Abstract
Advances in genetic methods have enabled the study of genes involved in human neurodegenerative diseases using Drosophila as a model system1. Most of these diseases, including Alzheimer's, Parkinson's and Huntington's disease are characterized by age-dependent deterioration in learning and memory functions and movement coordination2. Here we use behavioral assays, including the negative geotaxis assay3 and the aversive phototaxic suppression assay (APS assay)4,5, to show that some of the behavior characteristics associated with human neurodegeneration can be recapitulated in flies. In the negative geotaxis assay, the natural tendency of flies to move against gravity when agitated is utilized to study genes or conditions that may hinder locomotor capacities. In the APS assay, the learning and memory functions are tested in positively-phototactic flies trained to associate light with aversive bitter taste and hence avoid this otherwise natural tendency to move toward light. Testing these trained flies 6 hours post-training is used to assess memory functions. Using these assays, the contribution of any genetic or environmental factors toward developing neurodegeneration can be easily studied in flies.
Keywords: Neuroscience, Issue 49, Geotaxis, phototaxis, behavior, Tau
Protocol
1. Introduction
In this video, we will demonstrate two behavioral assays using Drosophila to demonstrate the measurement of locomotion and learning and memory functions that are compromised in neurodegenerative disorders. First, we will show how locomotor behavior can be measured using the negative geotaxis assay. Second, we will assess learning and memory abilities using positively-phototactic flies that are trained to associate light with an aversive bitter taste and then tested 6 hours later to assess memory functions. Finally, we will discuss the implications and limitations of these behavior assays.
2. Equipment and Reagents
- Fly work
- Stereo scope (Zeiss)
- Small paint brushes for pushing flies
- Drosophila carbon dioxide (CO2) anesthesia apparatus (Geneseesci, Inc)
- Negative Geotaxis Assay
- 28.5 x 95 mm Polystyrene Vial (Capitol Vial, Inc.)
- Mini-Alarm Timer/Stopwatch (VWR International)
- Sharpie pens
- Adhesive Tape
- Aversive Phototaxis Suppression Assay
- Quinine Hydrochloride (Sigma Aldrich; CAS Number: 6119-47-7)
- Distilled Water
- Digital measuring scale for precise dilution by weight
- Quick Disconnect Connectors, High-Density Polyethylene (NALGENE VWR Catalog No. 62868-021)
- 15 mL Centrifuge Tube (VWR International), cut at the 2 mL mark from closed end to fit with the Quick Disconnect Connector adaptor for light source
- Single-channel 200 μL pipetter to load quinine solution onto filter paper lining the chamber
- T-Maze (Simple Behavioral Systems; Figure 1)
- Aluminum Foil
- Light Source (Zeiss)
- A lamp with red light.
- Filter Paper
- Mini-Alarm Timer/Stopwatch (VWR International)
3. Preparing the Flies
Flies are maintained on standard cornmeal-agar-molasses-yeast medium at 25°C on a 12 hr light/dark cycle.
Virgin flies are isolated under carbon dioxide anesthesia on the day of eclosion. For aging studies, flies are maintained in groups of twenty per vial and transferred to a fresh vial every 3 days. It is important to use sibling flies from the same parental cross for aging studies, to minimize differences arising from genetic backgrounds.
For both the assays, flies are sexed and maintained in groups of ten flies per vial. The gender difference on behavior is significant and it is advisable not to mix male and female flies in a study as data might be hard to interpret and reproduce6.
For negative geotaxis assay, flies are sorted into groups of ten per vial and tested one hour after anesthesia. It is important for flies to recover completely from anesthesia as this might have an effect on locomotor activities.
For APS assay, flies of each group are placed in an empty polystyrene vial with water moistened filter paper for six hours before the assay is performed. This ensures that the flies are starved before the assay and will be more perceptive to aversive taste. Just before the assay is performed, each fly from a group of ten is placed in an empty 15mL centrifuge tube, which is then capped loosely, to avoid escape.
4. Negative Geotaxis Assay
Geotaxis is generally measured for ten to twenty groups of ten individuals of the same genotype or treatment (100-200 flies total for each genotype/treatment)7. Sort groups of ten female or male flies on a CO2 anesthesia apparatus and place each group in a separate vial. Allow at least one hour for the flies to recover from anesthesia.
Prepare the climbing apparatus for each group, such that two empty polystyrene vials are vertically joined by tape facing each other. It is important to make sure that the openings of the vials are perfectly aligned with each other to provide an even climbing surface for the flies.
For the lower vial, measure a vertical distance of 8 cm above the bottom surface and mark each vial by drawing a circle around the entire circumference of the vial.
Transfer a group of ten flies into the lower vial carefully preventing the escape of any fly. Immediately cover the lower vial with the top vial and tape securely near the contacting openings. Allow the flies to acclimatize to the new setting for 1 minute before conducting the assay.
Gently tap the flies down to the bottom of the vial and measure the number of flies that can climb above the 8-cm mark by 10 seconds after the tap.
Repeat this assay for the same group ten times, allowing for 1 minute rest period between each trial.
Record the number of flies per group that passed the 8-cm mark as a percentage of total flies.
5. Aversive Phototaxis Suppression Assay (APS Assay)
Originally adapted from Le Bourg and Buecher (2002)4, this assay exploits the positive phototactic behavior in flies to train them to associate light with aversive stimuli (in this case, bitter taste of quinine). After a training/conditioning phase, wild type flies will be able to associate the lighted area with aversive taste and avoid it. Flies with compromised learning capacities will fail to make this association. Furthermore, the APS assay can also be used to measure short term memory function of flies5 by subjecting already trained flies to the same test 6 hours post conditioning to test their ability in remembering the learned task.
- Preparing the Quinine Solution
- Dissolve quinine hydrochloride in distilled water to make a 0.1M stock solution (1.98g in 50mL distilled water). The stock solution can be kept at -20°C for up to a year in small aliquots
- Prepare a working solution of 1 μM by diluting the stock solution in distilled water.
- Preparing the T-Maze
- T-maze consists of the center column with the trap door and two independent chambers, a "dark" chamber and a "lighted" chamber (Figure 1).
- Chamber preparation: take two 15mL plastic centrifuge tubes and cut at 2 mL mark from the bottom closed end with a manual saw. Fit the connector adapters at each end, and seal the connection with parafilm. The adapter at the end of the tube serves as the input slot for the gooseneck light source. Connect one tube with the light source and this tube will be the "lighted" chamber. Wrap the other tube with aluminum foil to serve as the "dark" chamber.
- Add 180uL of either distilled water or quinine solution to filter paper and place in the light chamber.
- Assemble the T-maze by screwing the lighted and dark chambers on each side of the center column, with the trap door in the middle closed (Figure 1).
- Insert the light source into the lighted chamber.
- Training flies to suppress phototaxis with aversive stimuli
- Unscrew the dark chamber from the T-maze and transfer a single fly into this chamber and immediately screw the dark tube back to the maze.
- Turn off the lights in the room and turn on the red lamp.
- Allow the fly to acclimatize in the dark chamber for 30 seconds and slowly turn on the light source illuminating the lighted chamber. Slowly open the trap door that separates the two chambers.
- If the fly walks to the lighted chamber within 10 seconds, it is considered positively phototactic and is ready to be trained for the assay. Failure in phototaxis indicates problems in the visual system and flies with negative phototaxis need to be excluded from the assay.
- For Aversive Phototaxis Suppression (APS) training, tap the fly that demonstrated positive phototaxis back to the dark chamber, close the trap door and turn off the light. Allow 30 seconds of acclimatizing. During this time put filter paper with quinine solution into the lighted chamber. Slowly open the trap door and turn on the light. Allow the fly walk into the quinine coated lighted chamber. After one minute, tap the fly back to the dark chamber and repeat this 9 more times. (Typically, wild type flies avoid the lighted chamber after 3 to 5 training trials.)
- Immediately after training, five test trials will be conducted. In each test trial, allow 10 seconds after light is turned on for the trained fly to walk to the lighted chamber. Failure to walk to the lighted vial will be recorded as "Pass", which is equivalent to "task learned through reinforcement". The Pass rate over five consecutive trials is recorded and shown below in Figure 2 as PC0 (0 hr post conditioning).
- Assessing Short-Term Memory Function in Flies
- After training and initial PC0 recordings, each fly is placed back into its original food vial and kept aside for six hours.
- Six hours post training, subject each fly to 5 trials again in the same way as before, and record the number of times the fly avoids (pass) or goes into (fail) the lighted vial. This pass rate is recorded as PC6 (6 hrs post conditioning), which is an indicator of short term memory.
6. Data Interpretation and Statistical Analysis
- Negative Geotaxis Assay
- The raw data generated from this assay represents the number of flies crossing the 8-cm mark in 10 seconds in each group. Convert this to percentage and compute the average pass rate for each group over 10 sessions.
- Data is represented graphically as an average pass rate per group (genotype, treatment etc.) with the standard error of mean (SEM).
- Differences between groups can then be determined to be statistically significant or not using a Student T-test or ANOVA.
- Aversive Phototaxis Suppression Assay
- The raw data from this assay is represented as the number of times each fly avoids the lighted chamber in five successive trials. The pass rate is calculated as the percentage of successful avoidance trials over the total trials. Calculate the average pass rate in this manner for a minimum of 15 flies per group.
- Data is graphically represented as average pass rate PC0 (immediately after conditioning; "learning" indicator) or average pass rate PC6 (6 hours post conditioning; "memory" indicator) with respective SEM.
- Learning and memory indices are compared between groups with both wild type and "genetically-manipulated-benign" group (such as GFP overexpressing flies) appropriately.
7. Representative Results
In this video experiment, we are assessing learning and memory and motor deficits in flies overexpressing human Tau in the central nervous system and comparing their performance to those of flies overexpressing GFP. Neuronal overexpression of human Tau in Drosophila has been shown to cause severe vacuolization in the brain8 and lead to significant deficits in learning and memory functions9. As shown in Figure 2A, 10 day old male or female flies overexpressing human Tau have significant locomotor deficits compared to age-matched GFP overexpressing flies. In addition, these flies failed to associate light with bitter taste as shown by PC0 pass rate compared to the control group, as well as fail to remember this association as shown by a decline in PC6 pass rate (Figure 2B). This experiment further validates that Drosophila models can be used for studying human neurodegenerative conditions and recapitulating some of the behavioral phenotypes observed in humans and mammals.
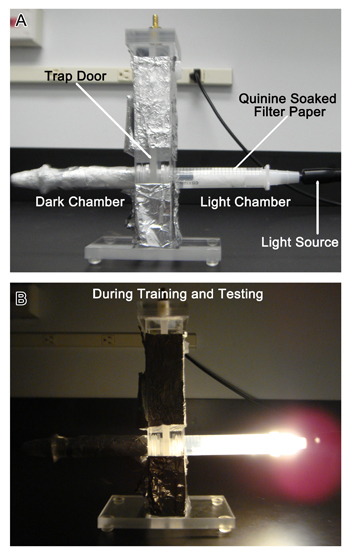 Figure 1. T-Maze Setup for Aversive Phototaxis Suppression Assay. A. The overall experimental setup for the APSA experiment with the light source connected to the "lighted" falcon tube, lined with filter paper and the "dark" tube on the left (covered in foil), separated by a trap door. B. During the training and testing phase, the fly is in the dark chamber and the trap door opened after the light is turned on in the lighted chamber.
Figure 1. T-Maze Setup for Aversive Phototaxis Suppression Assay. A. The overall experimental setup for the APSA experiment with the light source connected to the "lighted" falcon tube, lined with filter paper and the "dark" tube on the left (covered in foil), separated by a trap door. B. During the training and testing phase, the fly is in the dark chamber and the trap door opened after the light is turned on in the lighted chamber.
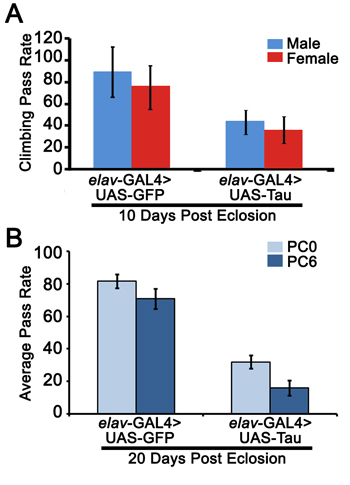 Figure 2. Representative results from the negative geotaxis and APS experiment using flies overexpressing GFP or Tau with a pan-neuronal driver. A. In the negative geotaxis assay, 10-day old male (blue column) or female (red column) flies overexpressing GFP showed higher climbing activity than Tau-overexpressing flies. B. 20-day old female flies overexpressing GFP showed better learning (PC0) and memory (PC6) functions than Tau overexpressing flies.
Figure 2. Representative results from the negative geotaxis and APS experiment using flies overexpressing GFP or Tau with a pan-neuronal driver. A. In the negative geotaxis assay, 10-day old male (blue column) or female (red column) flies overexpressing GFP showed higher climbing activity than Tau-overexpressing flies. B. 20-day old female flies overexpressing GFP showed better learning (PC0) and memory (PC6) functions than Tau overexpressing flies.
Discussion
Both negative geotaxis and APS assays are robust behavioral assays to measure changes in locomotor or learning and memory capacities arising from genetic or environmental manipulation. However, there are some disadvantages to these assays that await further optimization.
In negative geotaxis assay, mutations in certain genes can render severe motor dysfunctions in flies. In such cases, the flies can hardly climb the walls in response to gravity upon agitation. Other behavior assays that directly measure movement may be more appropriate. These assays include righting reflex behavior that measures paralysis and seizure10, and optomotor behavior11 that records loss of movement coordination.
In APS assay, the first hindrance can be flies randomly stopping at the entrance of the lighted chamber without moving forward. This becomes more of an issue if aging is one of the conditions studied, since older flies are less active in general and tend to stop and rest for long periods of time. In this case the experimenter has to provide mechanical stimuli to promote movement, which is not always effective. Second, one might argue that the failure to go to the lighted chamber results from a loss of positive phototaxis. This is rarely the case, since previous work has shown that flies turn back to light responsiveness once the negative reinforce is removed4. Third, the duration of contact with the aversive stimulus may vary from trial to trial. This variation can usually be reduced by encircling the inner wall of the chamber with soaked filter paper to prevent flies from avoiding the quinine. In addition, the experimenter can ensure that each fly spend enough time in contact with the filter paper by increasing the amount of time spent in the chamber from the otherwise noted 1 minute.
In summary, negative geotaxis and APS assays are fast and simple ways of assessing behavioral deficits and quantitatively indicate locomotor and learning and memory abilities in Drosophila. These assays are particularly useful in modeling human neurodegenerative conditions in Drosophila.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Dr. Charles Luetje for providing the T-maze and the behavior room. This work is supported by the American Heart Association (to Y.A.), the National Institute for Neurological Disorder and Stroke Grant R01NS64269 (to R.G.Z.), and the Pew Charitable Trust (to R.G.Z.).
References
- Bonini NM, Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annual Review of Neuroscience. 2003;26:627–656. doi: 10.1146/annurev.neuro.26.041002.131425. [DOI] [PubMed] [Google Scholar]
- Lloyd TE&, Taylor JP. Flightless flies: Drosophila models of neuromuscular disease. Ann N Y Acad Sci. 2010;1184:e1–e20. doi: 10.1111/j.1749-6632.2010.05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Animal Learning & Behavior. 2002;30:330–341. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Stidd R, Shaw PJ. Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav. 2009;8:377–389. doi: 10.1111/j.1601-183X.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen SP, Chan Y-B, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Neckameyer WS. Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J Neurobiol. 2002;50:245–261. doi: 10.1002/neu.10030. [DOI] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: Neurodegeneration Without Neurofibrillary Tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Mershin A, Pavlopoulos E, Fitch O, Braden BC, Nanopoulos DV, Skoulakis EM. Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learn Mem. 2004;11:277–287. doi: 10.1101/lm.70804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. Acetylcholinesterase mutants in Drosophila and their effects on the structure and function of the central nervous system. J Comp Neurol. 1980;189:741–774. doi: 10.1002/cne.901890409. [DOI] [PubMed] [Google Scholar]


