Abstract
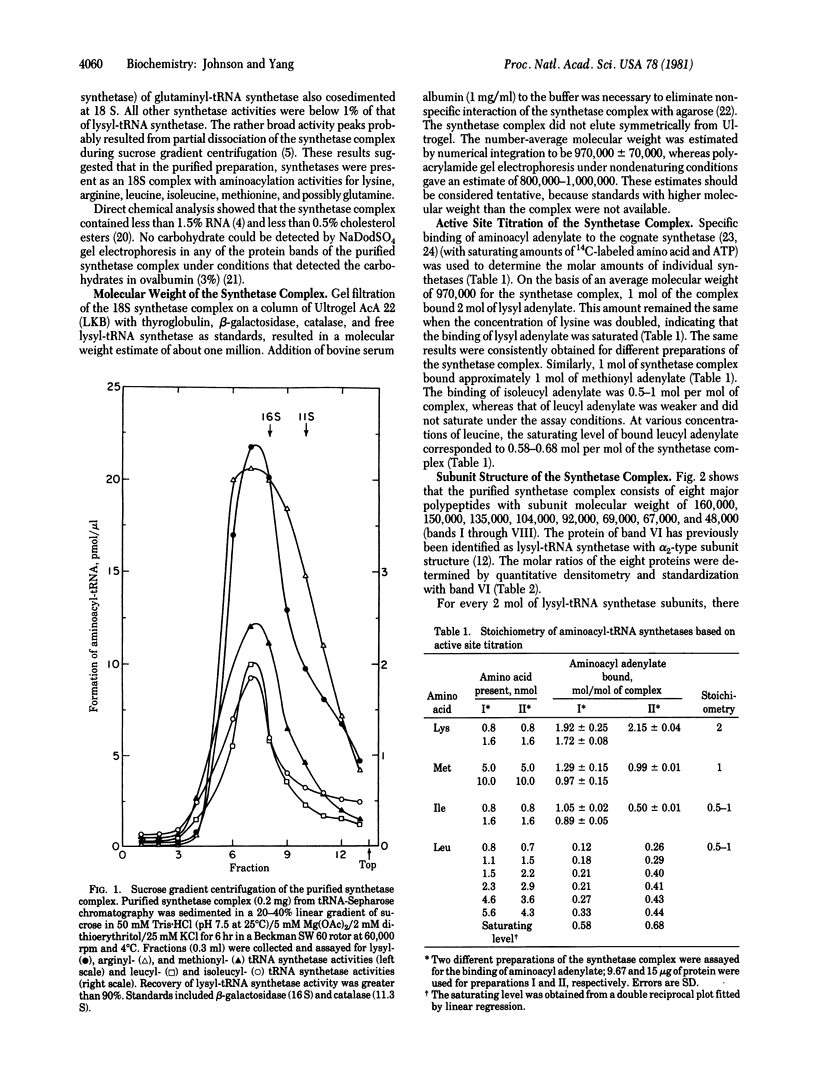
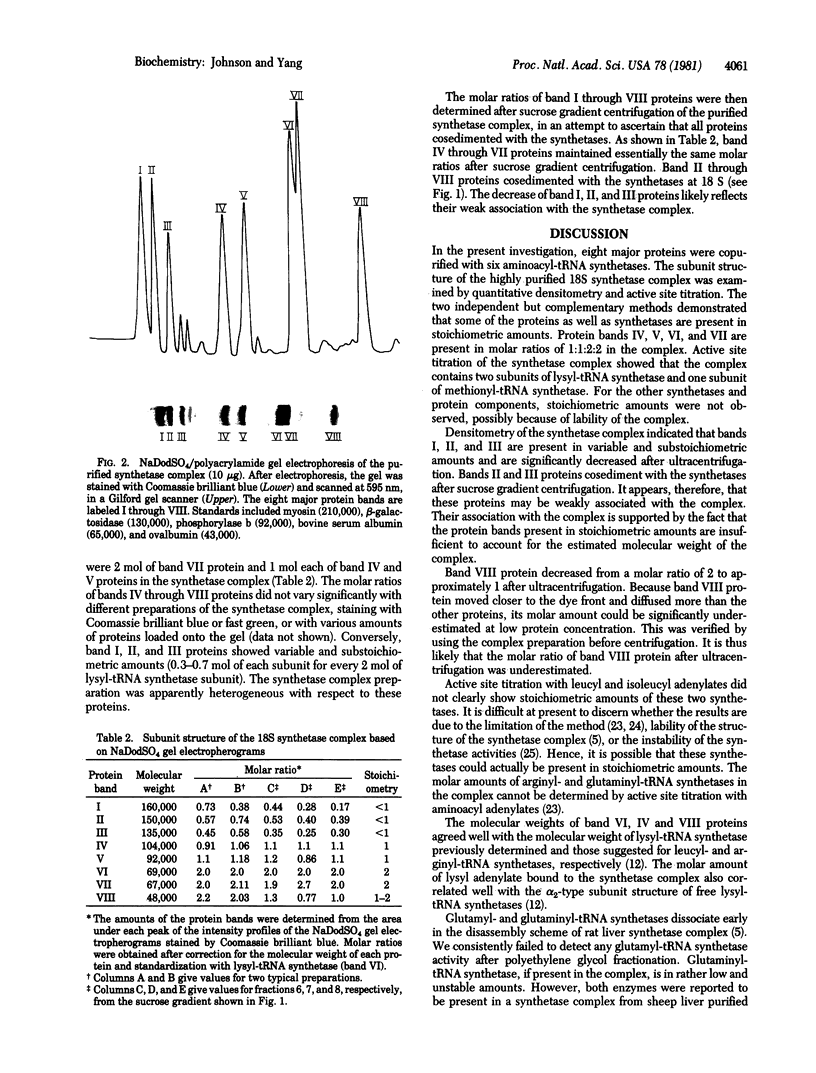
The particulate aminoacyl-tRNA synthetases of rat liver were copurified about 1000-fold with more than 20% yields for individual synthetase activities. Measurements of aminoacylation activities showed that lysyl-, arginyl-, leucyl-, isoleucyl-, and methionyl-tRNA synthetases in the purified complex cosedimented at 18 S. The molecular weight of the synthetase complex is about one million, as estimated by gel filtration. The stoichiometry of the synthetase in the complex was determined by active site titration with aminoacyl adenylates. Results indicate that the 18S synthetase complex contains one subunit of methionyl-tRNA synthetase and two subunits of lysyl-tRNA synthetase. Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate showed that the 18S synthetase complex contains eight major protein bands. Proteins with subunit molecular weights of 104,000, 92,000, 69,000, and 67,000 are present in molar ratios of 1:1:2:2, while proteins with subunit molecular weights of amounts. These results suggest that the particulate aminoacyl-tRNA synthetases exist as a heterotypic multienzyme complex with defined structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Simpson D. R., Chiang C. S., Andrulis I. L., Hatfield G. W. A role for asparaginyl-tRNA in the regulation of asparagine synthetase in a mammalian cell line. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2367–2369. doi: 10.1073/pnas.74.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Deutscher M. P. Complex of aminoacyl-transfer RNA synthetases. J Mol Biol. 1971 Aug 28;60(1):113–122. doi: 10.1016/0022-2836(71)90451-7. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Deutscher M. P. Lipids associated with the aminoacyl-transfer RNA synthetase complex. J Mol Biol. 1973 Feb 25;74(2):257–261. doi: 10.1016/0022-2836(73)90112-5. [DOI] [PubMed] [Google Scholar]
- Berg B. H. The role of cyclic 3',5'-AMP in the regulation of aminoacyl-tRNA synthetase activities in mouse uterus and liver following 17beta-oestradiol treatment. Activation of a phosphoaminoacyl-tRNA synthetase phosphatase by phosphorylation with cyclic 3',5'-AMP dependent protein kinase. Biochim Biophys Acta. 1978 Nov 21;521(1):274–287. doi: 10.1016/0005-2787(78)90270-8. [DOI] [PubMed] [Google Scholar]
- Boeker E. A., Cantoni G. L. Seryl transfer ribonucleic acid synthetase of Escherichia coli B, Enzyme-substrate interactions. Biochemistry. 1973 Jun 19;12(13):2384–2389. doi: 10.1021/bi00737a003. [DOI] [PubMed] [Google Scholar]
- Brevet A., Kellermann O., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 2. Agarose gel-filtration behaviour of the extensively purified high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):551–558. doi: 10.1111/j.1432-1033.1979.tb13287.x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Yang D. C. Affinity chromatography of rat liver aminoacyl-tRNA synthetase complex. Biochem Biophys Res Commun. 1978 Feb 28;80(4):709–714. doi: 10.1016/0006-291x(78)91302-5. [DOI] [PubMed] [Google Scholar]
- Denney R. M. Detection and partial purification of rapidly sedimenting forms of aminoacyl-transfer ribonucleic acid synthetases from human placenta. Arch Biochem Biophys. 1977 Sep;183(1):156–167. doi: 10.1016/0003-9861(77)90430-1. [DOI] [PubMed] [Google Scholar]
- Glinski R. L., Gainey P. C., Mawhinney T. P., Hilderman R. H. Evidence that lysyl- and/or arginyl-tRNA synthetases from rat liver contain carbohydrate. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1052–1061. doi: 10.1016/0006-291x(79)91515-8. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Hampel A., Enger M. D. Subcellular distribution of aminoacyl-transfer RNA synthetases in Chinese hamster ovary cell culture. J Mol Biol. 1973 Sep 15;79(2):285–293. doi: 10.1016/0022-2836(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Johnson D. L., Van Dang C., Yang D. C. Purification and characterization of lysyl-tRNA synthetase after dissociation of the particulate aminoacyl-tRNA synthetases from rat liver. J Biol Chem. 1980 May 10;255(9):4362–4366. [PubMed] [Google Scholar]
- Kellermann O., Brevet A., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 1. Extensive purification and characterization of the high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):541–550. doi: 10.1111/j.1432-1033.1979.tb13286.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore P. A., Jayme D. W., Oxender D. L. A role for aminoacyl-tRNA synthetases in the regulation of amino acid transport in mammalian cell lines. J Biol Chem. 1977 Nov 10;252(21):7427–7430. [PubMed] [Google Scholar]
- Remy P., Birmelé C., Ebel J. P. Purification of yeast phenylalanyl-tRNA synthetase by affinity chromatography, on a tRNA(Phe)-sepharose column. FEBS Lett. 1972 Oct 15;27(1):134–138. doi: 10.1016/0014-5793(72)80426-5. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Som K., Hardesty B. Isolation and partial characterization of an aminoacyl-tRNA synthetase complex from rabbit reticulocytes. Arch Biochem Biophys. 1975 Feb;166(2):507–517. doi: 10.1016/0003-9861(75)90414-2. [DOI] [PubMed] [Google Scholar]
- Spassky A., Busby S. J., Danchin A., Buc H. On the binding of tRNA to Escherichia coli RNA polymerase. Eur J Biochem. 1979 Aug 15;99(1):187–201. doi: 10.1111/j.1432-1033.1979.tb13245.x. [DOI] [PubMed] [Google Scholar]
- Ussery M. A., Tanaka W. K., Hardesty B. Subcellular distribution of aminoacyl-tRNA synthetases in various eukaryotic cells. Eur J Biochem. 1977 Feb;72(3):491–500. doi: 10.1111/j.1432-1033.1977.tb11272.x. [DOI] [PubMed] [Google Scholar]
- Van Dang C., Yang D. C. Disassembly and gross structure of particulate aminoacyl-tRNA synthetases from rat liver. Isolation and the structural relationship of synthetase complexes. J Biol Chem. 1979 Jun 25;254(12):5350–5356. [PubMed] [Google Scholar]
- Vennegoor C., Bloemendal H. Occurrence and particle character of aminoacyl-tRNA synthetases in the post-microsomal fraction from rat liver. Eur J Biochem. 1972 Apr 24;26(4):462–473. doi: 10.1111/j.1432-1033.1972.tb01788.x. [DOI] [PubMed] [Google Scholar]
- Welch G. R. On the role of organized multienzyme systems in cellular metabolism: a general synthesis. Prog Biophys Mol Biol. 1977;32(2):103–191. [PubMed] [Google Scholar]
- Yarus M., Berg P. On the properties and utility of a membrane filter assay in the study of isoleucyl-tRNA synthetase. Anal Biochem. 1970 Jun;35(2):450–465. doi: 10.1016/0003-2697(70)90207-1. [DOI] [PubMed] [Google Scholar]