Abstract
Acamprosate suppresses alcohol intake and craving in recovering alcoholics; however, the central sites of its action are unclear. To approach this question, brain regions responsive to acamprosate were mapped using acamprosate microimplants targeted to brain reward and circadian areas implicated in alcohol dependence. mPer2 mutant mice with nonfunctional mPer2, a circadian clock gene that gates endogenous timekeeping, were included, owing to their high levels of ethanol intake and preference. Male wild-type (WT) and mPer2 mutant mice received free-choice (15%) ethanol/water for 3 wk. The ethanol was withdrawn for 3 wk and then reintroduced to facilitate relapse. Four days before ethanol reintroduction, mice received bilateral blank or acamprosate-containing microimplants releasing ∼50 ng/day into reward [ventral tegmental (VTA), peduculopontine tegmentum (PPT), and nucleus accumbens (NA)] and circadian [intergeniculate leaflet (IGL) and suprachiasmatic nucleus (SCN)] areas. The hippocampus was also targeted. Circadian locomotor activity was measured throughout. Ethanol intake and preference were greater in mPer2 mutants than in wild-type (WT) mice (27 g·kg−1·day−1 vs. 13 g·kg−1·day−1 and 70% vs. 50%, respectively; both, P < 0.05). In WTs, acamprosate in all areas, except hippocampus, suppressed ethanol intake and preference (by 40–60%) during ethanol reintroduction. In mPer2 mutants, acamprosate in the VTA, PPT, and SCN suppressed ethanol intake and preference by 20–30%. These data are evidence that acamprosate's suppression of ethanol intake and preference are manifest through actions within major reward and circadian sites.
Keywords: reward, circadian rhythm, mper2 clock gene, relapse, mouse
the glutamate antagonist, acamprosate (Campral), is one of a limited number of antirelapse drugs approved by the Food and Drug Administration to help reduce relapse risk in recovering alcoholics (25, 53). Neurochemically, it is thought to act by attenuating increased glutamatergic neurotransmission associated with alcohol dependence and repeated episodes of withdrawal (53, 56). Notably, in mPer2 mutant mice with nonfunctional mPer2, elevated glutamatergic tonus, and raised ethanol intake, acamprosate depresses extracellular glutamate and drinking to wild-type (WT) levels (56). Independent of its role in the etiology of alcoholism, mPer2 is a negative transcription factor of a molecular feedback loop that gates endogenous circadian timekeeping (47, 56).
In humans, the degree of efficacy of acamprosate in treating alcohol dependence is significant, but variable. It is estimated that recovering alcoholics treated with acamprosate are ∼2–3 times more prone to remain abstinent, at least in the short term vs. placebo (34, 48). Characterization of acamprosate pharmacokinetics has revealed that acamprosate's actions may be restricted by limited transport across biological tissues and the development of tolerance (11, 53, 66). Also, little is known concerning the brain site(s) for acamprosate's suppressive action on ethanol intake, although there is some evidence that application of acamprosate into the nucleus accumbens core (a major component of the mesolimbic alcohol reward circuitry), can suppress ethanol intake in rats by interacting with accumbal glycine receptors and possibly by activating cholinergic transmission in the ventral tegmental area (VTA) (8, 9).
To address the question of the brain site(s) of acamprosate action, intracranial constant-release acamprosate-containing microimplants were used to map brain areas responsive to the suppressive effect of acamprosate on ethanol intake and preference during relapse. We examined the effects of the acamprosate implants in ethanol-preferring mPer2 mutant vs. wild-type (WT) mice to determine whether the propensity for excessive ethanol intake affects the degree of suppressive response to the drug. The effects of acamprosate treatment on the daily locomotor activity rhythm were also assessed to explore possible circadian rhythm-related actions of mPer2 mutation and drug treatment (3). Brain areas targeted with acamprosate in this study represent major alcohol reward [VTA, peduculopontine tegmentum (PPT), and nucleus accumbens (NA)] and circadian-related brain areas [intergeniculate leaflet (IGL) and suprachiasmatic nucleus (SCN)] (22, 26, 33, 37, 52, 61). These circadian areas were included because of the suspected role of circadian signaling in modulating ethanol reward and craving (3, 4, 47, 49, 56, 62, 63), and as such, are potential targets for acamprosate action.
MATERIALS AND METHODS
Animals
Adult, 8-wk-old homozygous mPer2 mutant male mice (strain:B6.129S7-MPER2tm1Brd/J) and wild-type (WT) mice [strain: B6(Cg)-Tyrc−2J/J] were produced from breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME). Both strains are back-crossed to C57BL/6. Animals were singly housed in polycarbonate cages under a 12:12-h light-dark photoperiod (LD) at a light intensity of 270 lux in a temperature-controlled vivarium (23°C) with food (Prolab 3000, PMI Feeds, St. Louis, MO) and water provided ad libitum. The experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Kent State Institutional Animal Care and Use Committee.
Acamprosate Implants
Bilateral acamprosate microimplants (1.25 mm × 0.75 mm; prepared from a homogeneous mixture of 300 mg acamprosate and 1 g beeswax [or beeswax alone for blank control implants]) were stereotaxically targeted to the lateral margin of the selected drug reward and circadian brain areas (stereotaxic coordinates derived from 1) VTA: AP = −3.08 mm from bregma, L = −0.75 mm from midline, and H = −4.00 mm from dura; 2) PPT: AP = −4.72 mm from bregma, L = −0.80 mm from midline, and H = −3.00 mm from dura; 3) NA: AP = +1.70 mm from bregma, L= −0.75 mm from midline, and H = −4.00 mm from dura; 4) IGL: AP = −2.30 mm from bregma, L = −2.40 mm from midline and H = −3.00 mm from dura; 5) SCN: AP = −0.46 mm from bregma, L = −0.03 mm from midline, and H = −5.50 mm from dura; and 6) Hippocampus: AP = −2.70 mm from bregma, L = −2.25 mm from midline, H = −2.00 mm from dura). For implantation, animals were anesthetized with pentobarbital sodium (Nembutal: 35.0 mg/kg) and pretreated with Marcaine (0.25% bupivicaine, 0.05 ml sc) and atropine (0.09% atropine, 0.10 ml ip) to manage localized pain and reduce respiratory occlusion, respectively. The microimplants were extruded from a stainless-steel 26-gauge cannula directly into the targeted brain regions. Following implantation, the skin was sutured with stainless-steel wound clips, and the animals were given 3 days of postoperative recovery prior to experimentation.
In Vitro Acamprosate Release
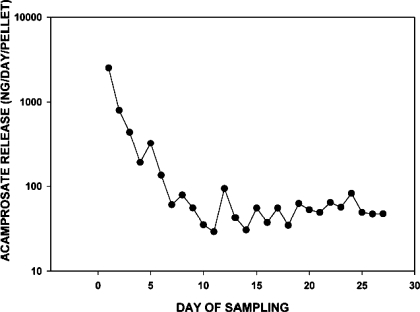
The daily rate of acamprosate release from the microimplants was determined in vitro by incubating the implants in 1.5 ml physiological saline at 37°C over 27 days. Each day, the saline medium was decanted and replenished with new medium. The daily samples were stored frozen prior to acamprosate analysis by UV spectrometry (Varian Cary 300 Bio; Varian, Santa Clara, CA) using an absorbance calibration curve generated at a wavelength of 201 nm. Release of acamprosate was asymptotic, reaching a relatively steady-state release rate of ∼50 ng·day−1·implant−1 by day 6 of incubation. This profile of acamprosate release is shown in Fig. 1.
Fig. 1.
Profile of in vitro acamprosate release from acamprosate microimplants incubated at 37°C in physiological saline sampled daily over 27 days.
Histological Evaluation of Acamprosate Implant Sites
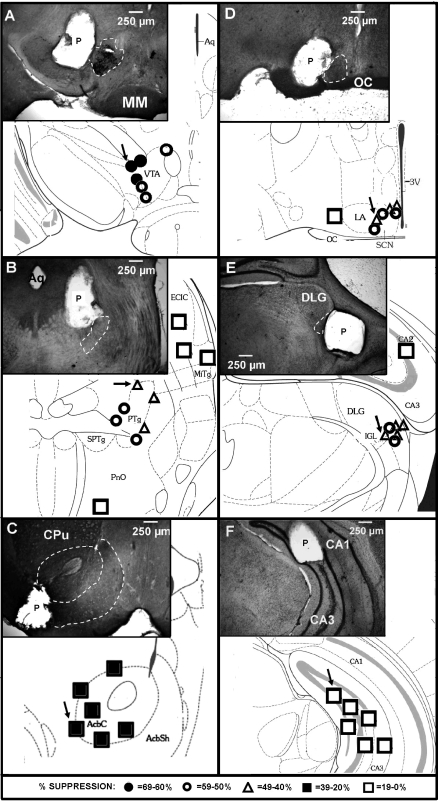
Animals were deeply anesthetized with Nembutal and intracardially perfused with 100 ml of 4% buffered paraformaldehyde (pH = 7.3). The brains were extracted and immersion-fixed in 4% paraformaldehyde for 24 h, followed by immersion in 30% sucrose for 24 h at 4°C. Cryostat SCN and hippocampal sections (40 μm-thick) were stained with cresyl violet. VTA, PPT, IGL, and NA (40 μm-thick) sections were incubated with rabbit polyclonal IgG antibodies to tyrosine hydroxylase for localization of dopaminergic neurons in the VTA and NA (Santa Cruz Biotechnology, Santa Cruz, CA), choline acetyltransferase for localization of cholinergic neurons in the PPT, and glutamate decarboxylase for localization of GABA neurons in the IGL (Millipore, Billerica, MA). Staining was visualized using Vectastain Elite ABC kit with 3,3-diaminobenzidine tetrahydrochloride as chromagen (Vector Laboratories, Burlingame, CA). Representative micropellet photomicrographs and overall micropellet placements are presented diagrammatically in Fig. 2.
Fig. 2.
Diagrammatic coronal brain sections with representative photomicrograph inserts illustrating the suppressive effects of acamprosate implants on ethanol intake in wild-type mice. Symbols represent the closest apposition of each pellet relative to its intended target site. Implants were aimed at reward areas (left): ventral tegmental area (VTA; A); peduculopontine tegmentum (PTg; B); and nucleus accumbens (AcbC/AchSh; C), and circadian areas (right): suprachiasmatic nucleus (SCN; D) and intergeniculate leaflet (IGL; E), and a control area (F), the hippocampus (CA1/CA3). The degree of suppressive response is represented by the different symbols (key provided at bottom of the figure). Arrows designate the microimplants shown in each photomicrograph. CPu, caudate putamen; DLG, dorsalolateral geniculate nucleus; ECIC, external cortex of the inferior colliculus; LA, lateroanterior hypothalamic nucleus; MiTg, microcellular tegmental nucleus; MM, medial mammillary nucleus; OC, optic chiasm; P, microimplant; Aq, cerebral aqueduct; PnO, pontine reticular nucleus oral part; SPTg, subpeduncular tegmental nucleus; 3V, third ventricle. [Adapted from Ref. 19.].
Circadian Activity Measurements
General circadian locomotor activity was measured using overhead passive infrared motion detectors interfaced with a computerized data acquisition system (Clocklab, Coulbourn Instruments, Whitehall, PA). Data were collected in 1-min bins, and activity onset associated with lights-off [designated as zeitgeber time (ZT) 12] was defined by the initial 6-min period that 1) coincided with an intensity of activity that exceeded 10% of the maximum rate for the day, 2) preceded by at least 4 h of activity quiescence, and 3) followed by at least 60 min of sustained activity. Activity offset was defined as a final period of activity that 1) immediately preceded by at least 60 min of activity and 2) was followed by at least 4 h of inactivity. Alpha (measured in hours) represented the length of the nighttime activity period between activity onset and offset. Total activity across alpha was calculated from normalized activity bout durations (measured in min) × activity bout numbers derived from the Clocklab data acquisition system.
Experimental Protocol
WT and mPer2 mutant mice (n = 12, each strain) received free-choice (15%) ethanol/water for 3 wk. The ethanol was withdrawn for 3 wk and then reintroduced to facilitate relapse. Ethanol preference was calculated as the percentage of daily ethanol intake relative to total fluid intake measured in 50-ml plastic, graduated vials to the nearest 0.25 ml (Fisher Scientific; Pittsburgh, PA). Four days before ethanol reintroduction, blank or acamprosate-containing microimplants were implanted into brain areas listed above (n = 6, for each area). Five weeks following ethanol reintroduction, animals were euthanized with pentobarbital overdose, and brains were immediately extracted to verify implant sites. General circadian activity was recorded during ethanol withdrawal and ethanol reintroduction.
Statistics
A repeated-measures ANOVA was used to compare raw values of ethanol intake and preference between strain (mPer2 mutant vs. WT) during ethanol introduction and reintroduction and raw values of water intake between strain, treatment, or brain area. A one-way ANOVA was used to compare circadian activity measures between strain. Student-Newman-Keuls post hoc analysis was executed where appropriate. Paired t-tests were used to compare ethanol intake, preference, and circadian activity measures prior to and during theoretical constant acamprosate (or blank) release over 4 wk. SPSS 16.0 (Chicago, IL) was used to analyze the data. In all cases, the level of significance was P < 0.05.
RESULTS
Strain-related differences in ethanol intake, preference, and circadian activity.
Baseline levels of ethanol intake and preference were 1.9- and 1.3-fold greater, respectively, in mPer2 mutant vs. WT mice (intake: F1,22 = 56.4; preference: F1,22 = 66.9; both, P < 0.01). Nocturnal activity onset was 2.1 ± 0.4 h earlier in the mPer2 mutant mice compared with WTs (ZT 10.4 ± 0.2 vs. ZT 12.5 ± 0.6, respectively; F1,22 = 42.1; P < 0.01). Alpha was extended by 1.7 ± 0.1 h in the mPer2 mutant vs. WT mice (F1,22 = 10.2; P < 0.01), and the mPer2 mutants were 1.4 times more active across the nocturnal active period compared with WTs (total activity: F1,22 = 16.8; bout duration: F1,22 = 48.1; both, P < 0.01).
Acamprosate Suppresses Ethanol Intake and Preference in Brain Reward Areas
Ventral tegmental area.
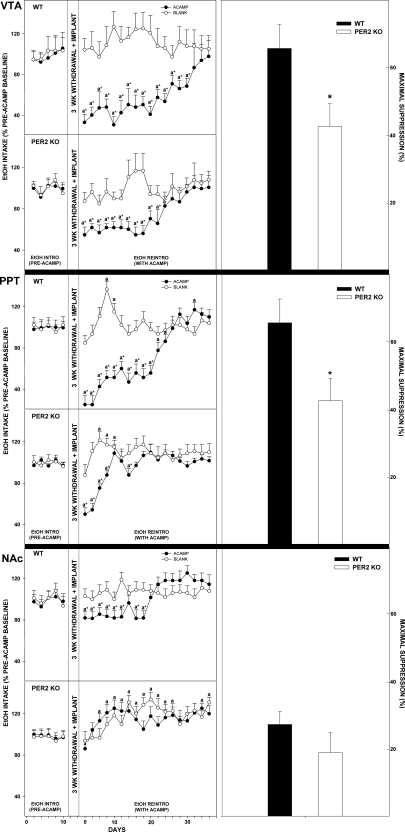
Acamprosate implants in the VTA of mPer2 mutants (calculated over total days of significant acamprosate effect) suppressed ethanol intake and preference from pretreatment levels by 38.7 ± 7.8% and 18.7 ± 4.3%, respectively, during ethanol reintroduction (both, P < 0.01; Figs. 3 and 4). In WTs, this treatment suppressed ethanol intake and preference by 60.5 ± 11.8% and 49.6 ± 9.6%, respectively (both, P < 0.01). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 15.6; preference: F1,22 = 18.3; both, P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both, P > 0.05).
Fig. 3.
Line graphs show percent change of ethanol intake ± SE from pretreatment levels in mice receiving acamprosate (ACAMP) or no acamprosate (BLANK) from microimplants in reward areas [ventral tegmental area (VTA), peduculopontine tegmentum (PPT), and nucleus accumbens (NAc)] during simulated relapse. aSignificant difference from pretreatment levels (P < 0.05). *Significant difference between treatment groups for any given day (P < 0.05). Bar graphs show means ± SE for the time point of maximal suppression of ethanol intake by acamprosate microimplants in WT vs. mPer2 mutant (PER2 KO) mice. *Significant difference between strains (P < 0.05).
Fig. 4.
Line graphs denote percent change of ethanol preference ± SE from pretreatment levels in mice receiving acamprosate (ACAMP) or no acamprosate (BLANK) from microimplants in reward areas (VTA, PPT, and NAc) during simulated relapse. aSignificant difference from pretreatment levels (P < 0.05). *Significant difference between treatment groups for a given day (P < 0.05). Bar graphs show means ± SE for the time point of maximal suppression of ethanol preference by acamprosate microimplants in WT vs. mPer2 mutant (PER2 KO) mice. *Significant difference between strains (P < 0.05).
Pedunculopontine tegmental area.
Acamprosate implants in the PPT of mPer2 mutants suppressed ethanol intake and preference from pretreatment levels by 16.5 ± 7.5% and 14.2 ± 3.4%, respectively, during ethanol reintroduction (both, P < 0.01; Figs. 3 and 4). In WTs, this treatment suppressed ethanol intake and preference by 45.6 ± 12.4% and 41.0 ± 3.8%, respectively (both, P < 0.01). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 22.4; preference: F1,22 = 17.2; both, P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both, P > 0.05).
Nucleus accumbens.
Acamprosate implants in the NA of mPer2 mutants did not change ethanol intake or preference from pretreatment levels during ethanol reintroduction (both, P > 0.05; Figs. 3 and 4). In WTs, this treatment suppressed ethanol intake and preference by 27.2 ± 11.2% and 22.0 ± 5.4%, respectively (both, P < 0.01). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 16.3; preference: F1,22 = 18.8; both, P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both, P > 0.05).
Acamprosate Acts Within Circadian Areas to Suppress Ethanol Intake and Preference
Suprachiasmatic nucleus.
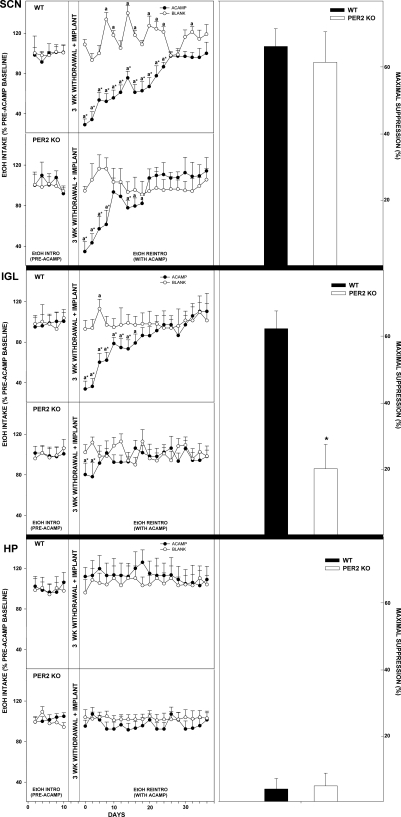
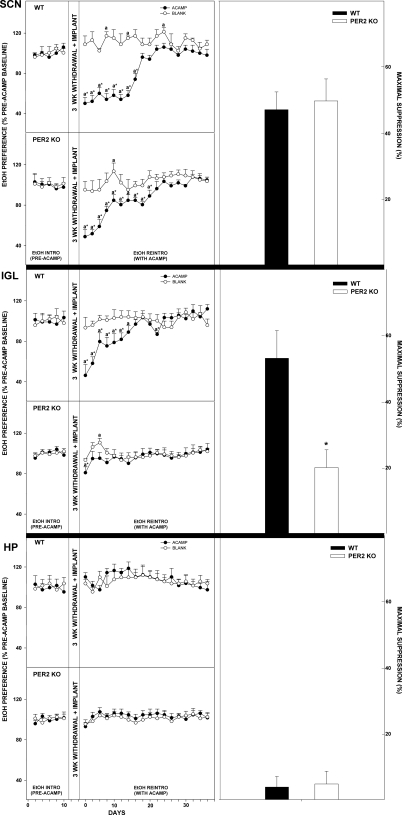
Acamprosate implants in the SCN of mPer2 mutants suppressed ethanol intake and preference from pretreatment levels by 36.3 ± 11.9% and 28.8 ± 4.7%, respectively, during ethanol reintroduction (both, P > 0.05; Figs. 5 and 6). In WTs, this treatment suppressed ethanol intake and preference by 48.7 ± 17.9% and 41.1 ± 5.9%, respectively (both, P < 0.01). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 40.0; preference: F1,22 = 9.4; both, P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both, P > 0.05).
Fig. 5.
Line graphs show percent change of ethanol intake ± SE from pretreatment levels in mice receiving acamprosate (ACAMP) or no acamprosate (BLANK) from microimplants in circadian areas [suprachiasmatic nucleus (SCN), intergeniculate leaflet (IGL), and also hippocampus (HP)] during simulated relapse. aSignificant difference from pretreatment levels (P < 0.05). *Significant difference between treatment groups for a given day (P < 0.05). Bar graphs denote means ± SE for the time point of maximal suppression of ethanol intake by acamprosate microimplants in WT vs. mPer2 mutant (PER2 KO) mice. *Significant difference between strains (P < 0.05).
Fig. 6.
Line graphs show percent change of ethanol preference ± SE from pretreatment levels in mice receiving acamprosate (ACAMP) or no acamprosate (BLANK) from microimplants in circadian areas (SCN, IGL, and HP) during simulated relapse. aSignificant difference from pretreatment levels (P < 0.05). *Significant difference between treatment groups for a given day (P < 0.05). Bar graphs show means ± SE for the time point of maximal suppression of ethanol preference by acamprosate microimplants in WT vs. mPer2 mutant (PER2 KO) mice. *Significant difference between strains (P < 0.05).
Intergeniculate leaflet.
Acamprosate implants in the IGL of mPer2 mutants did not change ethanol intake or preference from pretreatment levels during ethanol reintroduction (both, P > 0.05; Figs. 5 and 6). In WTs, this treatment suppressed ethanol intake and preference by 45.0 ± 19.6% and 28.8 ± 7.8%, respectively (both, P < 0.01). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 22.1; preference: F1,22 = 16.5; both, P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both, P > 0.05).
Acamprosate does not affect ethanol intake and preference in the hippocampus. Acamprosate implants in the hippocampus of mPer2 mutant and WT mice did not change ethanol intake or preference from pretreatment levels during ethanol reintroduction (all P > 0.05; Figs. 5 and 6). Higher values of ethanol intake and preference were observed in mPer2 mutant mice vs. WT mice throughout the acamprosate treatment (intake: F1,22 = 95.4; preference: F1,22 = 67.5; both P < 0.01). Blank implants in mPer2 mutants and WTs did not change ethanol intake or preference from baseline (both P > 0.05).
Effects of acamprosate on water intake.
Pretreatment levels of water intake did not differ between mPer2 mutant and WT mice (96.0+1.8 ml·kg−1·day−1 and 96.2 ± 1.6 ml·kg−1·day−1, respectively; F1,22 = 1.1; P > 0.05). Across all brain areas of implant and strain, there was a main effect for treatment, such that mice with acamprosate implants consumed more water compared with mice with blank implants (123.1 ± 4.4 ml·kg−1·day−1 and 103.8 ± 3.5 ml·kg−1·day−1, respectively; F1,22 = 46.5; P < 0.01). There were significant interactions for brain area × treatment (F5,22 = 14.1), brain area × strain (F5,22 = 17.5), and brain area × treatment × strain (F5,22 = 2.9). Within acamprosate treatment groups, there was a significant effect of strain, such that WT mice consumed more water compared with mPer2 mutants (125.9 ± 4.3 ml·kg−1·day−1 and 112.5 ± 3.3 ml·kg−1·day−1, respectively; F1,10 = 66.5; P < 0.01) and a significant effect for brain area of implant, such that mice with acamprosate implants in reward and circadian brain areas consumed more water compared with mice with acamprosate implants in the hippocampus (132.0 ± 3.1 ml·kg−1·day−1 and 94.8 ± 3.6 ml·kg−1·day−1, respectively; F5,10 = 8.5; P < 0.05, post hoc analyses). Water intake did not differ between blank treatment groups (P > 0.05).
Effects of acamprosate on circadian locomotor activity.
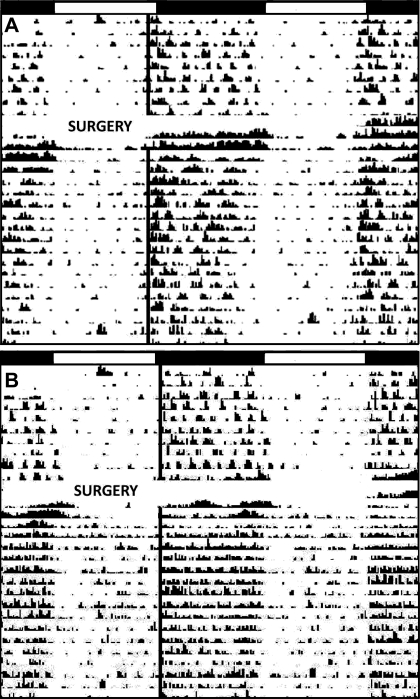
Acamprosate and blank implants in all targeted brain areas of mPer2 mutant and WT mice did not change the phase angle of entrainment to the LD cycle or alpha. However, there was a 1.3-fold increase in the amplitude of nighttime circadian activity rhythms from pretreatment levels in WT mice with acamprosate implants in the VTA and NAc (P < 0.05). Acamprosate implants in the SCN of mPer2 mutant and WT mice increased the amplitude of nighttime circadian activity from pretreatment levels by 1.2- and 2.3-fold, respectively (both P < 0.05; Fig. 7).
Fig. 7.
Representative actogram of a mPer2 mutant and WT mouse prior to and during the release of acamprosate from microimplants targeted to the suprachiasmatic nucleus (A: mPer2 mutant; B: wild-type). White-dark bars represent the light-dark photoperiods. The solid black line represents nighttime activity onset. “Surgery” represents micropellet implantation.
DISCUSSION
Acamprosate has been used clinically since 1989 as a pharmacological agent to help promote abstinence in alcohol-dependent patients. In the majority of clinical trials, this drug was shown to have a statistically significant effect over placebo in controlling relapse, at least during the initial period of abstinence (34, 48). In rodents, acamprosate's overall suppressive effect on ethanol intake is consistent across studies, despite wide differences in drug and/or ethanol treatment regimens. For example, in C57 mice subjected to the drinking-in-the-dark protocol to enhance nighttime ethanol intake, systemic administration of acamprosate (300 mg/kg ip) suppresses nighttime ethanol intake by 20% (26). Also in C57 mice, acamprosate (300 mg/kg ip) reduces overall daily ethanol intake under free-choice by up to 70% (3). In ethanol-preferring clock gene (mPer2) mutant mice, acamprosate (200 mg/kg and 300 mg/kg ip) suppresses free-choice ethanol intake by 60–77% (3, 56). In ethanol-preferring rats, acamprosate (200 mg/kg ip) suppresses ethanol intake by 20–40% (8, 59). Finally, as shown here, brain stimulation with acamprosate-containing microimplants in selected reward and circadian areas of C57 WT and mPer2 mutant mice reduces free-choice ethanol intake during induced relapse by 40–50%. The commonality of effects of acamprosate observed at the behavioral level between humans and rodents suggests that defining the central sites and neurophysiological actions of this drug from basic animal studies is useful for understanding the nature of its clinical action in humans.
Acamprosate Action in Brain Reward Areas
To our knowledge, the present study is the first to explore the effects of centrally administered acamprosate in modulating ethanol drinking behavior. In the first series of experiments, bilateral acamprosate implants were targeted to brain reward areas implicated in modulating ethanol intake and craving. These areas included the VTA and NA of the mesocorticolimbic system and the PPT of the mesopontine system. As revealed in rodent models of alcoholism, lesioning or pharmacologic manipulation of the VTA (18, 20), the NAc (10, 14), or the PPT (52) alters ethanol intake. In our experiments, the most pronounced response to acamprosate was in the VTA and PPT, where acamprosate decreased ethanol intake by 60% and preference by 40% in WT mice. At present, there is little direct neurochemical evidence for an action of acamprosate in these regions. However, such action, at least in the VTA, is plausible given evidence that ethanol activation in this area stimulates dopamine release in the NAc (36), which is also affected by acamprosate via modulation of VTA ACh receptors (8). Also, acamprosate inhibits NMDA (glutamatergic) receptor response in mesencephalic neurons, presumably containing elements of the VTA (1). Further, antagonism of NMDA receptors within the VTA has also been shown to reduce reward-seeking behaviors (31). Finally, acamprosate reduces behavioral ethanol withdrawal (2, 55), ameliorates glutamate neurotoxicity, and therefore, may limit the drive to drink (15, 28, 35) by acting in part through metabotropic glutamate type 5 receptors (mGlu5) (28, 38), which are also present in the VTA (46).
Acamprosate implants in the NAc of WT mice also significantly suppressed ethanol intake and preference (both by ∼20%), but to a lesser extent than in the other reward areas. Nevertheless, this suppression is significant with respect to previous observations that strychnine administration into the NAc of ethanol-preferring rats (to inhibit glycine receptors) completely blocked acamprosate suppression of ethanol intake. Also, previous studies have shown that the administration of acamprosate in the NAc increases levels of accumbal extracellular dopamine (8, 9) and dopamine reuptake transporters (11). Further, acamprosate dampens ethanol and NMDA-induced accumbal dopamine release (6, 44, 57). These results, together with those of the present study, strongly implicate the NAc as a target for acamprosate (8, 9), which is consistent with the role of the NAc in contributing to alcohol intake (43). It is notable that the degree of response to acamprosate in all three reward areas in mPer2 mutants was less robust than in WTs. It is possible that the enhanced drive for ethanol in mPer2 mutants rendered them less susceptible to acamprosate action. This is consistent with our previous study showing that intraperitoneal injection of acamprosate was somewhat less effective in suppressing ethanol intake in mPer2 mutants compared with WTs (3).
It should be noted that the present results do not rule out possible actions of acamprosate in other brain regions involved with ethanol drive and reward. One such area is the striatum/caudate nucleus, particularly since systemic acamprosate reduces extracellular glutamate in the ventral striatum of mPer2 mutant mice (56). Interestingly, one area in which acamprosate did not suppress drinking and preference is the hippocampus. This is surprising since the hippocampus participates in reinstatement of alcohol seeking via operant conditioning, and such seeking can be prevented by the activation of metabotropic glutamate receptors, which are affected by acamprosate (51, 65). Nevertheless, this negative result helps support the issue of anatomical specificity of the acamprosate-releasing implants.
Acamprosate action in brain circadian areas.
In the second series of experiments, acamprosate was targeted to brain sites involved in regulating circadian timing. Recent studies have shown that the circadian timing system regulates ethanol seeking and consumption as reflected in the distinct daily rhythm in ethanol intake that peaks at the beginning and end of the night in rodent models of alcoholism (3, 4, 16, 49, 62) and in the additional circadian phase of ethanol intake that aligns with a 2-h phase-advance in nighttime activity onset in mPer2 mutant vs. wild-type mice (3). This strain difference in circadian entrainment in mPer2 mutant vs. wild-type mice is further noted in this study. In mammals, behavioral and physiological circadian rhythms are generated and maintained by the SCN of the anterior hypothalamus (12, 17, 40). Major photic and nonphotic regulatory inputs to the SCN arise from the retina and the IGL, respectively (7, 21, 22, 26, 30). The circadian phase of alcohol intake could be regulated by signaling from the SCN to the VTA (37) and/or by clock gene activity within the reward areas (47, 63). In view of findings that 1) the SCN clock is directly disrupted by ethanol (4, 5, 45, 49, 50) and 2) the SCN and IGL possess transmitter systems sensitive to acamprosate, including ACh, glutamate, and glycine (29, 41, 58), these circadian regulatory areas represent potential substrates for acamprosate's modulation of ethanol intake. Results from the present study bear out this hypothesis, as acamprosate implants targeted to the SCN and IGL inhibited ethanol intake in WT mice to a similar degree as acamprosate implants in the VTA and PPT. Also, unlike other targeted regions, acamprosate in the SCN suppressed ethanol intake and preference in WTs and mPer2 mutants similarly. It is notable that actogram analysis of circadian locomotor activity revealed that acamprosate suppression of ethanol intake was associated with few effects on circadian rhythm parameters, which were limited to an increase in the nighttime rhythm amplitude, and, more importantly, did not perturb rhythm stability, phase angle of entrainment to the LD cycle, and the duration of nocturnal activity (alpha). This indicates that the suppression of alcohol intake is not a direct consequence of altered circadian timekeeping.
Acamprosate implants: site specificity, release kinetics, tolerance, and postdeprivation rebound.
The constant-release acamprosate microimplants were originally designed to map hypothalamic sites of action of another small molecule, melatonin, in mice (23, 24). The in vitro release profile is similar for acamprosate and melatonin, both having a similar molecular mass (181 and 232 g/mol, respectively). The melatonin implants had differential effects within various hypothalamic nuclei over a 7-wk period (23), and autoradiographic analysis of 3H-melatonin incorporated into the implants showed that spread of label was ∼0.2 mm, indicating reasonable site specificity of implant effect. Site specificity of acamprosate action is highlighted diagrammatically in Fig. 2. In this regard, the extent of acamprosate suppression of ethanol intake and preference in mice with mistargeted acamprosate microimplants was substantially less than in mice that received accurately targeted implants (10–15% vs. 45–60%, respectively).
The in vitro release of acamprosate from the implants is asymptotic, with near-constant output of ∼50 ng·day−1·ml−1 incubation medium apparent by day 7. This concentration (0.3 μM) is similar to that in the cerebrospinal fluid of recovering alcoholic patients taking the medication (0.5–1.5 μM) (64). In the brain, the concentration of acamprosate at the point of release from the implant would likely be considerably higher, probably within the range suitable for modulating activated NMDA receptor activity associated with ethanol withdrawal (13, 42). It is apparent that in the SCN, IGL, and PPT, the suppressive action of the acamprosate began to steadily decrease by ∼8 days of implantation, suggestive of developing drug tolerance, as drug release is constant at this time. In the VTA and NAc, the reduction in response to acamprosate began to occur by ∼20 days. In studies on acamprosate tolerance, the suppressive actions of acamprosate on voluntary ethanol intake were reversed by the second week of daily systemic administration of acamprosate in alcohol-preferring rat strains (11, 53), which is in line with the present observations.
In previous studies (32, 39, 54, 60), it has been observed that repeated episodes of withdrawal induce a postdeprivation elevation in ethanol intake, and promote a more compulsive vs. controlled state of ethanol intake, reminiscent of drinking in relapsed alcoholics. Thus, one methodological limitation of the present study is that is does not discriminate between compulsive vs. controlled ethanol intake (54, 60). Additional studies employing drinkometers with associated measurements of ethanol pharmacokinetics (3, 4, 49) under a long-term protocol of chronic ethanol intake and withdrawal will be important to determine whether acamprosate in circadian and reward areas suppresses compulsive and/or controlled ethanol intake.
Perspectives and Significance
In summary, this study presents the first demonstration of brain sites responsive to the suppressive effect of acamprosate on ethanol intake and preference. Specifically, these experiments have revealed that reward and circadian brain areas are responsive to acamprosate and that acamprosate's actions are modulated by the mPer2 clock gene. Thus, genetic and/or other factors that contribute to a higher drive for ethanol intake could affect the therapeutic efficacy of acamprosate treatment.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA-015948 and AA-017898 to R. A. Prosser and J. D. Glass.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Rainer Spanagel for his generous gift of acamprosate.
REFERENCES
- 1. Allgaier C, Franke H, Sobottka H, Scheibler P. Acamprosate inhibits Ca2+ influx mediated by NMDA receptors and voltage-sensitive Ca2+ channels in cultured rat mesencephalic neurones. Naunyn Schmiedebergs Arch Pharmacol 362: 440–443, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence, and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol 11: 775–793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiol Int In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and impairs daily locomotor activity in the mouse. Alcohol Clin Exp Res 34: 1266–1273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol impairs photic and serotonergic phase-resetting in the mouse. Alcohol Clin Exp Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cano-Cebrián MJ, Zornoza-Sabina T, Guerri C, Polache A, Granero L. Local acamprosate modulates dopamine release in the rat nucleus accumbens through NMDA receptors: an in vivo microdialysis study. Naunyn Schmiedebergs Arch Pharmacol 367: 119–125, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Card JP, Moore RY. Ventral lateral geniculate nucleus efferent to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J Comp Neurol 206: 390–396, 1982 [DOI] [PubMed] [Google Scholar]
- 8. Chau P, Lidö-Hölfödt H, Löf E, Söderpalm B, Ericson M. Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res 34: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Chau P, Stomberg R, Fagerberg A, Söderpalm B, Ericson M. Glycine receptors involved in acamprosate's modulation of accumbal dopamine levels: an in vivo microdialysis study. Alcohol Clin Exp Res 34: 32–38, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28: 2288–2298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowen MS, Adams C, Kraehenbuehl T, Vengeliene V, Lawrence AJ. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict Biol 10: 233–242, 2005 [DOI] [PubMed] [Google Scholar]
- 12. DeCoursey PJ, Krulas JR, Mele G, Holley DC. Circadian performance of suprachiasmatic nuclei (SCN)-lesioned antelope ground squirrels in a desert enclosure. Physiol Behav 62: 1099–1108, 1997 [DOI] [PubMed] [Google Scholar]
- 13. De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate. CNS Drugs 19: 517–537, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav 92: 335–42, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int 37: 509–533, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci USA 81: 3543–3546, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edgar DM, Dement WC, Fuller CA. Effects of SCN lesions on sleep and wake in squirrel monkeys: evidence for an opponent processes of sleep-wake regulation. J Neurosci 13: 1065–79, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358: 189–196, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed New York: Elsevier [Google Scholar]
- 20. Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348: 201–203, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Glass JD, Grossman JH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci 23: 7451–7460, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glass JD, Guinn J, Kaur G, Francl JM. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur J Neurosci 32: 1117–1126, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Glass JD, Lynch GR. Evidence for a brain site of melatonin action in the white-footed mouse, Peromyscus leucopus. Neuroendocrinology 34: 1–6, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Glass JD, Lynch GR. Melatonin: identification of sites of antigonadal action in mouse brain. Science 214: 821–823, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Gorwood P, Schumann G, Treutlein J, Adès J. Pharmacogenetics of alcohol-dependence. In: Gorwood P, Hamon M. (eds.). Psychopharmacogenetics. Springer: New York, pp 177–201, 2006 [Google Scholar]
- 26. Grossman GH, Farnbauch L, Glass JD. Regulation of serotonin release in the Syrian hamster intergeniculate leaflet region. Neuroreport 15: 103–106, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol drinking-in-the-dark in male C57BL/6J mice. Alcohol Clin Exp Res 32: 1992–1998, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res 26: 1779–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Hartgraves MD, Fuchs JL. NMDA receptor binding in rodent suprachiasmatic nucleus. Brain Res 640: 113–118, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Johnson RF, Moore RY, Morin LP. Lateral geniculate lesions alter circadian activity rhythms in the hamster. Brain Res Bull 22: 411–422, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 40: 119–126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koob GF, Roberts AJ, Schulteis G, Parson LH, Heyser CJ, Hyytiä P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22: 3–9, 1998 [PubMed] [Google Scholar]
- 34. Kranzler HR, Gage A. Acamprosate efficacy in alcohol-dependent partients: summary of results from three pivotal trials. Am J Addict 17: 70–76, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyld-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther 99: 79–94, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev 27: 713–20, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci 29: 748–760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res 32: 1105–1110, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res 30: 2017–2025, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Moore RY. Organization and function of a central nervous system oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc 42: 2783–2789, 1983 [PubMed] [Google Scholar]
- 41. Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett 150: 112–116, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res 22: 802–809, 1998 [PubMed] [Google Scholar]
- 43. Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA 108: 4459–64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol 437: 55–61, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience 152: 837–848, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Renoldi G, Calcagno E, Borsini F, Invernizzi RW. Stimulation of group I mGlu receptors in the ventrotegmental area enhances extracellular dopamine in the rat medial prefrontal cortex. J Neurochem 100: 1658–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev 38: 1249–1255, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev 8: CD004332, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol disrupts circadian behavior and photic phase-resetting in the hamster. Am J Physiol Regul Integr Comp Physiol 297: R729–R737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol 296: R411–R418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral spetum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology 165: 296–305, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Samson HH, Chappell A. Injected muscimol in pedunculopontine tegmental nucleus alters ethanol self-administration. Alcohol 23: 41–8, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci 29: 109–115, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol 34: 231–243, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W. Acamprosate and alcohol: I. effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 305: 39–44, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene mPer2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci 22: 521–527, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Stamp JA, Piggins HD, Rusak B, Semba K. Distribution of ionotropic glutamate receptor subunit immunoreactivity in the suprachiasmatic nucleus and intergeniculate leaflet of the hamster. Brain Res 756: 215–224, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Stromberg MF, Mackler SA, Volpicelli JR, O'Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol 23: 109–116, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addict Biol 14: 384–396, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Vrang N, Mrosovsky N, Mikkelsen JD. Afferent projections to the hamster intergeniculate leaflet demonstrated by retrograde and anterograde tracing. Brain Res Bull 59: 267–288, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. Alcohol Res Health 25: 94–100, 2001 [PMC free article] [PubMed] [Google Scholar]
- 63. Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms 24: 465–76, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Wilde MI, Wagstaff Acamprosate AJ. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs 53: 1038–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdale. J Neurosci 26: 9967–74, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zornoza T, Cano M, Polache A, Granero L. Pharmacology of acamprosate: an overview. CNS Drug Rev 9: 359–374, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]