Abstract
Although the use of antenatal glucocorticoids has resulted in decreased neonatal morbidity/mortality, recent animal studies have raised concerns regarding adverse effects of these medications on postnatal cardiovascular function. We hypothesized that antenatal betamethasone (Beta) exposure alters cerebral vascular reactivity in adult female sheep. We observed that K+-induced constriction was comparable in middle cerebral artery (MCA) from Beta-exposed animals and age-matched controls. Pressure-induced constriction was significantly attenuated in MCA from Beta-exposed compared with control sheep. Inhibition of NOS significantly augmented pressure-induced constriction in MCA from both Beta-exposed and control sheep whereas cyclooxygenase (COX) inhibition augmented pressure-induced constriction only in MCA from Beta-exposed sheep. Furthermore, NOS and COX inhibition significantly attenuated bradykinin (BK)-induced dilation in MCA from both Beta-exposed and control sheep. However, there appeared to be a greater contribution of both NOS and COX to BK-induced dilation in Beta-exposed compared with control MCA. Our findings demonstrate that fetal exposure to a clinically relevant course of Beta alters cerebral vascular tone and reactivity in adult female sheep.
Introduction
Glucocorticoid administration is the standard of care in mothers with preterm labor. However, little is known regarding the cardiac and vascular effects of these medications in adult life. Exposure of the ovine fetus to glucocorticoids is associated with altered kidney development and renal function (1–5). This altered kidney development/function has been linked to moderate elevation in fetal (6, 7) and postnatal (1, 8, 9) blood pressure (BP) in animal models. Altered coronary, femoral, mesenteric and brachial artery reactivity have been described in offspring exposed to glucocorticoids in utero (8, 10, 11). More recent studies have shown altered pulmonary vascular development in term rabbits after antenatal exposure to glucocorticoids (12).
After dexamethasone exposure in utero, fetal ovine middle cerebral arteries (MCA) develop tachyphylaxis when exposed to >10 nmol/L concentrations of endothelin-1 (13). Furthermore, Schwab and colleagues have demonstrated decreased regional cerebral blood flow and increased cerebral vascular resistance in fetal sheep exposed to Beta late in gestation (14). Thus, antenatal glucocorticoid exposure can alter cerebral vascular tone and reactivity in fetal life. Little is known regarding the effects of antenatal Beta exposure on the postnatal cerebral circulation, especially in adulthood. We hypothesized that a clinically relevant course of antenatal maternal Beta administration would alter (a) pressure-induced constriction, and (b) agonist-induced dilator responses in resistance-size MCA from adult female sheep.
Materials and Methods
Animals
All experimental protocols were performed in accordance with NIH guidelines for the use of experimental animals. This study was approved by the Animal Care and Use Committee at Wake Forest University School of Medicine.
Pregnant sheep received two intramuscular injections of Beta (1:1 mixture of Beta acetate and Beta phosphate; Celestone Soluspan®) in a manner similar to that used in the clinical setting for stimulating lung maturation, i.e. 0.17 mg/kg/dose 24 hours apart (maximum of 12 mg/day) starting on day 80 of gestation (0.6 of gestation which corresponds to early third trimester of human pregnancy). This gestational age was chosen to more closely approximate the period of human gestation at which antenatal glucocorticoids are used therapeutically. Lambs were delivered spontaneously, weaned at three months of age, and placed in sex-specific enclosures. Estrous-synchronized non-pregnant Beta-exposed (Beta; N=7) and age-matched control (C; N=6) sheep were euthanized under halothane general anesthesia at 18-months of age. Estrous synchronization was achieved by implanting sheep with Eazi-Breed CIDR Sheep (progesterone delivery device; Pfizer, Australia) for 14 days and the sheep were euthanized seven days after the implant was removed, i.e., at a stage of the estrous cycle where plasma estrogen levels are the lowest.
Isolation of MCA and pressurized arterial wall diameter measurements
After euthanasia, the brain was removed and transferred into cold (4°C) physiological saline solution (PSS; composition (in mmol/L): 118 NaCl, 4.5 KCl, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 11 dextrose, 2.5 CaCl2, and 0.26 EDTA; pH 7.4) aerated with gas containing 21% O2, 74% N2 and 5% CO2. MCA segments (150–250 μm internal diameter, 2–3 mm length) were dissected, cleaned of connective tissue, and transferred to the well of a 2-ml arteriograph (Instrumentation and Model Facility, University of Vermont, Burlington, VT) fitted with a borosilicate (FHC, Bowdoinham, ME) micropipette at both ends. Both the proximal and distal micropipettes were attached to 3-way Luer Stopcocks (Cole-Parmer, Vernon Hills, Illinois). The arteries were cannulated on the proximal pipette, secured with suture, gently flushed free of blood and then cannulated/secured on the distal pipette. The arteriograph containing the cannulated vessel was then transferred to the stage of an inverted microscope (Nikon) where the vessel diameter was viewed, measured, and continuously recorded using video edge detection equipment and data acquisition software, respectively (IonOptix Inc., Milton, MA, USA). After placing the arteriograph on the microscope stage, the proximal cannula was attached to the pressure reservoir and the intraluminal pressure increased to 10 mmHg to allow a gentle flushing (~ 2 min) of the intraluminal contents from the vessel. The 3-way stopcock on the distal cannula was then closed in order to pressurize the artery; thus all experimental protocols were conducted under “no-flow” conditions. In all experiments, pressurized arteries were continuously superfused with PSS (8–10 mL/min) aerated with gas containing 21% O2, 74% N2 and 5% CO2 at 37°C.
Study protocol
The vessels were equilibrated for a total of approximately 90 minutes. Arteries were initially pressurized to 10 mmHg (as described above) and allowed to equilibrate for 10 minutes, allowing the vessel to acclimate at 37°C. The intraluminal pressure was then increased to 60 mmHg to assess the development of pressure-induced constriction. Vessels exhibiting pressure-induced constriction at 60 mmHg were dilated with 0.1 μmol/L bradykinin (BK) to confirm endothelial function. After washing out BK with PSS and re-establishing a stable baseline lumen diameter, the pressurized vessels were constricted with a single dose of 50 mM KCl to confirm vascular smooth muscle (VSM) function. Vessels that did not exhibit a minimum of 20% pressure-induced constriction (at 60 mmHg), K+-induced constriction and BK-induced dilation were excluded from further study. Studies assessing the effect of antenatal beta on pressure-induced constriction and endothelium-dependent dilation were completed as described below.
Pressure-induced vasoconstriction
In these experiments, the effect of antenatal Beta exposure on pressure-induced constriction was determined both in the absence and presence of NOS and COX inhibitors. Since each vessel acted as its own experimental control, each arterial segment was exposed to three sequential pressure curves; each pressure curve consisted of a step-wise increase in intraluminal pressure from 10 to 120 mmHg. Changes in lumen diameter were recorded at each pressure.
In all experiments, the first pressure-diameter relationship acted as the “control” following which the tissues were allowed to recover for 10 minutes at 10 mmHg pressure. To determine the role of NOS and COX on pressure-induced constriction, the arterial segments were then incubated with either N-omega-nitro-L-arginine methyl ester (L-NAME; 100 μmol/L), a non-specific NOS inhibitor, or indomethacin (Indo; 10 μmol/L), a non-specific COX inhibitor, for 30 minutes and a second pressure curve was generated. Tissues were once again returned to 10 mmHg, allowed to recover for 10 minutes, and then incubated in Ca2+-free PSS with diltiazem (80 μmol/L), an L-type Ca2+-channel inhibitor, for 60 minutes to obtain the passive diameter for the pressure curves.
Pressure-induced constriction was expressed as a percent decrease of the fully dilated diameter of individual arteries at the same intravascular pressure. These values were obtained using the following equation:
where DP = the “passive” diameter of the artery in Ca2+-free PSS with diltiazem, and DA = the “active” diameter of the artery in response to the stimulus (change in intraluminal pressure) in Ca2+-containing PSS in the absence/presence of L-NAME or Indo. In addition, the passive diameter value obtained at 60 mmHg was used to assess the % pressure-induced constriction with 50mM K+ reported in Figure 1.
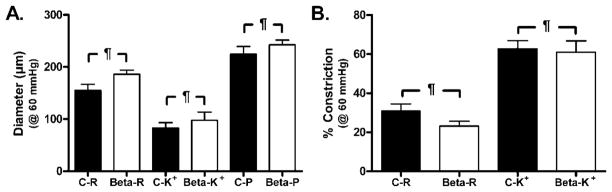
Figure 1.
Effect of 50 mmol/L KCl (K+) on (A) vascular diameter and (B) arterial constriction in C and Beta MCA pressurized at 60 mmHg. A: There was no significant difference between resting (R) (C-R vs. Beta-R) and passive (P) (C-P vs. Beta-P) diameters in MCA from C and Beta sheep. Furthermore, addition of 50 mmol/L K+ caused a comparable decrease in arterial diameter in both C-K+ and Beta-K+ MCA. (N: C=5; beta-exposed=7; p=n.s.). B: Percent pressure-induced constriction was similar in MCA from C and Beta sheep. Additionally, 50 mmol/L K+ resulted in a comparable degree of constriction in both C-K+ and Beta-K+ MCA. (N: C=5; beta-exposed=7; p=n.s.).
Endothelium-dependent dilations
In these experiments, the effect of antenatal steroid exposure on endothelium-dependent dilations was determined in the absence and presence of either NOS or COX inhibitors. As described above, each vessel acted as its own experimental control. These experiments were performed at a single intraluminal pressure of 60 mmHg. After a stable lumen diameter was obtained at 60 mmHg, control and Beta-exposed MCA were exposed to increasing concentrations of BK (0.01–10 nmol/L) and vessel diameters recorded for each concentration of BK (“control” values). After completion of the “control” curve, the arterial segments were washed with PSS and the vessel diameter allowed to return to baseline. After a 30-minute recovery period, tissues were incubated with either L-NAME (100 μmol/L) or Indo (10 μmol/L) for 30 minutes to assess the contribution of NOS and COX, respectively, to BK-induced dilations. After completion of the second BK concentration-response relationship, the vessels were washed, allowed to equilibrate with a stable lumen diameter following which the vessels were incubated in Ca2+-free PSS with diltiazem (80 μmol/L) for 60 minutes to obtain a passive diameter at 60 mmHg. The results from these experiments were expressed as percent reversal of constriction. These values were obtained using the following equation:
where DV = the diameter of the artery in response to BK in Ca2+-containing PSS.
Chemicals
All chemicals were purchased from Sigma Chemicals (St. Louis, MO). Drug stock solutions were prepared as follows: BK was prepared daily from 1.0 mmol/L aliquots made in an aqueous stock solution. Aliquots were diluted to 0.1 μmol/L and serial dilutions were made to the desired concentrations. L-NAME (100 μmol/L) and Indo (10 μmol/L) were prepared daily in fresh PSS at the desired concentration.
Statistical analyses
Data are expressed as mean ± SEM. In instances where multiple arterial segments from a single animal were used for a particular experimental protocol, the results obtained from all experiments were averaged to denote a single “N”. K+- and pressure-induced constriction were analyzed using either a paired t-test, one or two way ANOVA, or with a repeated measure model with within-group contrasts, as appropriate. Comparisons between groups were analyzed with a post hoc Newman Keul’s multiple comparison test at the 0.05 level of significance. Differences between concentration-response curves to BK, in the absence and presence of L-NAME or Indo, were determined using GraphPad Prism’s preprogrammed nonlinear regression curve fitting model which compares the following three parameters of a dose-response curve: bottom (baseline response), top (peak response), and pD2 (−log EC50) values. Individual parameters and/or the BK dose response curves were considered significantly different from one another if the three parameter comparison test was ≤0.05 level of significance. All statistical analyses were performed using GraphPad Prism version 5.03 (GraphPad Software, Inc., San Diego, CA). Where appropriate, the level of statistical significance is indicated as follows: p<0.05 (*), p<0.01 (**), p<0.001 (§) and not significant (¶). Symbols adjacent to vertical bars (e.g., Figure 2A and B: | §) indicate differences between experimental groups.
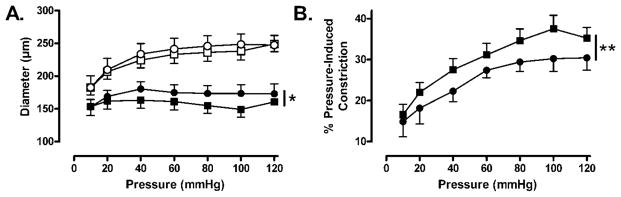
Figure 2.
Effect of increasing intraluminal pressure (10–120 mmHg) on vascular diameter (A) and arterial constriction (pressure-induced constriction; B) in C and Beta MCA. A: MCAs from both C (□) and Beta (○) sheep had comparable passive diameters at all pressures studied. In contrast, C arteries (■) constricted more, and thus had a smaller lumen diameter in response to a stepwise increase in intraluminal pressure when compared with Beta MCA (●). (N: C=5, beta-exposed=6; p<0.05) B: A stepwise increase in intraluminal pressure resulted in significantly greater pressure-induced constriction in C (■) compared with Beta (●) MCA. (N: C=5, beta-exposed=6; p<0.01)
Results
VSM depolarization with KCl
At an intraluminal pressure of 60 mmHg, MCA from Control (C) and Beta sheep had similar resting (R) diameters (Figure 1A: C-R=155±11 μm vs. Beta-R=186±8 μm; p=n.s.) and passive (P) diameters (Figure 1A: C-P=224±14 μm vs. Beta-P=242±9 μm; C:N=5, Beta: N=7; p=n.s.). Furthermore, addition of 50 mmol/L KCl (K+) resulted in a comparable decrease in arterial diameter (Figure 1A: C-K+=83±10 μm vs. Beta-K+=97±16 μm; p=n.s.) and % arterial constriction (Figure 1B: C-K+=62±4% vs. Beta-K+=61±6%; C:N=5, Beta: N=7; p=n.s.) in both control and Beta-exposed sheep.
Contribution of NOS and COX to pressure-induced constriction
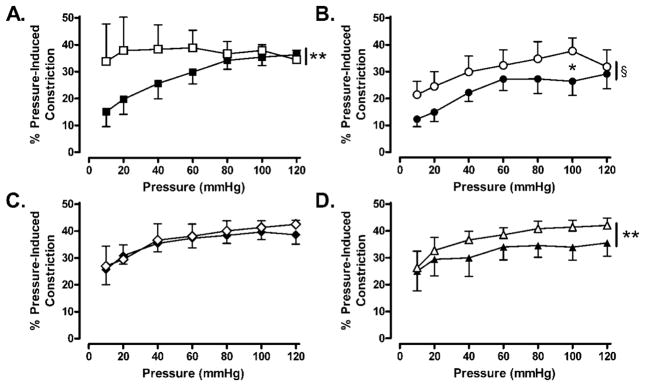
Our next series of experiments was designed to assess pressure (10–120 mmHg)-induced constriction in MCA from control and Beta-exposed sheep. As shown in Figure 2A, the passive diameters of MCA from both control and Beta-exposed animals were comparable at all pressures studied whereas the active diameters of Beta-exposed MCA were significantly greater than control MCA (control N=5; Beta-exposed N=6; p<0.05). Pressure-induced constriction was significantly attenuated in Beta-exposed MCA compared with control MCA (Figure 2B: control N=5; beta-exposed N=6; p<0.01). Inhibition of NOS with L-NAME (100 μmol/L) caused augmentation in pressure-induced constriction in both control (Figure 3A: N=4; p<0.01) and Beta-exposed (Figure 3B: N=5; p<0.001) MCA. In contrast, inhibition of COX with Indo (10 μmol/L) had no effect on pressure-induced constriction in control MCA (Figures 3C: N=5; p=n.s.) but significantly augmented pressure-induced constriction in Beta-exposed MCA (Figure 3D: N=5; p<0.01).
Figure 3.
Effect of NOS and COX inhibition on pressure (10–120 mm Hg)-induced constriction in C and Beta MCA. A and B: Inhibition of NOS with L-NAME (100 μmol/L) caused an increase in pressure-induced constriction in both C (A: N=4; C: ■; C+L-NAME: □; p<0.01) and Beta (B: N=5; Beta: ●; Beta-L-NAME: ○; p<0.001) MCA. C and D: Inhibition of COX with Indo (10 μmol/L) had no effect on pressure-induced constriction in MCA form C sheep (C: N=5; C: ◆; C+L-NAME: ◇; p=n.s.) but caused significant augmentation of pressure-induced constriction in Beta MCA (D: N=5; Beta: ▲; Beta+L-NAME: △; p<0.01).
Contribution of NOS and COX to BK-induced dilations
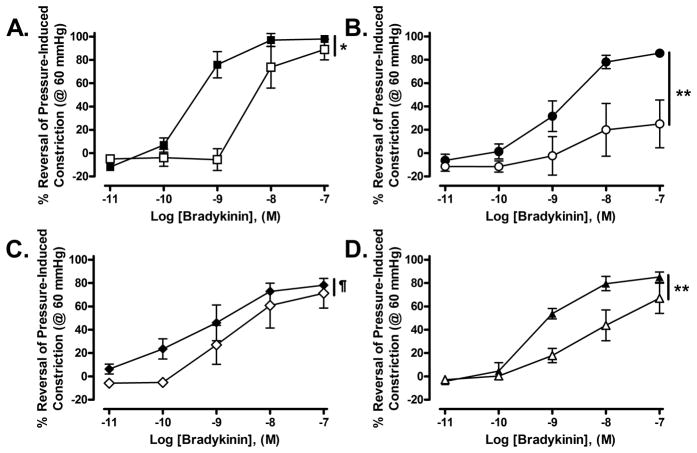
Inhibition of NOS with L-NAME (100 μmol/L) significantly inhibited BK-induced reversal of pressure-induced constriction (developed at 60 mmHg) in both control (Figure 4A: three-parameter curve fit: p<0.021; N=4) and Beta-exposed (Figure 4B: pD2: Beta-exposedthree-parameter curve fit: p<0.0001; N=4) MCA. However, the maximal dilation to BK was significantly reduced only in beta-exposed MCA (Figure 4B). Inhibition of COX with Indo (10 μmol/L) had no effect on BK-induced reversal of pressure-induced constriction in control MCA (Figure 4C: three parameter curve fit: p=0.1022; N=4) but significantly altered the BK-induced reversal of tone in Beta-exposed MCA (Figure 4D: three parameter curve fit: p=0.0032; N=5). Unlike L-NAME, Indo did not affect the maximal dilation to BK in Beta-exposed animals.
Figure 4.
Effect of NOS and COX inhibition on the concentration-dependent reversal of pressure-induced constriction (at 60 mmHg) by BK (0.01–100 nmol/L) in C and Beta MCA. A: In C MCA, inhibition of NOS with L-NAME (100 μmol/L) significantly shifted the BK dose-response curve to the right without affecting maximal dilation to BK. (N=4; C: ■; C+L-NAME: □; pD2: C = −9.50±0.09 vs. C+L-NAME = −8.33±0.32, p=0.012; Top: C = 100.40±3.19 vs. C+L-NAME = 98.40±15.90, p=0.9005; Bottom: C = +17.83±4.60 vs. C+L-NAME = −10.23±9.53, p=0.499; concentration response curves were found to be significantly different using a three-parameter curve fit: p=0.021). B: In Beta MCA, inhibition of NOS with L-NAME (100 μmol/L) significantly shifted the BK dose-response curve to the right and reduced maximal dilation to BK. (N=4; Beta: ●; Beta+L-NAME: ○; pD2: Beta = −8.84±0.05 vs. Beta+L-NAME = −8.59±0.088, p=0.049; Top: Beta= 88.16±1.74 vs. Beta+L-NAME = 26.70±1.41; p<0.0001, Bottom: Beta = −6.70±1.582 vs. Beta+L-NAME = 12.53±1.06, p=0.014; concentration response curves were found to be significantly different using a three-parameter parameter curve fit: p<0.0001). C: In C MCA, inhibition of COX with Indo (10 μmol/L) had no effect on the BK dose-response curve. (N=4; C: ◆; C+Indo: ◇; pD2: C = −9.56±0.16 vs. C+Indo = −9.06±0.36, p=0.2577; Top: C = 76.66±3.22 vs. C+Indo = 69.99±9.86, p=0.5056; Bottom: C = 3.74±4.96 vs. C+Indo = −9.60±10.29, p=0.3152; concentration response curves were not different as assessed using a three-parameter curve fit: p=0.1022). D: In Beta MCA, inhibition of COX with Indo (10 μmol/L) shifted the BK dose-response curve significantly to the right without affecting maximal dilation to BK. (N=5; Beta: ▲; Beta+Indo: △; pD2: Beta = −9.26±0.05 vs. Beta+Indo = −8.39±0.19, p=0.005; Top: Beta = 85.31±1.80 vs. Beta+Indo = 67.51±5.78, p=0.026; Bottom: Beta = −7.42±2.13 vs. Beta+Indo = −0.67±3.67, p=0.1631; concentration response curves were found to be significantly different using a three-parameter parameter curve fit: p=0.0032).
Discussion
To the best of our knowledge, this is the first study to evaluate alterations in adult cerebral vascular pressure-induced constriction and reactivity after fetal exposure to a clinically relevant course of Beta via maternal administration of the drug. Our data demonstrate the following findings in MCA from adult female sheep exposed to antenatal Beta: (1) comparable K+-induced constriction, (2) attenuation of pressure-induced constriction in Beta-exposed MCA, and (3) apparent alterations in the contribution of NOS and COX to pressure-induced constriction and BK-induced reversal of pressure-induced constriction in Beta-exposed MCA.
Based on the prognostic advantage to preterm infants after maternal administration of antenatal glucocorticoids, the NIH consensus statements of 1994 and 2000 recommend a single course of antenatal glucocorticoids for pregnant women at risk for preterm delivery between 24 to 34 weeks of gestation (15, 16). Despite the routine use of glucocorticoids in women in preterm labor, the long-term vascular consequences of this therapy have not been well characterized. Accumulating animal data demonstrate adverse postnatal outcomes including fetal growth restriction (17, 18), altered renal development (1–3, 19), elevated BP (1), and altered vascular reactivity (8, 10) when the developing fetus is exposed to glucocorticoids. Previous studies have also demonstrated altered cerebral vascular reactivity in ovine fetuses exposed antenatally to glucocorticoids (13, 14). However, the effects of antenatal glucocorticoid exposure on adult cerebral vascular tone and reactivity are not well understood at present.
Here, we describe the effects of fetal exposure to Beta on pressure-induced constriction and vascular reactivity in the cerebral circulation. We first assessed vascular smooth muscle (VSM) function by exposing MCA to 50 mmol/L KCl. VSM depolarization with 50 mmol/L KCl caused comparable constriction in control and Beta-exposed MCA (Figure 1) suggesting that exposure of the female fetus to Beta does not affect voltage-dependent Ca2+ channel activity in adult MCA. Similarly, other investigators have demonstrated comparable K+-induced constriction in cerebral and femoral arteries after fetal exposure to dexamethasone (13) and coronary arteries after fetal exposure to Beta (8, 20, 21). Recently, Segar has demonstrated similar K+-induced constriction in femoral and mesenteric arteries from early dexamethasone-exposed 10–14 day old lambs (22). In contrast, exposure of the ovine fetus to multiple courses of dexamethasone increases the maximal constriction of fetal femoral arteries to 125 mmol/L KCl (10). This discrepancy in K+-induced constriction observed by Molnar may be related to multiple courses or the particular glucocorticoid (dexamethasone) administered, and/or the age of the fetus/sheep at the time of study.
We next determined the effect of antenatal Beta exposure on pressure-induced constriction and the contribution of NOS and COX signaling to pressure-induced constriction in adult female MCA. Contrary to expectations, our data demonstrate attenuation in pressure-induced constriction in Beta-exposed compared with control MCA (Figure 2). Schwab and colleagues have previously shown increased cerebral vascular resistance 24 and 48 hours after fetal exposure to Beta (14). This difference in results may be related to the age of the animal at the time of study (18 months vs. 129–130 days), gestational age at which Beta was administered (80–81 days vs. 128 days), and the route of administration (maternal vs. fetal). Our data further indicate that pressure-induced constriction in MCA from control animals is modulated primarily by NOS activity with little apparent contribution by COX products. In contrast, pressure-induced constriction is modulated by both NOS and COX activity in Beta-exposed adult female sheep (Figure 3). Thus, there appears to be an alteration in the relative contribution of endogenous NOS and COX vasodilatory products modulating pressure-induced vasoconstriction in Beta-exposed MCA. Similarly, Angeles et. al. has demonstrated that attenuation of 5-hydroxytryptamine-induced constrictions in fetal carotid arteries incubated with dexamethasone can be reversed with Indo, suggesting that there is increased contribution of COX products in the modulation of carotid artery reactivity after exposure to dexamethasone (23).
We next determined the effect of NOS and COX on BK-induced reversal of pressure-induced constriction in control and Beta-exposed MCA. Inhibition of NOS with L-NAME shifted the BK dose-response curve significantly to the right; however, the maximal dilation to BK was attenuated only in Beta-exposed MCA (Figures 3A and 3B). Similar to our findings, Roghair has demonstrated attenuated adenosine-induced relaxation and decreased endothelial NOS staining in coronary arteries from newborn lambs exposed to dexamethasone early in gestation (21). In contrast, Molnar and colleagues have shown enhanced acetylcholine-induced relaxation but unchanged eNOS RNA and protein levels in femoral arteries from 5 month old sheep exposed to dexamethasone in utero starting on day 103 of gestation (24). It is possible that the differences in eNOS protein expression observed in these 2 studies are related to the timing of fetal exposure to glucocortiocids as well as the vascular bed studied. Inhibition of COX with Indo shifted the BK response curve significantly to the right in MCA from Beta-exposed offspring (Figure 4D), but did not affect BK-induced reversal of pressure-induced constriction in MCA from control offspring (Figure 4C). These data indicate that COX-derived vasodilator products play a greater role in BK-induced dilation in MCA from Beta-exposed offspring compared with age-matched controls. Furthermore, these data suggest an apparent shift in the relative contribution of both NOS and COX to the modulation of BK-induced dilation in Beta-exposed compared with control MCA. We speculate that the altered contribution of NOS is associated with an increased contribution of COX vasodilatory products in MCA from Beta-exposed female sheep.
In summary, maternal administration of a clinically relevant course of Beta results in attenuated pressure-induced constriction and altered vascular reactivity in adult female MCA. Our data demonstrate comparable K+-induced constriction in control and Beta-exposed MCA. Fetal exposure to Beta results in a shift in the contribution of NO and prostaglandin modulation of pressure-induced constriction and BK-induced dilation in MCA. Our results provide supportive evidence for the concept of fetal programming after prenatal exposure to glucocorticoids, with subsequent development of maladaptive cardiovascular responses in adulthood.
Acknowledgments
Financial Support: This study was supported by startup funds from Brenner Children’s Hospital [to D.M.E.], and grants HL068728 [to J.P.F.] and P01 HD047584 [to J.C.R.]
Abbreviations
- Beta
Betamethasone
- C
Control
- COX
Cyclooxygenase
- Indo
Indomethacin
- L-NAME
N omega-nitro-L-arginine methyl ester
- MCA
Middle cerebral artery
- VSM
Vascular Smooth Muscle
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58:510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 2.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig. 2006;13:174–180. doi: 10.1016/j.jsgi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Kantorowicz L, Valego NK, Tang L, Figueroa JP, Chappell MC, Carey LC, Rose JC. Plasma and renal renin concentrations in adult sheep after prenatal betamethasone exposure. Reprod Sci. 2008;15:831–838. doi: 10.1177/1933719108318599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential Effects of Clinical Doses of Antenatal Betamethasone on Nephron Endowment and Glomerular Filtration Rate in Adult Sheep. Reprod Sci. 2010;17:186–195. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segar JL, Bedell KA, Smith OJ. Glucocorticoid modulation of cardiovascular and autonomic function in preterm lambs: role of ANG II. Am J Physiol Regul Integr Comp Physiol. 2001;280:R646–R654. doi: 10.1152/ajpregu.2001.280.3.R646. [DOI] [PubMed] [Google Scholar]
- 7.Kutzler MA, Coksaygan TC, Ferguson AD, Nathanielsz PW. Effects of maternally administered dexamethasone and acute hypoxemia at 0.7 gestation on blood pressure and placental perfusion in sheep. Hypertens Pregnancy. 2004;23:75–90. doi: 10.1081/PRG-120028283. [DOI] [PubMed] [Google Scholar]
- 8.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–R88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 9.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molnar J, Nijland MJ, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0.7, 0.75, and 0.8 gestation in sheep. Am JPhysiol Regul Integr Comp Physiol. 2002;283:R561–R567. doi: 10.1152/ajpregu.00031.2002. [DOI] [PubMed] [Google Scholar]
- 11.Pulgar VM, Figueroa JP. Antenatal betamethasone administration has a dual effect on adult sheep vascular reactivity. Pediatr Res. 2006;60:705–710. doi: 10.1203/01.pdr.0000246481.05231.17. [DOI] [PubMed] [Google Scholar]
- 12.Roubliova XI, Lewi PJ, Vaast P, Jani JC, Verbeken EK, Tibboel D, Deprest JA. Effects of betamethasone on peripheral arterial development in term fetal rabbit. Pediatr Pulmonol. 2008;43:795–805. doi: 10.1002/ppul.20870. [DOI] [PubMed] [Google Scholar]
- 13.Docherty CC, Kalmar-Nagy J, Engelen M, Koenen SV, Nijland M, Kuc RE, Davenport AP, Nathanielsz PW. Effect of in vivo fetal infusion of dexamethasone at 0.75 GA on fetal ovine resistance artery responses to ET-1. Am J Physiol Regul Integr Comp Physiol. 2001;281:R261–R268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- 14.Schwab M, Roedel M, Anwar MA, Muller T, Schubert H, Buchwalder LF, Walter B, Nathalielsz W. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol. 2000;528:619–632. doi: 10.1111/j.1469-7793.2000.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health Consensus Development Panel. [Accessed, June 18, 2010];The effect of antenatal steroids for fetal maturation on perinatal outcomes-interim draft statement. 1994 Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsnihcdc&part=A12756.
- 16.National Institutes of Health Consensus Development Panel. [Accessed, June 18, 2010];Antenatal corticosteroids revisited: repeat courses. 2000 doi: 10.1016/s0029-7844(01)01410-7. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsnihcdc&part=A20680. [DOI] [PubMed]
- 17.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–184. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 19.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roghair RD, Lamb FS, Miller FJ, Jr, Scholz TD, Segar JL. Early gestation dexamethasone programs enhanced postnatal ovine coronary artery vascular reactivity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R46–R53. doi: 10.1152/ajpregu.00165.2004. [DOI] [PubMed] [Google Scholar]
- 21.Roghair RD, Segar JL, Sharma RV, Zimmerman MC, Jagadeesha DK, Segar EM, Scholz TD, Lamb FS. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1169–R1176. doi: 10.1152/ajpregu.00369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segar JL, Roghair RD, Segar EM, Bailey MC, Scholz TD, Lamb FS. Early gestation dexamethasone alters baroreflex and vascular responses in newborn lambs prior to hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;291:R481–488. doi: 10.1152/ajpregu.00677.2005. [DOI] [PubMed] [Google Scholar]
- 23.Angeles DM, Chang M, Leong V, Oberg KC, Pearce WJ. Dexamethasone Alters Vascular Reactivity by Enhancing COX-Related Vasodilatation in Fetal Ovine Carotids. Biol Neonate. 2006;90:1–8. doi: 10.1159/000090340. [DOI] [PubMed] [Google Scholar]
- 24.Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol. 2003;547:61–66. doi: 10.1113/jphysiol.2002.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]