Abstract
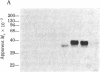
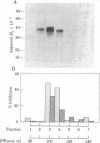
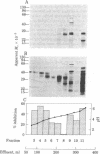
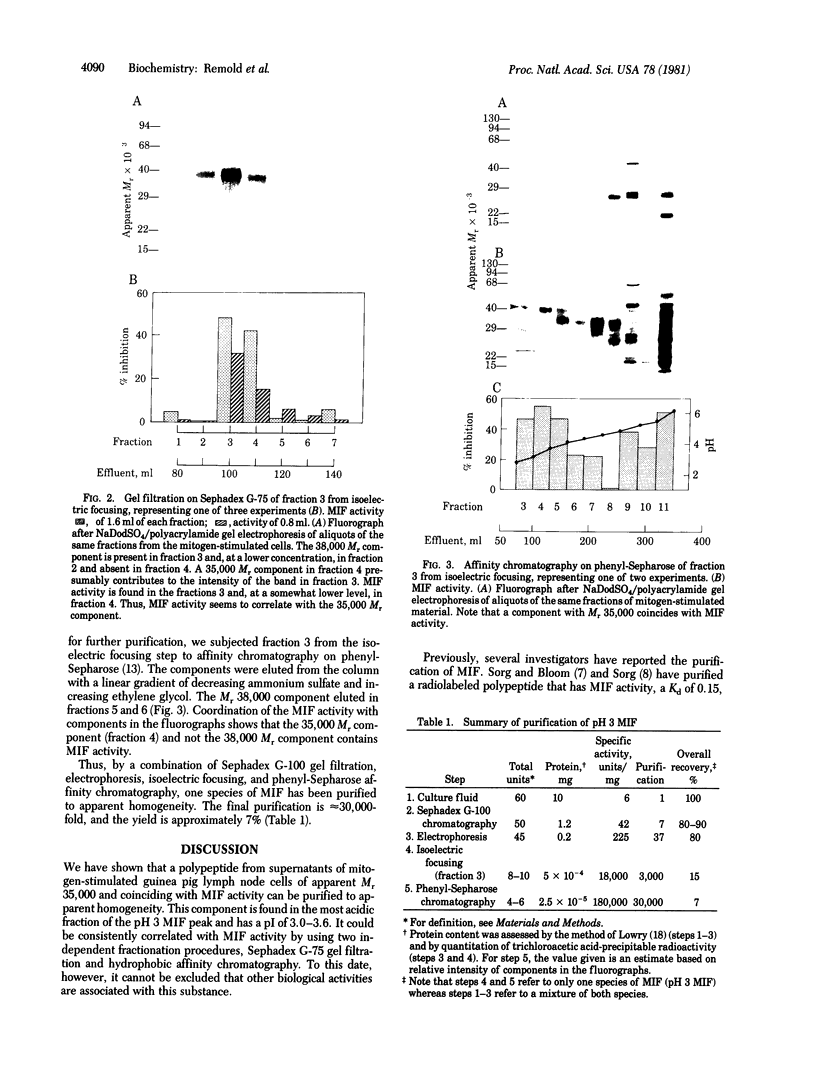
Macrophage migration inhibitory factor (MIF) from the guinea pig was recently shown to reside in two discrete and separable proteins referred to as pH 3 MIF and pH 5 MIF. One subfraction of pH 3 MIF has now been purified to apparent homogeneity from supernatants of stimulated lymph node cells. To monitor purification, biosynthetically radiolabeled MIF was prepared. Sensitized lymphocytes were stimulated in the presence of [3H]leucine by concanavalin A to produce radiolabeled mediators. MIF was purified approximately 30,000-fold from the culture fluid by using gel filtration, sucrose density gradient electrophoresis, isoelectric focusing, and hydrophobic affinity chromatography. This procedure yielded a single 3H-labeled polypeptide with an apparent Mr of 35,000 that coincides with MIF activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block L. H., Jaksche H., Bamberger S., Ruhenstroth-Bauer G. Human migration inhibitory factor: purification and immunochemical characterization. J Exp Med. 1978 Feb 1;147(2):541–553. doi: 10.1084/jem.147.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Hydrophobic interaction of human, mouse, and rabbit interferons with immobilized hydrocarbons. J Biol Chem. 1976 Dec 10;251(23):7620–7625. [PubMed] [Google Scholar]
- David J. R., David R. R. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300–449. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geczy C. L., Geczy A. F., De Weck A. L. Antibodies to guinea pig lymphokines. II. Suppression of delayed hypersensitivity reactions by a "second generation" goat antibody against guinea pig lymphokines. J Immunol. 1976 Jul;117(1):66–72. [PubMed] [Google Scholar]
- Geczy C. L., Geczy A. F., De Weck A. L. Antibodies to guinea pig lymphokines. IV. Suppression of the mixed leukocyte culture reaction by anti-lymphokine globulin. J Immunol. 1976 Nov;117(5 PT2):1824–1831. [PubMed] [Google Scholar]
- Heck L. W., Remold-O'Donnell E., Remold H. G. DFP-sensitive polypeptides of the guinea pig peritoneal macrophage. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1576–1583. doi: 10.1016/0006-291x(78)91401-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Remold H. G., Katz A. B., Haber E., David J. R. Studies on migration inhibitory factor (MIF): recovery of MIF activity after purification by gel filtration and disc electrophoresis. Cell Immunol. 1970 May;1(1):133–145. doi: 10.1016/0008-8749(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Remold H. G., Mednis A. D. Two migration inhibitory factors with different chromatographic behavior and isoelectric points. J Immunol. 1977 Jun;118(6):2015–2019. [PubMed] [Google Scholar]
- Rubinstein M., Rubinstein S., Familletti P. C., Miller R. S., Waldman A. A., Pestka S. Human leukocyte interferon: production, purification to homogeneity, and initial characterization. Proc Natl Acad Sci U S A. 1979 Feb;76(2):640–644. doi: 10.1073/pnas.76.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C., Bloom B. R. Products of activated lymphocytes. I. The use of radiolabeling techniques in the characterization and partial purification of the migration inhibition factor of the guinea pig. J Exp Med. 1973 Jan 1;137(1):148–170. doi: 10.1084/jem.137.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C., Klinkert W. Chemical characterization of products of activated lymphocytes. Fed Proc. 1978 Nov;37(13):2748–2753. [PubMed] [Google Scholar]
- Sorg C. Radioactive labelling and characterization of the products of activated mouse lymphocytes. Eur J Biochem. 1975 Jul 1;55(2):423–430. doi: 10.1111/j.1432-1033.1975.tb02178.x. [DOI] [PubMed] [Google Scholar]