Abstract
Recombinant vectors based on a non-pathogenic human parvovirus, the adeno-associated virus 2 (AAV2) have been developed, and are currently in use in a number of gene therapy clinical trials. More recently, a number of additional AAV serotypes have also been isolated, which have been shown to exhibit selective tissue-tropism in various small and large animal models1. Of the 10 most commonly used AAV serotypes, AAV3 is by far the least efficient in transducing cells and tissues in vitro as well as in vivo.
However, in our recently published studies, we have documented that AAV3 vectors transduce human liver cancer - hepatoblastoma (HB) and hepatocellular carcinoma (HCC) - cell lines extremely efficiently because AAV3 utilizes human hepatocyte growth factor receptor as a cellular co-receptor for binding and entry in these cells2,3.
In this article, we describe the steps required to achieve high-efficiency transduction of human liver cancer cells by recombinant AAV3 vectors carrying a reporter gene. The use of recombinant AAV3 vectors carrying a therapeutic gene may eventually lead to the potential gene therapy of liver cancers in humans.
Keywords: Medicine, Issue 49, Adeno-associated virus, viral vectors, gene transfer, gene expression, liver cancer, gene therapy
Protocol
1. Packaging of Recombinant Adeno-associated Virus Serotype 3 (rAAV3) Vectors
Go to the website: "http://www.geguchadze.com/calc" and calculate the amount of DNA.
Pre-mix plasmids pHelper (Adenovirus helper), pACG2c3 (AAV helper) and pdsAAV-CB-EGFP (rAAV vector) in medium without FBS and antibiotics. Total volume should be 8,750 μL for ten 15 cm2 plates.
Add directly 1,250 μL of PEI (0.1% m/v, Polysciences, Cat# 23966, pH 4.5) into DNA solution in Step 1.2. Vortex the DNA-PEI solution for a few seconds.
Incubate DNA-PEI solution to form a complex for 5 min at room temperature (RT).
Add 1 mL of DNA-PEI solution per 15 cm2 plate.
Remove medium after 4 hours, replacing with fresh 25 mL complete medium+10%FBS (C-DMEM).
Seventy two hours post-transfection, scrape cells and pour into a 250 mL conical centrifuge tube.
Spin at 3000 rpm, at 4°C for 10 min. Pour off supernatant.
Re-suspend the cell pellet in 5 mL of RB TMS Buffer (50 mM Tris-HCl, 150 mM NaCl, pH 8.0). Transfer to a new 15 mL conical tube.
Freeze in dry ice-ethanol bath for 10 min and thaw at 37°C for 10 min. Repeat three times.
Add 1 μL of 4.8 M MgCl2 and 2 μL of Benzonase (25 U/ μL, Novagen, Cat# 70664-3). Vortex and incubate in 37°C for 40 min.
Spin down at 4,000 rpm, at 4°C for 40 min and collect the supernatant (clarified lysate).
In a 13 mL Quick-Seal centrifuge tube (Beckman, Cat# 342413), load iodixanol gradient (OptiPrep, Cat# 1114542) from top to bottom: 2 mL of 15%, 2 mL of 25%, 1.5 mL of 40% and 1.5 mL of 60% iodixanol. Load the supernatant (Step 1.12) on the top of iodixanol gradient. Fill the tubes with RB TMS Buffer.
Centrifuge at 75,000 rpm for 1 hour at 16°C using Beckman 90Ti rotor.
Collect the 40% iodixanol fraction by inserting a syringe at 40-60% boundary.
Transfer the 40% iodixanol fraction in one 50 mL conical tube and make up to 40 mL with Buffer A (20 mM Tris, 15 mM NaCl, pH 8.5).
Assemble the filtration apparatus using HiTrap Q HP column (GE Healthcare, Cat# 17-1154-01). Wash the column with the following buffer: 25 mL of Buffer A (20 mM Tris, 15 mM NaCl, pH 8.5); 25 mL of Buffer B (10 mM Tris, 500 mM NaCl, pH 8.5); 50 mL of Buffer A; 40 mL of virus sample in Buffer A; 50 mL of Buffer A (20 mM Tris, 15 mM NaCl, pH 8.5); 20 mL of Buffer C (20 mM Tris, 175 mM NaCl, pH 8.5);
Collect Buffer C in a 20 mL Apollo Concentrators (Orbital Biosciences). Centrifuge at 3,000 rpm, at 4°C for 10 min.
Discard the flow-through and add 20 mL of chilled PBS and centrifuge at 3,000 rpm, at 4°C for 10 min.
Discard the flow-through and add 500 μL of chilled PBS. Wash the vectors off from the membrane and transfer to silicone-treated Eppendorf tubes and store frozen in small aliquots at -80°C.
2. Determination of rAAV3 Vector Titers
Thaw 10 μL of purified virus in an Eppendorf tube on ice. Add 40 μL of ddH2O.
Add 0.2 μL of Benzonase (25 U/ μL, Novagen, Cat# 70664) and incubate at 37°C for 1 hour.
Take 50 μL of plasmid standard (0.2 ng/ μL) in another Eppendorf tube.
Add 50 μL of 100 mM NaOH in each tube and incubate in 65°C for 30 min.
Chill on ice immediately for at least 5 min.
Cut transfer membrane (Millipore, Cat# INYC00010) according to Bio-Dot Filter Paper (Bio-Rad, Cat# 1620161). Rinse membrane in 10x SSC with three filter papers for 20 min.
Set slot blot SF Apparatus (Bio-Dot, Cat# 170-6542). Connect to a vacuum flask and add 100 μL of 10x SSC to slot blot apparatus.
Add 100 μL of 20x SSC and 1 μL of 6x loading dye to each of the samples in Step 2.5.
Load 100 μL of each samples onto the membrane in the slot blot apparatus.
Add another 100 μL of 10x SSC into each of the samples.
Repeat Steps 2.9 and 2.10 until all the sample goes through the membrane.
Disassemble the slot blot apparatus and remove the membrane. Put it into Inspector Linker SL-1000 UV Crosslinker. Turn on "Energy", "Optimal Crosslink" (show 1200) and push "Start".
Boil 1 mL of salmon sperm (SS) DNA (Fisher, Cat# NC9753983) for 5 min. Chill on ice for at least 5 min.
Prepare the pre-hybridization solution by mixing 27.5 mL of ddH2O, 15 mL of 20x SSC, 5 mL of 50x Denhardt's solution, 1.25 mL of 20% SDS, 0.25 mL of PolyA and 1 mL of SS DNA from Step 2.13.
Suspend the membrane in 25 mL of pre-hybridization solution in a capped glass cylinder and incubate at 65°C in a hybridization chamber overnight.
Dilute 50 ng DNA templates in 10 μL of ddH2O. Boil at 100°C for 5 min and chill on ice immediately for at least 5 min.
Centrifuge at 3,000 rpm at RT for 1 min. Add 2 μL of 10 mM 3 dNTPs (lacking dCTP), 2 μL of Hexanucleotide Mix (Roche, Cat# 11277081001), 1 μL of Klenow (Roche, Cat# 11008404001) and 5 μL of α-32P-dCTP. Incubate at 37°C for 10 min.
Centrifuge G-50 Columns (GE Healthcare, Cat# 27-5330-01) at 3,000 rpm for 2 min. load the probe in Step 2.17 into the column. Centrifuge at 3,000 rpm for 2 min and collect the flow-through.
Count radioactivity. Boil the probe at 100°C for 5 min and chill on ice immediately for at least 5 min.
Add the probe (6x105 cpm/ mL) into the pre-hybridization solution with membrane.
Incubate overnight at 65°C in a hybridization chamber.
Discard the hybridization solution and rinse the membrane in 2x SSC+0.1%SDS at RT for 15 min.
Discard the solution and rinse the membrane in 0.1x SSC+0.1%SDS at 65°C for 30 min.
Discard the solution and rinse the membrane in 0.1x SSC at RT briefly.
Expose the film at -80°C for 6 hours and develop the film. A typical result is shown in Figure 1.
3. rAAV3 Vector-mediated Transduction and Transgene Expression in Human Liver Cancer Cells
Seed 1x104 cells (either Huh7 or Hep293TT) in each well of 96-well plate. Incubate in a humidified CO2 incubator for at least 18 hours.
Remove medium from the 96-well plate.
Wash cells twice with 50 μL of medium without FBS and antibiotics and remove.
Add 50 μL of medium without FBS and antibiotics and rAAV vectors at 5,000 vgs/cell.
Incubate the plates in the CO2 incubator for 4 hours.
Discard the medium. Wash cells twice with 50 μL of complete medium and remove.
Add 150 μL of C-DMEM to each well and incubate the plate in CO2 incubator for 72 hours, and visualize the EGFP expression using a fluorescence microscope (DMI 4000B; Leica Microsystems).
Images from three wells of virus-infected cells are analyzed quantitatively by ImageJ analysis software (NIH, Bethesda, MD, USA).
Transgene expression is assessed as total area of green fluorescence (pixel2) per visual field. A typical result is shown in Figure 2.
Transgene expression can also be determined by Flow Cytometry.
4. Representative Results:
Following the protocol outlined above, one can generate AAV serotype vectors efficiently. The typical yield is ~500 μL containing ~1011 vgs/ μL of purified vector stock. The purity of the vector stock is determined by comparing hybridization signals on quantitative DNA slot blots, with and without Benzonase digestion. Purified rAAV3 vectors transduce human liver cancer - hepatoblastoma (HB) and hepatocellular carcinoma (HCC) - cell lines efficiently.
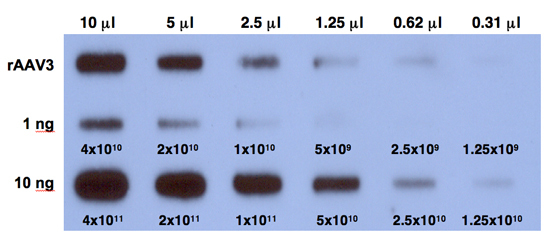 Figure 1. Quantitative DNA slot-blot analysis for determining the titers of rAAV3 vectors. Two-fold dilutions of purified viral stocks, digested with Benzonase (top row) were analyzed on quantitative DNA slot blots with 32P-labeled EGFP-specific DNA probe. The titer of AAV3 vector was determined by comparison with 1 ng (middle row) or 10 ng (bottom row) of AAV-EGFP plasmid standards loaded on the membrane. The numbers correspond to DNA copies.
Figure 1. Quantitative DNA slot-blot analysis for determining the titers of rAAV3 vectors. Two-fold dilutions of purified viral stocks, digested with Benzonase (top row) were analyzed on quantitative DNA slot blots with 32P-labeled EGFP-specific DNA probe. The titer of AAV3 vector was determined by comparison with 1 ng (middle row) or 10 ng (bottom row) of AAV-EGFP plasmid standards loaded on the membrane. The numbers correspond to DNA copies.
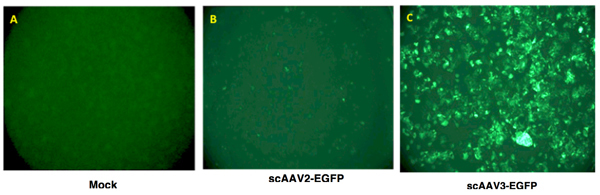 Figure 2. Recombinant AAV vector-mediated transgene expression in human liver cancer cells. Hep293TT cells, a recently established human hepatoblastoma cell line4, were either mock-infected (left panel), or transduced with 5,000 vgs/cell of either scAAV2-EGFP or scAAV3-EGFP vectors at 37°C for 2 hours. Transgene expression was visualized 72 hours post-transduction using a fluorescence microscope.
Figure 2. Recombinant AAV vector-mediated transgene expression in human liver cancer cells. Hep293TT cells, a recently established human hepatoblastoma cell line4, were either mock-infected (left panel), or transduced with 5,000 vgs/cell of either scAAV2-EGFP or scAAV3-EGFP vectors at 37°C for 2 hours. Transgene expression was visualized 72 hours post-transduction using a fluorescence microscope.
Discussion
Recombinant vectors based on a non-pathogenic human parvovirus, the adeno-associated virus (AAV) have been developed, and are currently in use in a number of gene therapy clinical trials5. Previously, of the 10 most commonly used AAV serotypes, the transduction efficiency of AAV3 vectors in general has been reported to be particularly low, both in vitro and in vivo1. However, our recent observation that AAV3 vectors transduce human liver cancer cell lines exceedingly well, as determined quantitatively by flow cytometry2, and that AAV3 utilizes the human hepatocyte growth factor receptor (hHGFR) as a cellular co-receptor for binding and entry in these cells3, strongly suggest a selective tissue-tropism of AAV3 for human liver cells in general, and human liver cancer cells in particular. However, since AAV3 vectors also transduce normal human hepatocytes efficiently2, their potential use in cancer gene therapy application in vivo would be negatively impacted. One possible strategy to circumvent this potential problem involves transcriptional-targeting of cancer cells by identifying a gene product that is selectively produced by the tumor, but not by normal hepatocytes. For example, previous studies have shown that serum levels of α-fetoprotein (AFP) are used as a specific marker for tracking the presence, progression, and/or reoccurrence of certain types of liver cancers, since normal hepatocytes generally produce very small amounts of this protein. Indeed, we have used AAV3 vectors containing the AFP promoter to target transgene expression in liver cancer cells, but not in normal hepatocytes2, and studies are currently underway to test the efficacy of this approach in a murine xenograft model of liver cancer. In our additional studies, we have observed that the transduction efficiency of various AAV serotype vectors can be significantly augmented by site-directed mutagenesis of surface-exposed tyrosine residues in the viral capsids6-14. Since 6 of 7 surface-exposed tyrosines are also conserved in AAV3, we have performed site-directed mutagenesis of these residues and observed that the transduction efficiency of tyrosine-mutant AAV3 vectors is significantly enhanced in human liver cancer cells (unpublished data). Studies are also currently underway to evaluate the safety and efficacy of the tyrosine-mutant AAV3 vectors in mouse xenograft models for human hepatoblastoma and hepatocellular carcinoma, and if successful, the optimal tyrosine-mutant AAV3 serotype vectors may prove to be useful for targeting human liver cancers for the potential gene therapy.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Drs. R. Jude Samulski and Xiao Xiao for their kind gifts of recombinant AAV3 and AAV-EGFP plasmids, respectively, and Dr. Gail Tomlinson for generously providing Hep293TT cells. This research was supported in part by Public Health Service grant grants R01 HL-076901, R01 HL-097088, and P01 DK-058327 (Project 1) from the National Institutes of Health (to AS).
References
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, Liu C, Srivastava A, Stacpoole PW. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet & Metabol. 2009;98:289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, Zhong L, Agbandje-McKenna M, Srivastava A. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther. 2010;21:1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TT, Rakheja D, Hung JY, Hornsby PJ, Tabaczewski P, Malogolowkin M, Feusner J, Miskevich F, Schultz R, Tomlinson GE. Establishment and characterization of a cancer cell line derived from an aggressive childhood liver tumor. Pediatr Blood & Cancer. 2009;53:1040–1047. doi: 10.1002/pbc.22187. [DOI] [PubMed] [Google Scholar]
- Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, Li B, Herzog RW, Srivastava A. Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum. Gene Ther. 2010;21:271–283. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jayandharan GR, Li B, Ling C, Ma W, Srivastava A, Zhong L. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21:1527–1543. doi: 10.1089/hum.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss MA, Smith LJ, Zhong L, Srivastava A, Wong KKJr, Chatterjee S. Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2. Hum Gene Ther. 2010;21:1129–1136. doi: 10.1089/hum.2010.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C, Zhang W, Yuan Z, Shin JH, Li J, Jayandharan GR, Zhong L, Srivastava A, Xiao X, Duan D. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, Jayandharan GR, Ling C, Zolotukhin I, Ma W, Zolotukhin S, Srivastava A, Zhong L. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojano-Dirain C, Glushakova LG, Zhong L, Zolotukhin S, Muzyczka N, Srivastava A, Stacpoole PW. An animal model of PDH deficiency using AAV8-siRNA vector-mediated knockdown of pyruvate dehydrogenase E1α. Mol Genet & Metabol. 2010;101:183–191. doi: 10.1016/j.ymgme.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, Chiodo V, Neeley AW, Govindasamy L, Bennett A, Agbandje-McKenna M. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011. Forthcoming. [DOI] [PMC free article] [PubMed]


