Abstract
Percutaneous thermal ablation is an emerging treatment option for many tumors of the abdomen not amenable to conventional treatments. During a thermal ablation procedure, a thin applicator is guided into the target tumor under imaging guidance. Energy is then applied to the tissue until temperatures rise to cytotoxic levels (50-60 °C). Various energy sources are available to heat biological tissues, including radiofrequency (RF) electrical current, microwaves, laser light and ultrasonic waves. Of these, RF and microwave ablation are most commonly used worldwide.
During RF ablation, alternating electrical current (~500 kHz) produces resistive heating around the interstitial electrode. Skin surface electrodes (ground pads) are used to complete the electrical circuit. RF ablation has been in use for nearly 20 years, with good results for local tumor control, extended survival and low complication rates1,2. Recent studies suggest RF ablation may be a first-line treatment option for small hepatocellular carcinoma and renal-cell carcinoma3-5. However, RF heating is hampered by local blood flow and high electrical impedance tissues (eg, lung, bone, desiccated or charred tissue)6,7. Microwaves may alleviate some of these problems by producing faster, volumetric heating8-10. To create larger or conformal ablations, multiple microwave antennas can be used simultaneously while RF electrodes require sequential operation, which limits their efficiency. Early experiences with microwave systems suggest efficacy and safety similar to, or better than RF devices11-13.
Alternatively, cryoablation freezes the target tissues to lethal levels (-20 to -40 °C). Percutaneous cryoablation has been shown to be effective against RCC and many metastatic tumors, particularly colorectal cancer, in the liver14-16. Cryoablation may also be associated with less post-procedure pain and faster recovery for some indications17. Cryoablation is often contraindicated for primary liver cancer due to underlying coagulopathy and associated bleeding risks frequently seen in cirrhotic patients. In addition, sudden release of tumor cellular contents when the frozen tissue thaws can lead to a potentially serious condition known as cryoshock 16.
Thermal tumor ablation can be performed at open surgery, laparoscopy or using a percutaneous approach. When performed percutaneously, the ablation procedure relies on imaging for diagnosis, planning, applicator guidance, treatment monitoring and follow-up. Ultrasound is the most popular modality for guidance and treatment monitoring worldwide, but computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used as well. Contrast-enhanced CT or MRI are typically employed for diagnosis and follow-up imaging.
Keywords: Medicine, Issue 49, Thermal ablation, interventional oncology, image-guided therapy, radiology, cancer
Protocol
1. Introduction/Case Presentation:
Example case: 50 yr old female with retroperitoneal leiomyosarcoma s/p primary resection. Had evidence of metastatic disease in the liver and lung that responded to chemotherapy. Had a single chemo-refractory hepatic lesion. Multidisciplinary discussion led to ablation as a possible treatment option, given the patient's young age, lack of comorbidities and paucity of alternative chemotherapeutic treatment options. The lesion was biopsied prior to treatment to confirm metastatic disease. Planning ultrasound demonstrated the lesion in the inferior and posterior aspect of the left hepatic lobe. Given its proximity to pancreas and bowel, the need for hydrodissection was reviewed with the patient at the time of consultation.
2. Patient selection
- One of the most important aspects of the procedure is to ensure that the patient is an appropriate candidate. This involves a multi-factorial assessment of both tumor and patient related factors including:
- Tumor type, size, location and focality (Figure 1).
- Patient co-morbidities and overall health.
Patients should be discussed at a multi-disciplinary tumor board to determine the available treatment alternatives and choose the most efficacious and appropriate option.
The most appropriate ablation modality is variable and dependent on many different factors as well, such as: location of the tumor within the organ, proximity of adjacent structures and the associated need for precision, experience of the operator, presence of underlying liver disease or bleeding diatheses. All of these factors and more must be considered to determine and choose the most appropriate ablation modality.
Patient must be well informed regarding the procedure, expected risks, possibility of a complete treatment, and likely benefits. Therefore, we consult with every patient prior to the procedure, usually at the time of planning ultrasound as below.
An overall assessment of the patient and procedure will determine the degree of sedation/anesthesia that is both safe and efficacious for the case.
3. Pre-treatment planning
We perform a pre-ablation planning ultrasound to determine optimal patient positioning and approach.This also helps us determine whether or not other imaging modalities will be required for guidance and more notably, monitoring. It is often helpful to have CT available at the time of the ablation procedure for an overview evaluation of the target tumor, ablation zone, and adjacent structures.
We also assess the need for adjuvant techniques at this time with an emphasis on safety and efficacy (Figure 2).
During this time we determine the number of applicators likely to be needed to complete the treatment as well as their optimal placement.
4. Ablation procedure
- Patient preparation
- Factors to be considered/evaluated include: bowel preparation, pre/periprocedural antibiotics, renal/liver function, IV access, need for an arterial line for blood pressure monitoring, etc. These determinations are made on a case-by-case basis, but there are some general principles that can be followed and these will be discussed.
- General anesthesia is common, but ablation can often be performed with deep sedation, or even conscious sedation in some scenarios. Should be optimized to the individual patient.
- Equipment setup (note that many aspects of the setup are vendor dependent)
- Will show an example of RF, cryoablation, and MW ablation equipment and setup including ground pad placement, connections and testing. For this case, radiofrequency ablation will be utilized.
- The ablation session
- Guidance
- Accurate and appropriate image-guided applicator placement is critical. Since this often includes multiple applicator placements, particularly for larger tumors, there are many factors to be considered and there are several 'tricks' that can be employed (Figure 3).
- Adjuvant techniques
- When performing percutaneous ablation, there are often adjacent structures that may be vulnerable to thermal damage. This has led to the development of hydrodissection and other displacement techniques, which allow physical, thermal, and electrical protection when applied appropriately. We will review the use of these techniques.
- We will also discuss other treatments/techniques that have been shown to, or theoretically can increase efficacy and safety of the treatment.
- Monitoring
- US, CT, and MRI all have specific strengths and weaknesses regarding their ability to effectively and precisely monitor the growing ablation zone. Because of this, we often utilize a multi-modality approach with both US and CT. This allows us to perform the procedure safely and effectively.
- Post-ablation assessment
- The post-ablation assessment allows the determination of the efficacy of the therapy. When and how it is performed is variable and will depend on availability of resources, practicality of administering US or CT contrast, and uncertainty regarding the technical success of the treatment (Figure 4). At our institution, we perform a contrast-enhanced CT on suitable patients immediately after the ablation session has been concluded. This allows us assess for complete treatment (with immediate re-treatment while the patient is still under anesthesia if necessary), establish a post procedure baseline and identify any early complications. However, many institutions perform the initial assessment a month or more after the ablation session.
- Follow-up
- The interval and optimal modality for follow up evaluation varies depending on tumor type and patient factors. We will discuss various factors that influence this decision and describe an accepted protocol (Figure 5).
5. Outcome:
The procedure was successfully carried out using a combination of ultrasound guidance with CT and ultrasound monitoring. A direct injection of 5% dextrose in water (D5W) was used for hydrodissection (Fig. 2). Ultrasound was utilized for guidance since the tumor was easily visualized by ultrasound imaging but not with non-contrast CT (as is often the case with liver lesions). In our experience, placing applicators with ultrasound guidance is faster than with CT. Ultrasound does not use ionizing radiation and is therefore safe for both the patient and personnel, and gives real-time feedback of the tumor and applicator location allowing accurate and consistent placement. Since accurate targeting is so critical to the success of the procedure, applicator guidance often determines whether or not the procedure will result in a complete treatment.
A combination of ultrasound and CT monitoring was utilized. Ultrasound allows real-time evaluation of the developing ablation while CT gives a global overview of applicator placement and proximity to adjacent structures, allowing superior assessment of the safety of the applicator placement and the likelihood of collateral damage to nearby structures. Thus, the combination of ultrasound and CT imaging results in a safer treatment.
D5W was utilized for hydrodissection for two main reasons: it acts as both a physical and electrical barrier to heating and, therefore, prevents damage to adjacent structures (i.e. pancreas and bowel), and intraperitoneal D5W has been shown to decrease post-procedural pain in patients undergoing liver tumor RF ablation. Thus, this intervention makes the procedure both safer and more comfortable for the patient. Follow up imaging shows that the procedure resulted in a complete treatment of the tumor with no associated complications and there was only minimal patient discomfort.
Liver tumor ablation is applied most commonly to hepatocellular carcinoma in cirrhotic patients, and in limited hepatic metastatic disease in patients with colon, breast or neuroendocrine tumors. Patients with other tumor types who have hepatic metastatic disease often have systemic disease and may not benefit from ablation; these patients are considered on a case-by-case basis. There is a relative paucity of long-term data in patients with metastatic sarcoma. The decision to proceed with ablation in this case was made given that all the other sites of disease in this young patient responded to chemotherapy with a single refractory hepatic lesion. Although this may control her disease in the short term and give her a reprieve from chemotherapy, with a tumor of this type, cure is unlikely. The patient later developed an additional liver lesion and two pulmonary nodules. All were treated with ablation. The patient is currently under continued surveillance.
6. Representative Results:
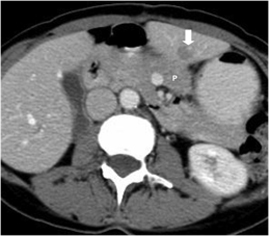 Figure 1. Pre-procedure CT demonstrating a low attenuation lesion in the left hepatic lobe (arrow) abutting the pancreas (P) with duodenum located just inferior and stomach just lateral.
Figure 1. Pre-procedure CT demonstrating a low attenuation lesion in the left hepatic lobe (arrow) abutting the pancreas (P) with duodenum located just inferior and stomach just lateral.
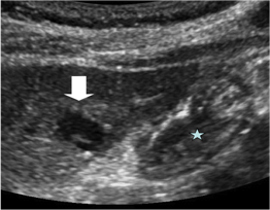 Figure 2. Planning ultrasound with the patient in the supine position demonstrates a hypoechoic lesion (arrow) closely abutting the adjacent stomach (star) and pancreas (not visible). Therefore, a decision was made to place an NG tube for decompression during the procedure and use hydrodissection to protect the posterior structures.
Figure 2. Planning ultrasound with the patient in the supine position demonstrates a hypoechoic lesion (arrow) closely abutting the adjacent stomach (star) and pancreas (not visible). Therefore, a decision was made to place an NG tube for decompression during the procedure and use hydrodissection to protect the posterior structures.
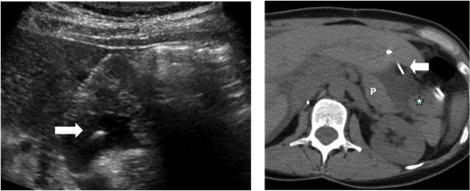 Figure 3. Ultrasound image (left) demonstrates hydrodissection needle (arrow) extending through the left hepatic lobe in to the space between the liver and the pancreas with infusion of fluid. Abdominal CT (right) shows the needle in place (arrow) with accumulation of fluid between the liver and pancreas (P) with posterior displacement of the stomach (star).
Figure 3. Ultrasound image (left) demonstrates hydrodissection needle (arrow) extending through the left hepatic lobe in to the space between the liver and the pancreas with infusion of fluid. Abdominal CT (right) shows the needle in place (arrow) with accumulation of fluid between the liver and pancreas (P) with posterior displacement of the stomach (star).
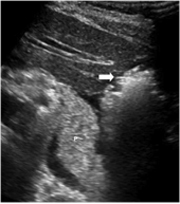 Figure 4. Ultrasound image obtained during ablation demonstrates the gas cloud in the lateral left lobe (arrow). A thin layer of fluid can be observed between the liver and the pancreas (P).
Figure 4. Ultrasound image obtained during ablation demonstrates the gas cloud in the lateral left lobe (arrow). A thin layer of fluid can be observed between the liver and the pancreas (P).
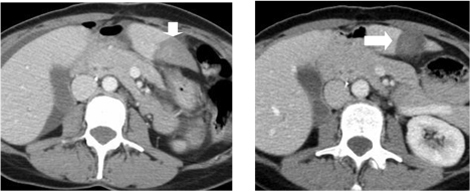 Figure 5. Immediate post-procedure CT (left) demonstrates the ablation zone (arrow). The ablation zone has contracted on the one-month follow-up CT (right, arrow).
Figure 5. Immediate post-procedure CT (left) demonstrates the ablation zone (arrow). The ablation zone has contracted on the one-month follow-up CT (right, arrow).
Discussion
Thermal tumor ablation is a safe and effective treatment for many abdominal tumors that are refractory to surgery, or have failed treatment with radiotherapy/chemotherapy. In addition, it is fast becoming an accepted first-line therapy for some patients. The benefits include: an exceptional safety profile, a more rapid convalescence than traditional surgical therapies, decreased costs, and excellent local control rates in appropriately triaged patients. Percutaneous procedures are generally feasible, especially when using techniques such as hydrodissection to improve safety and targeting of the tumor, thus limiting the morbidity even further. This technique has been utilized in practice for several decades now and many of the questions regarding long-term outcomes and oncological efficacy are being answered with encouraging results. Thermal ablation will certainly play an important and growing role in the management of oncology patients over the coming years.
Disclosures
CLB: Shareholder and consultant for NeuWave Medical, Inc, which develops ablation technologies. Consultant for Triagenics, LLC.
Acknowledgments
The authors would like to thank Fred Lee, Jr., for his leadership with the clinical tumor ablation program; and Lisa Sampson, Jan Ketzler and Marci Center for their assistance with the research and clinical programs at the University of Wisconsin Madison.
References
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, Marsland TA, Michelson R, Poston GJ, Schrag D, Seidenfeld J, Benson AB. American society of clinical oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J. Clin. Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of interventional radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20:3–8. doi: 10.1016/j.jvir.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Stasi MDi, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of ct-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189:429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- Solazzo SA, Liu Z, Lobo SM, Ahmed M, Hines-Peralta AU, Lenkinski RE, Goldberg SN. Radiofrequency ablation: importance of background tissue electrical conductivity--an agar phantom and computer modeling study. Radiology. 2005;236:495–502. doi: 10.1148/radiol.2362040965. [DOI] [PubMed] [Google Scholar]
- Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- Lubner MG, Brace CL, Hinshaw JL, Lee FTJ. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:192–203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano A, Huang Y, Meloni MF, Lee FTJ, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973. doi: 10.1118/1.3432569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J. Urol. 2008;180:844–848. doi: 10.1016/j.juro.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FTJ. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol. 2008;191:1159–1168. doi: 10.2214/AJR.07.3706. [DOI] [PubMed] [Google Scholar]
- Atwell TD, Farrell MA, Callstrom MR, Charboneau JW, Leibovich BC, Frank I, Patterson DE. Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol. 2007;188:1195–1200. doi: 10.2214/AJR.06.1152. [DOI] [PubMed] [Google Scholar]
- Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89:1396–1401. doi: 10.1046/j.1365-2168.2002.02292.x. [DOI] [PubMed] [Google Scholar]
- Callstrom MR, Atwell TD, Charboneau JW, Farrell MA, Goetz MP, Rubin J, Sloan JA, Novotny PJ, Welch TJ, Maus TP, Wong GY, Brown KJ. Painful metastases involving bone: percutaneous image-guided cryoablation--prospective trial interim analysis. Radiology. 2006;241:572–580. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]


