metastasis. However, both 99mTc-MIBI and 18F-FDG are non-melanoma-specific and have relatively high false positive rates of 16% and 17% respectively, making their utility in staging melanoma debatable (88-93). Peptides that target the MC1-R and melanin, have the advantage of being melanoma-specific, which should result in high selectivity and fewer false positives. While the imaging sensitivity of radiolabeled melanoma-selective peptides remains to be determined, their greatest potential lies in radiotherapy. The development of matched-pair imaging/therapeutic melanoma-targeting peptides or peptide labeled with therapeutic radionuclides that emit imageable photons are critical to the success of peptide-targeted radiotherapy. Imaging patients prior to therapy will allow clinicians to accurately determine the tumor uptake and clearance of the peptide labeled with the therapeutic radionuclide, yielding accurate patient-specific dosimetry. Most peptides labeled with therapeutic radionuclides clear out the body via the kidneys, making the kidneys the dose-limiting normal organ. Peptide imaging will allow physicians to determine renal clearance kinetics and dosimetry, which will greatly impact the administered therapeutic dose. Patient-specific dosimetry determined by peptide imaging will improve the safe and efficacious application of peptide-targeted radionuclide therapy, which is essential for its acceptance as a mainstream treatment option.
7. CONCLUSIONS
Based on published preclinical studies, there is an optimistic outlook for peptide-targeted radionuclide therapy for melanoma. Selective melanoma uptake of 188Re-(Arg11)CCMSH, 177Lu- and 212Pb-labeled DOTA-Re(Arg11)CCMSH and 188Re-HYNIC-4B4 coupled with their rapid whole-body clearance resulted in successful tumor treatment with no or minimal normal organ toxicity. Minimizing non-specific radioactivity in the kidneys remains a major challenge for all peptide-targeted radionuclide therapy applications. The ultimate clinical success of peptide-targeted radionuclide therapy is inextricably linked to reducing the dose to the kidney. It appears that using radionuclide with appropriate particle ranges will improve therapeutic efficacy while minimizing damage to the surrounding normal tissues. Peptide-targeted radionuclide therapy for melanoma will likely contribute in an adjuvant setting, once the primary tumor has been surgically removed, to treat metastatic deposits and for treatment of end-stage disease. The lack of effective treatments for metastatic melanoma and end stage disease underscores the necessity to develop and implement new treatment strategies, such as peptide-targeted radionuclide therapy.
8. ACKNOWLEDGMENTS
The authors express their gratitude to the grants support by National Cancer Institute P50 Imaging Center Grant P50-CA-103130, Department of Energy Grant DOE-FG0296ER61661, UNM-LANL MOU on Research and Education Grant 2R76T, American Foundation for Pharmaceutical Education Grant 3R48E, American Cancer Society Institutional Research Grant IRG-92-024 and New Mexico Technology Research Collaborative Grant 3R44N.
BIOGRAPHY
Yubin Miao obtained his B.S. degree in Chemistry at Beijing Normal University in 1992. He earned his Ph.D. degree with Professor Boli Liu at Beijing Normal University in 1997, studying techenetium-99m-labeled organic molecules for brain perfusion imaging. He then spent 3 years to carry out postdoctoral research with Professor Thomas P. Quinn at the University of Missouri-Columbia in the development of peptide radiopharmaceuticals for melanoma imaging and therapy. He was appointed as Research Assistant Professor in the Department of Internal Medicine at the University of Missouri-Columbia in 2003. In the Spring of 2006, he joined the faculty in the College of Pharmacy at the University of New Mexico, where he is now an Assistant Professor. His research interests include G protein-coupled receptor-targeting peptide radiopharmaceuticals for molecular imaging and targeted radionuclide therapy of cancer.
Thomas P. Quinn received his B.S. degree in Biochemistry at the University of Miami in Coral Gables, FL in 1983. He earned his Ph.D. degree with Dr. Duane Grandgenett in Cell and Molecular Biology at St. Louis University, St. Louis, MO in 1988. Quinn studied antibody engineering and combinatorial ligand discovery as a postdoctoral fellow with Dr. Dan Fowlkes at the University of North Carolina, Chapel Hill, NC. Later, he studied de novo protein design, molecular modeling and structural characterization with Drs. David and Jane Richardson at Duke University, Durham, NC as a NIH postdoctoral fellow. Quinn joined the faculty in the Department of Biochemistry at the University of Missouri, Columbia, MO in the Fall of 1991, where he is now a Professor of Biochemistry and Radiology, Director of the University of Missouri-Columbia Structural Biology Core, and a member of the Radiopharmaceutical Sciences Institute and Nuclear Sciences and Engineering Institute. His research interests include radiopharmaceutical chemistry with an emphasis on radiolabeled peptides, combinatorial ligand discovery using bacteriophage display, and in vivo molecular imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–6. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Marghood AA, Slade J, Salopek TG, Kopf AW, Bart RS, Rigel DS. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma. Cancer. 1995;75:707–14. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275–80. doi: 10.1001/archderm.135.3.275. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Atkins MB, Buzaid AC, Cascinelli N, Coit DG, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 5.Del Prete SA, Maurer LH, O’Donnell J, Forcier RJ, LeMarbre P. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep. 1984;68:1403–1405. [PubMed] [Google Scholar]
- 6.Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus Dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–51. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CM, Buzaid AC. Systematic treatments for advanced cutaneous melanoma. Oncology. 1995;9:1149–58. [PubMed] [Google Scholar]
- 9.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localization and therapy with radiopeptides. Curr Med Chem. 2000;7:971–94. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 10.Volkert WA, Goeckeler WF, Ehrhardt GJ, Ketring AR. Therapeutic radionuclides: production and decay property consideration. J Nucl Med. 1991;32:174–85. [PubMed] [Google Scholar]
- 11.Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, Heppeler A, et al. A powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med. 1997;24:792–95. doi: 10.1007/BF00879669. [DOI] [PubMed] [Google Scholar]
- 12.Otte A, Mueller-Brand J, Dellas S, Nitzsche EU, Herrmann R, Maecke HR, et al. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet. 1998;351:417–8. doi: 10.1016/s0140-6736(05)78355-0. [DOI] [PubMed] [Google Scholar]
- 13.Zamora PO, Bender H, Gulhke S, Marek MJ, Knapp FFJ, Rhodes BA, et al. Pre-clinical experience with Re-188-RC-160, a radiolabeled somatostatin analog for use in peptide-targeted radiotherapy. Anticancer Res. 1997;17:1803–8. [PubMed] [Google Scholar]
- 14.De Jong M, Breeman WAP, Bernard BF, Bakker WH, Schaar M, van Gameren A, et al. [177Lu-DOTA0, Tyr3]octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer. 2001;92:628–33. doi: 10.1002/1097-0215(20010601)92:5<628::aid-ijc1244>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Freitas JE, Gross MD, Ripley S, Shapiro B. Radionuclide diagnosis and therapy of thyroid cancer: current status report. Semin Nucl Med. 1985;15:106–31. doi: 10.1016/s0001-2998(85)80021-0. [DOI] [PubMed] [Google Scholar]
- 16.Hoefnagel CA. Radionuclide therapy revisited. Eur J Nucl Med. 1991;18:408–31. doi: 10.1007/BF02258432. [DOI] [PubMed] [Google Scholar]
- 17.Tatro JB, Wen Z, Entwistle ML, Atkins MB, Smith TJ, Reichlin S, et al. Interaction on an α-melanocyte stimulating hormone-diptheria toxin fusion protein with melanotropin receptors in human metastases. Cancer Res. 1992;52:2545–8. [PubMed] [Google Scholar]
- 18.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-α-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–706. [PubMed] [Google Scholar]
- 19.Eberle AN, Froidevaux S. Radiolabeled α-melanocyte-stimulating hormone analogs for receptor-mediated trageting of melanoma: from tritium to indium. J Mol Recognit. 2003;16:248–54. doi: 10.1002/jmr.633. [DOI] [PubMed] [Google Scholar]
- 20.Miao Y, Whitener D, Feng W, Owen NK, Chen JQ, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled α-melanocyte stimulating hormone peptide analogues. Bioconjugate Chem. 2003;14:1177–84. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 21.Miao Y, Quinn TP. Alpha-melanocyte stimulating hormone peptide-targeted melanoma imaging. Frontiers in Bioscience. 2007;12:4514–24. doi: 10.2741/2406. [DOI] [PubMed] [Google Scholar]
- 22.Hearing V. The melanosome: the perfect model for cellular responses to the environment. Pigment Cell Res. 2000;13(Suppl. 8):23–34. doi: 10.1034/j.1600-0749.13.s8.7.x. [DOI] [PubMed] [Google Scholar]
- 23.Ings RM. The melanin binding of drugs and its implications. Drug Metab Rev. 1984;15:1183–212. doi: 10.3109/03602538409033561. [DOI] [PubMed] [Google Scholar]
- 24.Fogarty RV, Tobin JM. Fungal melanins and their interactions with metals. Enzyme Microbial Technol. 1996;19:311–7. doi: 10.1016/0141-0229(96)00002-6. [DOI] [PubMed] [Google Scholar]
- 25.Dadachova E, Nosanchuk JD, Shi L, Schweitzer AD, Frenkel A, Nosanchuk JS, et al. Dead cell in melanoma tumors provide abundant antigen or targeted delivery of ionizing radiation by mAb to melanin. Proc Natl Acad Sci. 2004;101:14865–70. doi: 10.1073/pnas.0406180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, Wolbers JG, Kwekkeboom DJ. Effects of Therapy with [177Lu-DOTA0, Tyr3]Octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47:1599–606. [PubMed] [Google Scholar]
- 27.Giblin MF, Jurisson SS, Quinn TP. Synthesis and characterization of rhenium-complexed alpha-melanotropin analogs. Bioconjugate Chem. 1997;8:347–53. doi: 10.1021/bc9700291. [DOI] [PubMed] [Google Scholar]
- 28.Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium metal coordination. Proc Natl Acad Sci USA. 1998;95:12814–8. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JQ, Giblin MF, Wang N, Jurisson SS, Quinn TP. In vivo evaluation of 99mTc/188Re-labeled linear alpha-melanocyte stimulating hormone analogs for specific melanoma targeting. Nucl Med Biol. 1999;26:687–93. doi: 10.1016/s0969-8051(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 30.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–7. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 31.Miao Y, Owen NK, Darrell R, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re labeled α-melanocyte stimulating hormone peptide analogue in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–9. [PubMed] [Google Scholar]
- 32.Dadachova E, Model T, Schweitzer AD, Byran RA, Zhang T, Mints L, et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanoma in a mouse model of disease. Cancer Biother Radiopharm. 2006;21:117–29. doi: 10.1089/cbr.2006.21.117. [DOI] [PubMed] [Google Scholar]
- 33.Miao Y, Hoffman TJ, Quinn TP. Tumor targeting properties of 90Y and 177Lu labeled alpha-melanocyte stimulating hormone peptide analogues in a murine melanoma model. Nucl Med Biol. 2005;32:485–93. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Miao Y, Shelton T, Quinn TP. Therapeutic efficacy of a 177Lu labeled DOTA conjugated α-melanocyte stimulating hormone peptide in a murine melanoma-bearing mouse model. Cancer Biother Radiopharm. 2007;22:333–41. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- 35.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOA-ReCCMSH(Arg11), a cyclic peptide analogue of α-MSH. J Med Chem. 2005;48:2985–92. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 36.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, et al. Synthesis and biological evaluation of 64Cu-labled rhenium-cyclized α-MSH peptide analogs using a cross-bridged cyclam chelator. J Nucl Med. 2007;48:64–72. [PubMed] [Google Scholar]
- 37.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for MicroPET imaging of melanocortin 1 receptor expression. Bioconjugate Chem. 2007;18:765–72. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, et al. Melanoma therapy via peptide-targeted alpha-radiation. Clin Cancer Res. 2005;11:5616–21. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 39.Eberle AN, Hübscher W. α-Melanotropin labeled at its tyrosine2 residue: synthesis and biological activities of 3′-iodotyrosine2-,3′-125iodotyrosine2-,3′,5′-diiodotyrosine2-, and (3′,5′-3H2)-tyrosine2-α-melanotropin, and of related peptides. Helv Chim Acta. 1979;62:2460–83. [Google Scholar]
- 40.Eberle AN, Verin V Jäggin, Solca F, Siegrist W, Küenlin C, Bagutti C, et al. Biologically active monoidoinated α-MSH derivatives for receptor binding studies using human melanoma cells. J Recept Res. 1991;11:311–22. doi: 10.3109/10799899109066410. [DOI] [PubMed] [Google Scholar]
- 41.John CS, Bowen WD, Saga T, Kinuya S, Vilner BJ, Baumgold J, et al. A malignant melanoma imaging agent: synthesis, characterization, in vitro binding and biodistrubution of iodine-125-(2-piperidinylaminoethyl)4-iodobenzamide. J Nuc Med. 1993;34:2169–75. [PubMed] [Google Scholar]
- 42.Garg PK, Alston KL, Welsh PC, Zalutsky MR. Enhanced binding and inertness to dehalogenation of α-melanotropic peptides labeled using n-succinimidyl 3-iodobenzoate. Bioconjugate Chem. 1996;7:233–9. doi: 10.1021/bc960001+. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Z, Chen J, Quinn TP, Jurisson SS. Radioiodination of rhenium cyclized alpha-melanocyte-stimulating hormone resulting in enhanced radioactivity localization and retention in melanoma. Cancer Res. 2004;64:1411–18. doi: 10.1158/0008-5472.can-03-0193. [DOI] [PubMed] [Google Scholar]
- 44.De Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, et al. Yttrium-90 and indium-111 labelling, receptor binding and biodstribution of {DOTA0, D-Phe1, Tyr3}octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nuc Med. 1997;24:368–71. doi: 10.1007/BF00881807. [DOI] [PubMed] [Google Scholar]
- 45.De Jong M, Breeman WA, Bakker WH, Kooij PP, Bernard BF, Hofland LJ, et al. comparison of 111In-labeled somatostatin analogues for tumor scintigraphy and radionuclide therapy. Cancer Res. 1998;58:437–41. [PubMed] [Google Scholar]
- 46.Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [111In-DTPA0]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–22. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 47.Anthony LB, Woltering EA, Espanan GD, Cronin MD, Maloney TJ, McCarthy KE. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32:123–32. doi: 10.1053/snuc.2002.31769. [DOI] [PubMed] [Google Scholar]
- 48.Ginj M, Hinni K, Tshumi S, Schulz S, Maecke H. Trifunctional somatostatin-based derivatives designed for targeted radiotherapy using auger electron emitters. J Nucl Med. 2005;46:2097–103. [PubMed] [Google Scholar]
- 49.Chen JQ, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, et al. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: A novel cyclic-peptide analog with improved tumor targeting properties. J Nucl Med. 2001;42:1847–55. [PubMed] [Google Scholar]
- 50.Cheng Z, Chen J, Owen N, Miao Y, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: Synthesis and biological evaluation of 111In labeled DOTA conjugated rhenium cyclized α-MSH analogs. J Med Chem. 2002;45:3048–56. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 51.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–706. [PubMed] [Google Scholar]
- 52.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A gallium-labeled DOTA-alpha-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–23. [PubMed] [Google Scholar]
- 53.Froidevaux S, Eberle AN. DOTA alpha-melanocyte-stimulating hormone analogues for imaging metastatic melanoma lesions. Ann N Y Acad Sci. 2003;994:378–83. doi: 10.1111/j.1749-6632.2003.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 54.Miao Y, Benwell K, Quinn TP. 99mTc and 111In-labeled alpha-melanocyte stimulation hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 55.Garg PK, Alston KL, Welsh PC, Zalutsky MR. Enhanced binding and inertness to dehalogenation of α-melanotropic peptides labeled using N-succinimidyl-3-iodobenzoate. Bioconjugate Chem. 1996;7:233–9. doi: 10.1021/bc960001+. [DOI] [PubMed] [Google Scholar]
- 56.Vaidyanathan G, Zalutsky MR. Fluorine-18-labeled [Nle4, D-Phe7]-alpha-MSH, an alpha-melanocyte stimulating hormone analogue. Nucl Med Biol. 1997;24:171–8. doi: 10.1016/s0969-8051(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 57.Cheng Z, Zhang L, Graves E, Xiong Z, Dandekar M, Chen X, et al. Small-animal PET of melanocortin 1 receptor expression using a 18F-labeled α-melanocyte-stimulating hormone analog. J Nucl Med. 2007;48:987–94. doi: 10.2967/jnumed.107.039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schubiger PA, Alberto R, Smith A. Vehicles, chelators and radionuclides: Choosing the “building blocks” of an effective therapeutic radioimmunoconjugate. Bioconjugate Chem. 1996;7:165–79. doi: 10.1021/bc950097s. [DOI] [PubMed] [Google Scholar]
- 59.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, Curcio MJ, Miederer M, Bander NH, Scheinberg DA. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–40. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 60.De Jong M, Breeman WAP, Bernard BF, Bakker WH, Visser TJ, Kooij PPM, et al. Tumor response after [90Y-DOTA0,Tyr3]-octreotide radionuclide therapy in a transplantable rate tumor model is dependent on tumor size. J Nucl Med. 2001;42:1841–46. [PubMed] [Google Scholar]
- 61.De Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32:133–40. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 62.Teuissen JJM, Kwekkeboom DJ, De Jong M, Esser JP, Valkema R, Krenning EP. Peptide receptor radionuclide therapy. Best Pract Res Clin Gastroenterol. 2005;19:595–616. doi: 10.1016/j.bpg.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 63.De Jong M, Breeman WAP, Bernard BF, Bakker WH, Schaar M, van Gameren A, et al. [177Lu-DOTA0, Tyr3]octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer. 2001;92:628–33. doi: 10.1002/1097-0215(20010601)92:5<628::aid-ijc1244>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 64.McDevitt MR, Sgouros G, Finn RD, Humm JL, Jurcic JG, Larson SM, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–51. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 65.Zalutsky MR, Bigner D. Radioimmunotherapy with alpha-particle emitting radioimmunoconjugates. Acta Oncologica. 1996;35:373–9. doi: 10.3109/02841869609101654. [DOI] [PubMed] [Google Scholar]
- 66.Larsen RH, Akabani G, Welsh P, Zalutsky MR. The cytotoxicity and microdosimetry of Astatine-211-labeled chimeric monoclonal antibodies in human glioma and melanoma cells. Radiat Res. 1998;149:155–62. [PubMed] [Google Scholar]
- 67.Behr T, Behe M, Lohr M, Sgouros G, Angerstein C, Wehrmann E, et al. Therapeutic advantages of Auger electron-over beta-emitting radiometals or radioiodine when conjugated to internalizing antibodies. Eur J Nucl Med. 2000;27:753–65. doi: 10.1007/s002590000272. [DOI] [PubMed] [Google Scholar]
- 68.Sawyer TK, Staples DJ, Catrucci AML, Hadley ME, Al-Obeeidi FA, Cody WL, et al. Alpha-melanocyte stimulating hormone message and inhibitory sequences: Comparative structure activity studies on melanocytes. Peptides. 1990;11:351–7. doi: 10.1016/0196-9781(90)90092-j. [DOI] [PubMed] [Google Scholar]
- 69.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. [PubMed] [Google Scholar]
- 70.Wong W, Minchin RF. Binding and internalization of melanocyte stimulating hormone receptor ligand [Nle4, D-Phe7]-α-MSH in B16 melanoma cells. Int J Biochem Cell Biol. 1996;28:1223–32. doi: 10.1016/s1357-2725(96)00074-x. [DOI] [PubMed] [Google Scholar]
- 71.Nosanchuk JD, Valadon P, Feldmesser M, Casadevall A. Melanization of cryptococcus neoforms in murine infection. Mol Cell Biol. 1999;19:745–50. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassfjell S, Brechbiel MW. The development of the α-particle emitting radionuclides 212Bi and 213Bi and their decay chain related radionuclides, for therapy applications. Chem Rev. 2001;101:2019–36. doi: 10.1021/cr000118y. [DOI] [PubMed] [Google Scholar]
- 73.Leichner PK. Radiation dosimetry of monoclonal antibodies: practical considerations. In: Henkin RE, Boles MA, Dillehay GL, et al., editors. Nuclear Medicine. Mosby; St. Louis: 1996. pp. 558–62. [Google Scholar]
- 74.Acher RN, Friedman AM, Hines JJ. An improved generator for the production of Pb-212 and Bi-212 from Ra-224. Appl Radiat Isot. 1988;39:283–6. doi: 10.1016/0883-2889(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 75.Mirzadeh S, Kumar K, Gansow OA. The chemical fate of Bi-212-DOTA formed by β-decay of Pb(DOTA) Radiochim Acta. 1993;60:1–10. [Google Scholar]
- 76.Jones SB, Tiffany LJ, Garmestani K, Gansow OA, Kozak RW. Evaluation of dithiol chelating agents as potential adjuvants for anti-IL-2 receptor lead or bismuth a radioimmunotherapy. Nucl Med Biol. 1996;23:105–13. doi: 10.1016/0969-8051(95)02006-3. [DOI] [PubMed] [Google Scholar]
- 77.Chen JQ, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mTechnetium-labeled cyclic α-melanocyte stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–58. [PubMed] [Google Scholar]
- 78.Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiation Oncol Biol Phys. 2001;49:379–89. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 79.Moadel RM, Weldon RH, Katz EB, Lu P, Mani J, Stahl M, et al. Positherapy: targeted nuclear therapy of breast cancer with 18F-2-deoxy-2-fluor-D-Glucose. Cancer Res. 2005;65:698–702. [PubMed] [Google Scholar]
- 80.Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin MG, Orsi F, et al. Biokinetics and dosimetry in patients administered with 111In-DOTA-Tyr3-octreotide; implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med. 1999;26:887–96. doi: 10.1007/s002590050462. [DOI] [PubMed] [Google Scholar]
- 81.Otte A, Heppeler A, Behe M, Jermann E, Powell P, Maecke HR, et al. Yttrium-90 DOTATOC: first clinical trial. Eur J Nucl med. 1999;26:1439–47. [PubMed] [Google Scholar]
- 82.Moll S, Nickeleit V, Mueller-Brand J, Brunner FP, Maecke HR, Mihatsch MJ. A new case of renal thrombotic microangiopathy: yttrium 90-DOTATOC internal radiotherapy. Am J Kidney Diseases. 2001;37:847–51. doi: 10.1016/s0272-6386(01)80135-9. [DOI] [PubMed] [Google Scholar]
- 83.Boerman, Otto, Oyen, Wim JG, Corstens, Frans HM. Between the Scylla and Charybdis of peptide radionuclide therapy: hitting the tumor and saving the kidney. Eur J Nucl Med. 2001;28:1447–9. doi: 10.1007/s002590100597. [DOI] [PubMed] [Google Scholar]
- 84.Behr TM, Béhé M, Kuge G, Gotthardt M, Schipper ML, Gratz S. Nephrotoxicity versus anti-tumour efficacy in radiopeptide therapy: facts and myths about the Scylla and Charybdis. Eur J Nucl Med. 2002;29:277–9. doi: 10.1007/s00259-001-0713-1. [DOI] [PubMed] [Google Scholar]
- 85.Jaggi JS, Kappel BJ, McDevitt MR, Sgouros G, Flombaum CD, Cabassa C, et al. Efforts to control the errant products of targeted in vivo generator. Cancer Res. 2005;65:4888–95. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 86.Gotthardt M, van Eerd-Vismale J, Oyen WJ, De Jong M, Zhang H, Rolleman E, Maecke HR, Béhé M, Boerman O. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med. 2007;48:596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 87.Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, Quinn TP. 203Pb-labeled alpha-melanocyte stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008 doi: 10.2967/jnumed.107.048553. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alonso O, Martinez M, Delgado L, De Leon A, De Boni D, Lago G, et al. Staging of regional lymph nodes in melanoma patients by means of 99mTc-MIBI scintigraphy. J Nucl Med. 2003;44:1561–65. [PubMed] [Google Scholar]
- 89.Acland KM, Healy C, Calonje E, O’Doherty M, Nunan T, Page C, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of micrometastases of primary cutaneous malignant melanoma. J Clinical Oncology. 2001;19:2674–8. doi: 10.1200/JCO.2001.19.10.2674. [DOI] [PubMed] [Google Scholar]
- 90.Mijnhout SG, Hoekstra OS, van Tulder MW, Teule GJ, Deville WL. Systemic review of he diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer. 2001;91:1530–42. [PubMed] [Google Scholar]
- 91.Krug B, Dietlein M, Groth W, Strutzer H, Psaras T, Grossmann A, et al. Fluor-18-fluorodeoxyglucose positron emission tomography (FDG-PET) in malignant melanoma. Acta Radiologica. 2000;41:446–52. doi: 10.1080/028418500127345668. (2000) [DOI] [PubMed] [Google Scholar]
- 92.Wagner JD, Schauwecker D, Davidson D, Logan T, Coleman JJ, Hutchins G, et al. Inefficacy of F-18 fluorodeoxy-D-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer. 2005;104:570–9. doi: 10.1002/cncr.21189. [DOI] [PubMed] [Google Scholar]
- 93.Fuster D, Chiang S, Johnson G, Schuchter LM, Zhuang H, Alavi A. Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma. J. Nucl Med. 2003;45:1323–7. [PubMed] [Google Scholar]
- 94.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;249:1537–40. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 95.Sgouros G, Ballangrud AM, Jurcic JG, McDevitt MR, Humm JL, Erdi YE, et al. Pharmacokinetics and dosimetry of an alpha-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J Nucl Med. 1999;40:1935–46. [PubMed] [Google Scholar]