Abstract
Purpose
Despite aggressive screening, patients with hereditary renal cancers can present with large, multifocal tumors. We present oncologic outcomes in hereditary renal cell carcinoma patients treated with partial nephrectomy for multifocal solid tumors with the largest lesion greater than 4 cm.
Materials and Methods
Between 1995 and 2008, we identified 58 patients with hereditary RCC treated at our institution with partial nephrectomy for solid tumors greater than 4cm. The data collected included demographic parameters, tumor size, tumor pathology, and laterality. Overall survival and metastasis-free survival were calculated based on the information from the most recent follow up evaluation and imaging.
Results
The cohort included 58 patients consisting of 41 (71%) patients with VHL, 10 (17%) patients with BHD, and 7 (11%) with HPRC. The mean age was 43.7 (range 18–63) and the mean largest tumor size was 5.3 cm (range 4–13). The mean number of resected kidney tumors was 6.4 (range 1–44). There was a predominance of nuclear grade 2 tumors 51 (85%) and a predominance of clear cell histology 44 (73%), followed by papillary type I histology 7 (11.7%). Overall survival of the cohort was 93% and metastasis-free survival was 96.5% at the median follow up of 45 months (range 2–163).
Conclusions
The metastasis-free and overall survival of our patients is similar to those reported in the literature series for patients undergoing partial nephrectomy for T1B tumors in the sporadic population. The presence of multifocality does not affect oncologic outcomes at an intermediate follow up. Partial nephrectomy can be offered to hereditary patients presenting with multifocal tumors greater than 4 cm.
Keywords: partial nephrectomy, multifocality, renal cell carcinoma, clinical stage T1B, outcomes
The management of renal cell carcinoma in patients with multifocal renal tumors and hereditary syndromes predisposing to formation of RCC is challenging. Not only are these patients predisposed to develop synchronous bilateral multifocal tumors in the kidneys at an early age, most patients will manifest a recurrence or de novo tumor formation even after an aggressive partial nephrectomy.1–3 In order to avoid the surgical morbidity of repeat renal interventions and to decrease the likelihood of the development of metastatic disease, an approach has been developed in this patient population to recommend surgical removal of renal tumors with partial nephrectomy when the largest tumor reaches 3 cm in size.4,5 However, despite aggressive screening, some of these patients will present with larger tumors due to delayed diagnosis or co-morbidities. While recent data have demonstrated that partial nephrectomy for T1b tumors is oncologically equivalent to radical nephrectomy and may even confer a survival benefit in the sporadic population, to our knowledge, there are no studies that evaluated oncologic outcomes for clinical T1b renal masses in the presence of multifocal disease.6–9
Our ongoing clinical experience and aggressive nephron sparing approach to patients with multifocal and hereditary kidney cancer has provided a unique opportunity to evaluate the oncologic outcomes of partial nephrectomy in patients with clinical T1b or greater RCC in presence of multifocal disease.
Material and Methods
Between 1995 and 2008, 60 planned partial nephrectomies were performed at our institution on 58 patients who presented with multifocal renal masses with the largest solid renal tumors greater than 4cm. All patients were evaluated on a National Cancer Institute Institutional Review Board (NCI IRB) approved protocol. All patients had known germline mutations of VHL, BHD, or Met genes, leading to clinical diagnosis of VHL, BHD or HPRC, respectively. The data analyzed included demographics, admission history and physical, operative reports, discharge summaries, progress notes, records of all subsequent operative interventions, and most recent imaging. Pathology reports were reviewed for number of tumors resected, pathologic stage, highest grade, and histology of the largest tumor removed.
All patients underwent pre-operative evaluation with computerized tomography of chest as well as CT or MRI of the abdomen and pelvis. At the time of surgery no patients had preoperative or intraoperative suspicion of locally advanced or metastatic disease. Regardless of the tumor size and multifocality, all planned NSS were successfully performed in all patients.
All patients were followed at regular intervals following surgery, starting at 3 months after the procedure and at least once yearly after the first visit. Each visit included clinical and laboratory examination with complete blood count, routine chemistries, liver function tests, as well as imaging with computerized tomography of the chest, and either contrasted CT or MRI of the abdomen and pelvis.
Oncologic outcomes were evaluated by overall survival, cancer-specific survival, metastasis-free survival, and need for subsequent surgical intervention on the operated renal unit. Overall survival was determined by chart review and telephone contact, and death from any cause was verified using the Social Security Death Index. Cancer specific mortality and metastasis-free survival was attributed to patients with documented evidence of cancer progression as the cause of death. Survival data was obtained on the entire cohort and no patient was lost to follow up. Probabilities of overall and cancer specific survival were depicted using the Kaplan-Meier method.
Results
Patient characteristics are shown in Table 1. A total of 60 operations were performed on 58 patients. Two patients presented with T1b tumors bilaterally and underwent staged bilateral partial nephrectomy. Eight patients underwent prior partial nephrectomy on the ipsilateral kidney while 20 patients underwent prior partial nephrectomy on the contralateral kidney. Twenty patients underwent subsequent partial nephrectomy on the contralateral kidney and only one underwent subsequent radio frequency ablation. Of note, none of the prior or subsequent interventions were performed for tumors larger than 4cm. The mean age of the patients was 44 years (range 19 to 64) and the majority of patients were affected with VHL. The majority of patients were women and more surgeries were performed on the right side.
Table 1.
Patient Characteristics
| No. pts | 58 |
| No kidneys | 60 |
| Mean age (range) | 43.7 (18.5 – 63.3) |
| Sex (%) | |
| Male | 22 (38) |
| Female | 36 (62) |
| Distribution of Hereditary RCC patients (%) | |
| VHL | 41 (71) |
| BHD | 10 (17) |
| HPRC | 7 (12) |
| Laterality (%) | |
| Right | 35 (58) |
| Left | 25 (42) |
Tumor pathologic characteristics are shown in table 2. The patients had up to 44 tumors removed and the largest tumor size ranged from 4.1 to 18 cm (mean 5.3 cm). The majority of surgeries (87%) were performed for pathologic stage T1b and most tumors were clear cell RCC (73%). Fuhrman nuclear grade 2 was the most predominant (85%).
Table 2.
Tumor Pathologic Characteristics
| Mean no. of tumors resected (range) | 6.4 (1–44) |
| Mean cm largest tumor size (range) | 5.3(4–13) |
| Fuhrman nuclear grade of the largest tumor (%) | |
| 1 | 3 (5) |
| 2 | 51 (85) |
| 3 | 6 (10) |
| Distribution of histological subtype of RCC (%) | |
| Clear cell | 44 (73.3) |
| Papillary, type1 | 7 (11.7) |
| Chromophobe | 5 (8.3) |
| Hybrid | 3 (5) |
| Oncocytic | 1 (1.7) |
| TNM Staging | |
| T1b(%) | 52 (87) |
| T2 (%) | 8 (13) |
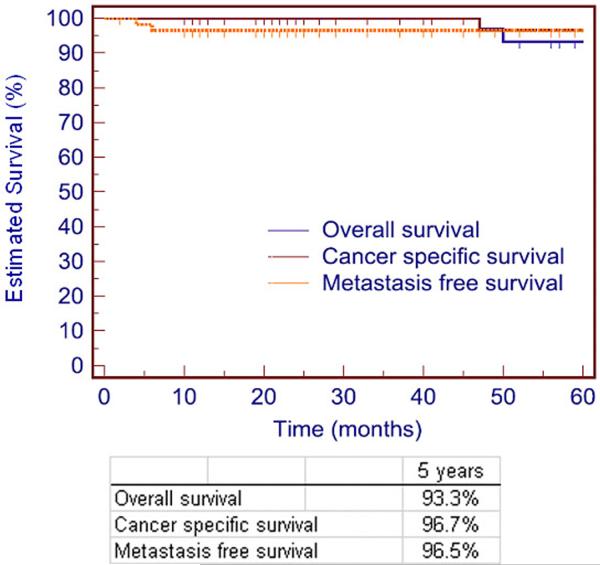
Median follow up for the entire cohort was 45 months (range 2 to 163). Figure 1 shows the overall, cancer specific and metastasis free survival in all 58 patients. The overall survival of the cohort was 93.3% at 5 years. Two patients (3.5%) had died of non-tumor related causes and 1 (1.7%) died of advanced metastatic disease. Cancer specific survival of the cohort was 96.7% and metastasis free survival was 96.5% at 5 years. Of the 58 patients, 2 (3.4%) developed distant metastasis. The details of patients with metastatic disease are shown in Table 3. Five patients (8.6%) required repeat intervention by open partial nephrectomy at a median time to re-intervention of 55 months (range 48 to 101). No patient underwent subsequent nephrectomy for any reason.
Figure 1.
Overall, cancer specific and metastasis free survival of 58 patients treated with NSS
Table 3.
Follow-up of 2 patients with metastasis after NSS
| Patient # | 1 | 2 |
| Hereditary Classification | VHL | VHL |
| Time until mets after NSS (mo) | 6 | 4 |
| Largest tumor size (cm) | 4.5 | 5.8 |
| Pathologic type | clear cell | clear cell |
| Nuclear Grade | 3 | 2 |
| Localization of metastasis | lung, scalp, retroperitoneum | lung (3 Mets) |
| Treatment | TKI | Metastatectomy for all 3 lesions |
| Follow up time (mo) | 41 | 60 |
| Outcome | Death due to cancer progression | Alive, no evidence of metastatic disease |
Discussion
While the initial indications for NSS were limited to patients with solitary kidney and chronic kidney disease, a number of investigators have shown the oncologic equivalency of NSS for T1b disease with that of radical nephrectomy.8–14 One of the main reasons for continued expansion of the role of NSS is our ongoing recognition of the importance of the preservation of renal function.6 As the role of nephron sparing surgery is continuously being expanded, it is imperative to meticulously evaluate oncologic efficacy. The current study evaluates oncologic outcomes of patients with two potentially adverse risk factors: multifocality and tumors larger than 4cm.
While the overall survival of our cohort was similar to other reports of partial nephrectomy for T1b RCC in the sporadic population, the metastatic rate in our cohort was the lowest when compared to other series (Table 4). There are several potential explanations for this observation. First, it is possible that the shorter follow up in our study did not allow enough time for the development of metastatic disease. While this is possible, most of the oncologic failures with RCC are typically observed in the first two years, although late development of metastatic disease may occur years after initial surgery.13,15 Consistent with the notion of early failures, two patients in our cohort developed metastatic disease within the first year after the surgery. The second explanation for the slightly lower metastatic rate in our cohort may be due to less biologically aggressive tumors with multifocal or hereditary RCC. Although it may be a reasonable explanation, the rate of metastatic events for tumors under 3 cm in our population is similarly low as a rate in a sporadic population with the same tumor size.16 Additionally, RCC used to be the most common cause of death in hereditary patients with as many as 46% dying from metastatic RCC.17 Of importance, as many as 21% of patients with tumors between 4–6cm and 50% of those with tumors between 6–10cm developed metastatic disease in our earlier study of the hereditary cohort.18 Therefore, attributing a lower rate of metastatic disease to a low aggressiveness of RCC in multifocal or sporadic population is unlikely to be the case. A third possible explanation for the lowest metastatic rate may come from the histological distribution of tumors in our cohort, with 27% consisting of non-clear histology. Nevertheless, Table 2 demonstrates that the overall percentage of pathologic diagnosis in our population is similar to those observed in the sporadic population.
Table 4.
Results after NSS for RCC greater than 4cm in published series
| Reference | No. Pts | Follow up (mos) | % Metastasis | % Overall Survival |
|---|---|---|---|---|
| Hafez et al13 | 175 | 47 | 16 | 86 |
| Leibovich et al9 | 91 | 106 | 4.4 | 98 |
| Carini et al12 | 71 | 74 | 14.9 | 85.1 |
| Becker etal11 | 69 | 74 | 5.8 | 94.9 |
| Patard et al26 | 65 | 62.5 | 7.1 | 93.8 |
| Peycelon et al14 | 61 | 70.7 | 19.7 | 92 |
| Antonelli et al10 | 52 | 54 | 5.3 | 93 |
| Present series | 58 | 44 | 3.5 | 93.3 |
There are likely two reasons why the metastatic rate in our study is lower than in other series listed in Table 4. One reason may be due to meticulous pre-operative assessment for metastatic disease, including a dedicated chest CT. Presence of suspicion for metastatic disease would preclude the entry criteria in this study. The second reason may be due to the absence of the most aggressive variants of familial renal cancer syndromes, such as Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC), TFE3 or TFE-3B in our cohort. These patients are likely to develop metastatic disease earlier or may not have been offered a partial for larger lesions with the suspicion of aggressive histology. Of note, among the patients with clear cell carcinoma in our cohort, the rate of metastatic disease was similar to other studies for T1b tumors (2 out of 41; 5%).
Most importantly, however, is the finding that the presence of both larger tumors and multifocality did not worsen the oncologic outcomes in this patient population. As mentioned earlier, many investigators have reported on the oncologic equivalency of NSS for T1b disease with that of radical nephrectomy.8–14 Additionally, Memorial Sloan Kettering Cancer Center and the Mayo Clinic groups have demonstrated no effect on recurrence or survival in patients with multifocal RCC treated with radical nephrectomy.19,20 The current study combines the two adverse risk groups of larger lesions and multifocality and demonstrates similar oncologic control to other published series for patients with either large solitary tumors or multifocal RCC.9–14,19,20
We also compared the outcomes between patients treated for miltifocal lesions with tumors greater than 4 cm by partial or radical nephrectomy in our institution. In review of the NCI patients over the same time period, among patients with VHL, BHD and HPRC without evidence of metastatic or locally advanced disease, we identified only 3 patients that underwent radical nephrectomy. Because of such a small number of events the comparison would not be appropriate. Nevertheless, it should be noted that at last follow up, all three are alive and have no evidence of locally advanced or metastatic disease.
Evaluating oncologic outcomes in patients with hereditary or multifocal RCC is challenging. Metastasis-free and cancer-specific survival provides the best measures of oncologic efficacy, since local recurrence in this population is impractical to assess. In fact, it is not possible to differentiate between a true local recurrence due to a persistence of a previously resected tumor, development of a new tumor(s), or finally detecting those tumors that were present at the time of surgery but were too small to appreciate with present pre- and intraoperative imaging modalities. Only if the new tumor is detected in a location completely remote from the resection site and the genetic studies confirm the absence of a common clonal origin of the recurrent tumor, can one comfortably conclude that the so-called “recurrence” is actually a reflection of multifocality. In our patients we have performed a partial nephrectomy with resection of as many as 44 tumors, and therefore found it impractical to make definitive conclusions on the reason for recurrence. Most likely, however, it is the presence of multifocality and the propensity to form renal lesions that is responsible for “local recurrence” in this patient population. Indeed, it has been shown that as many as 600 solid tumors and 1100 cysts may be found at the time of nephrectomy in patients with VHL, 2,400 tumors in patients with HPRC, and dozens of tumors in BHD patients.4,21,22
Five patients (8.6%) required subsequent intervention in the form of ipsilateral repeat partial nephrectomy at a median of 55 months (range 48 to 101). No patients experienced loss of a renal unit at the time of the most recent follow-up. Interestingly, the median time to subsequent surgery in this cohort is similar to our previously reported re-intervention interval of 50 months for similar patients with multifocal RCC.2 It appears that the presence of the largest lesion greater than 4 cm in the cohort of this study did not influence the interval for repeat intervention (55 vs 50 months).
The necessity and risks for repeat intervention must be carefully weighed against the risks of renal replacement therapy. Johnson et al reported a 19.6% major perioperative complication rate in a cohort of hereditary patients undergoing repeat partial nephrectomy. Of 47 patients in that study, only 2 required long term hemodialysis and there were 3 losses of the renal unit. In a study evaluating outcomes of salvage partial nephrectomy in patients who had undergone a minimum of 2 prior partial nephrectomy we reported a 46% major complication rate with a renal unit loss rate of 23%.23 Most important, however, is the fact that in the above two studies more than 95% of patients were alive and metastasis-free at 56 and 25 months, respectively. Contrary to outcomes of our patients, Goldfarb et al reported a 5 year survival rate of only 65% in VHL patients who were treated with bilateral nephrectomy and renal replacement therapy.24 The average ages of our cohort and patients reported by Goldfarb were similar (43.7 vs 36, respectively). The significant morbidity, mortality, and effects on quality of life, associated with dialysis makes this form of renal replacement therapy a last resort. In addition, options for kidney transplantation in hereditary patients may be limited due to the presence of renal carcinoma or other co-morbidities. In the general population, when a transplant is performed the graft survival is 73.9% at 5 years, and patient survival is 85.8% at 5 years.25 These results further underscore our aggressive surgical management strategy for bilateral recurrent RCC.
The present study has inherent limitations. It is a retrospective study and oncologic outcomes analyses are based on intermediate follow up data. We could not compare this cohort to patients who underwent radical nephrectomy for T1b due to the limited number of radical nephrectomies in this cohort performed at our institution. Also, since this paper predominantly focuses on oncologic outcomes of the management of multifocal RCC and the cohort spanned a time period of 13 years, the surgical techniques and perioperative outcomes have not been reported. We have previously described our technique, perioperative outcomes, operative time, clamp time, transfusion requirements in prior publications.2,4,5,23Nevertheless, our data support utilization of partial nephrectomy in patients with multifocal RCC and clinical stage T1B.
Conclusion
Nephron preservation is increasingly used in patients with T1B lesions with acceptable oncological outcomes. Our study supports utilization of NSS in hereditary patients who present with T1b or larger tumors even in the setting of tumor multifocality. The metastatic rate and cancer specific survival of patients with multifocal RCC and T1b lesions are similar to their sporadic counterparts.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We acknowledge Georgia F. Shaw for her invaluable contributions in the preparation of this manuscript.
References
- 1.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 2.Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180:89. doi: 10.1016/j.juro.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbach F, Novick AC, Zincke H, Miller DP, Williams RD, Lund G, et al. Treatment of renal cell carcinoma in von Hippel-Lindau disease: a multicenter study. J Urol. 1995;153:1812. [PubMed] [Google Scholar]
- 4.Walther MM, Choyke PL, Weiss G, Manolatos C, Long J, Reiter R, et al. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma. J Urol. 1995;153:913. [PubMed] [Google Scholar]
- 5.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001;165:777. [PubMed] [Google Scholar]
- 6.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minervini A, Serni S, Giubilei G, Lanzi F, Vittori G, Lapini A, et al. Multiple ipsilateral renal tumors: retrospective analysis of surgical and oncological results of tumor enucleation vs radical nephrectomy. Eur J Surg Oncol. 2009;35:521. doi: 10.1016/j.ejso.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell RE, Gilbert SM, Murphy AM, Olsson CA, Benson MC, McKiernan JM. Partial nephrectomy and radical nephrectomy offer similar cancer outcomes in renal cortical tumors 4 cm or larger. Urology. 2006;67:260. doi: 10.1016/j.urology.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 9.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli A, Cozzoli A, Nicolai M, Zani D, Zanotelli T, Perucchini L, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7cm. Eur Urol. 2008;53:803. doi: 10.1016/j.eururo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Becker F, Siemer S, Hack M, Humke U, Ziegler M, Stockle M. Excellent long-term cancer control with elective nephron-sparing surgery for selected renal cell carcinomas measuring more than 4 cm. Eur Urol. 2006;49:1058. doi: 10.1016/j.eururo.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Carini M, Minervini A, Lapini A, Masieri L, Serni S. Simple enucleation for the treatment of renal cell carcinoma between 4 and 7 cm in greatest dimension: progression and long-term survival. J Urol. 2006;175:2022. doi: 10.1016/S0022-5347(06)00275-8. [DOI] [PubMed] [Google Scholar]
- 13.Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999;162:1930. doi: 10.1016/S0022-5347(05)68071-8. [DOI] [PubMed] [Google Scholar]
- 14.Peycelon M, Hupertan V, Comperat E, Renard-Penna R, Vaessen C, Conort P, et al. Long-term outcomes after nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2009;181:35. doi: 10.1016/j.juro.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161:33. doi: 10.1016/s0022-5347(01)62052-4. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Hill JR, Babayev Y, Cronin A, Kaag M, Kundu S, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182:41. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 18.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172:63. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 19.Richstone L, Scherr DS, Reuter VR, Snyder ME, Rabbani F, Kattan MW, et al. Multifocal renal cortical tumors: frequency, associated clinicopathological features and impact on survival. J Urol. 2004;171:615. doi: 10.1097/01.ju.0000106955.19813.f6. [DOI] [PubMed] [Google Scholar]
- 20.Crispen PL, Boorjian SA, Lohse CM, Sebo TS, Cheville JC, Blute ML, et al. Outcomes following partial nephrectomy by tumor size. J Urol. 2008;180:1912. doi: 10.1016/j.juro.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 21.Ornstein DK, Lubensky IA, Venzon D, Zbar B, Linehan WM, Walther MM. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. J Urol. 2000;163:431. [PubMed] [Google Scholar]
- 22.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26:1542. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bratslavsky G, Liu JJ, Johnson AD, Sudarshan S, Choyke PL, Linehan WM, et al. Salvage partial nephrectomy for hereditary renal cancer: feasibility and outcomes. J Urol. 2008;179:67. doi: 10.1016/j.juro.2007.08.150. [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb DA, Neumann HP, Penn I, Novick AC. Results of renal transplantation in patients with renal cell carcinoma and von Hippel-Lindau disease. Transplantation. 1997;64:1726. doi: 10.1097/00007890-199712270-00017. [DOI] [PubMed] [Google Scholar]
- 25.United Network for Organ Sharing. 2009 9-16-2009. [Google Scholar]