Abstract
Roux-en-Y gastric bypass is the most effective therapy for morbid obesity. This study investigated how gastric bypass affects intake of and preference for high-fat food in an experimental (rat) study and within a trial setting (human). Proportion of dietary fat in gastric bypass patients was significantly lower 6 yr after surgery compared with patients after vertical-banded gastroplasty (P = 0.046). Gastric bypass reduced total fat and caloric intake (P < 0.001) and increased standard low-fat chow consumption compared with sham controls (P < 0.001) in rats. Compared with sham-operated rats, gastric bypass rats displayed much lower preferences for Intralipid concentrations > 0.5% in an ascending concentration series (0.005%, 0.01%, 0.05%, 0.1%, 0.5%, 1%, 5%) of two-bottle preference tests (P = 0.005). This effect was demonstrated 10 and 200 days after surgery. However, there was no difference in appetitive or consummatory behavior in the brief access test between the two groups (P = 0.71) using similar Intralipid concentrations (0.005% through 5%). Levels of glucagon-like peptide-1 (GLP-1) were increased after gastric bypass as expected. An oral gavage of 1 ml corn oil after saccharin ingestion in gastric bypass rats induced a conditioned taste aversion. These findings suggest that changes in fat preference may contribute to long-term maintained weight loss after gastric bypass. Postingestive effects of high-fat nutrients resulting in conditioned taste aversion may partially explain this observation; the role of GLP-1 in mediating postprandial responses after gastric bypass requires further investigation.
Keywords: food preference, taste, aversion, GLP-1, glucagon-like peptide-1
the epidemic of obesity causes significant harm to patients and puts an enormous burden on public healthcare systems (2). Lifestyle and available pharmacotherapy do not sufficiently resolve obesity-related comorbidities to offer a benefit in mortality or morbidity (16, 27). Gastric bypass surgery reduces body weight and provides maintained long-term weight loss. It offers the resolution of obesity-related comorbidities (such as type 2 diabetes mellitus) and a reduction in obesity-related mortality (1, 27, 28). Mechanisms by which gastric bypass causes weight loss are not fully understood, but include reduced hunger (5), increased satiety (5), increased energy expenditure (9, 32), and altered taste (10, 23, 34), as well as reduced preference for foods with a high-fat and sugar content (12, 21, 25, 37).
Patients reach satiety earlier after gastric bypass surgery (14) and report a reduced desire to consume fatty food as they no longer find it enjoyable (14). Two studies reported that total fat intake is lower after gastric bypass partly because of a reported disinterest in desserts and ice cream (6, 7). However, the same authors failed to find a consistent change in pre- vs. postoperative percentage fat intake (6, 7). A randomized controlled trial comparing gastric bypass and vertical-banded gastroplasty confirmed a reduced intake of high-fat foods 1 yr after gastric bypass (21). Similarly, in a rat model, gastric bypass appears to decrease affective responses to corn oil and sucrose. For example, it has been reported that following gastric bypass, rats display significantly fewer positive oromotor taste reactivity responses to 1.0 M sucrose (25). Furthermore, after gastric bypass, rats decrease their licking to high concentrations of sucrose (12, 25, 37) and corn oil (25). Given that taste plays a large role in food selection, these findings implicate some involvement of the gustatory system.
Three categories of taste processing have been described (30): First, stimulus identification (sensory) is the detection or discrimination of sensory signals arising from taste cell activation. Second, ingestive motivation (reward/aversion) involves processes receiving gustatory input that promote or discourage ingestion. Third, digestive preparation refers to taste-triggered physiological reflexes (e.g., salivation) that protect oral tissues, aid digestion and assimilation, and facilitate homeostasis. Affective responses to taste stimuli, which can be considered an example of ingestive motivation, can be both conditioned and unconditioned. It remains unclear which of these three domains might be involved in changes in food preference after gastric bypass surgery. For example, gastric bypass surgery could potentially act directly on central gustatory pathways associated with feeding and reward through gut hormonal intermediaries. Alternatively, gastric bypass could also alter the sensory signal in such a way that the intensity or the quality was changed. This, in turn, could lead to an unconditioned change in palatability. Finally, if gastric bypass causes visceral malaise consequential to ingestion of fat stimuli, then it is possible that the palatability of fat stimuli could change through a learning process. In fact, gastric bypass has been shown to increase postprandial levels of gut hormones including peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) (9, 17, 18, 25); peripheral PYY and GLP-1 administration in mice activates neurons in brainstem areas that have been suggested to mediate effects of certain classes of aversive stimuli (3, 13) and have been shown to be effective at conditioning taste aversions (24, 33). These potential mechanisms underlying the changes toward an altered fat preference after gastric bypass are not mutually exclusive.
In this study, we investigated changes in fat preference and intake after gastric bypass in obese humans and rats weighing between 350 and 500 g. We used data from a randomized controlled trial between gastric bypass and vertical-banded gastroplasty to establish the importance of the phenomenon in humans. We also used an established rat model to assess fat preference because it allows us greater latitude in behavioral, endocrine, and molecular measurements while providing a logical bridge with reports of changes in human taste preference following surgery. Thus, the aims of our study were first, to evaluate human patients 6 yr after being randomized to gastric bypass or vertical-banded gastroplasty and, second, to use a rat model to further investigate 1) preference for solid high-fat versus low-fat chow, 2) preference for increasing fat concentrations in a liquid preparation early and late after gastric bypass, 3) licking responses to increasing fat concentrations in a liquid preparation in a brief access test that minimizes postingestive consequences, and 4) whether reduced preference for fat might be due to induction of conditioned taste aversion.
MATERIALS AND METHODS
Humans
In this study, 16 patients (11 female) were included from a prospective clinical trial which randomized patients to gastric bypass and vertical-banded gastroplasty during 2000–2001 (22). Between 12/2006 and 06/2007, nine gastric bypass and seven vertical-banded gastroplasty patients were included at an average of 6 yr after surgery (range 5.8–6.8 yr). The study protocol was approved by the local ethics committee of the University of Gothenburg (reference no. 359–09) and the study was conducted according to the principles of the Helsinki declaration. All participants gave their written permission to participate in the study.
Both operations were performed laparoscopically as described previously (22). The validated Swedish Obese Subjects study questionnaire was used for dietary assessment (20). The questionnaires included 49 questions on ordinary food consumption patterns during the past 3 mo, with the emphasis on portion size and day of week. Amounts of snack foods and sweets or candies were quantified using sizes for preconfectioned packages as sold in Sweden. Bread-type, thickness, and contents of sandwiches were described in detail, owing to the large contribution of sandwiches in the Swedish diet. The amounts of food reported by the subjects were converted into grams from which daily intake of energy and 29 different nutrients were computed. In addition, a short questionnaire form was used to explore whether the patient avoided certain foods. Included were direct questions (e.g., Do you eat whole meat?) and open questions (e.g., Do you avoid eating any foods? Why?).
Animals
Male Wistar rats (Harlan Laboratories, Blackthorn, UK; Elevage Janvier, Le-Genest-St. Isle, France) weighing between 350 and 500 g were individually housed under a 12:12-h light-dark cycle at a room temperature of 21 ± 2°C. Water and standard chow were available ad libitum, unless otherwise stated. All experiments were performed under a license issued by the Home Office, UK (no. PL70-6669) or approved by the Veterinary Office of the Canton, Zurich, Switzerland. Numbers and preoperative age of animals, grouping details and postoperative time of investigation for each experiment are outlined in Table 1.
Table 1.
Numbers, grouping, age of animals and approximate time of investigation for each experiment as used in the studies
| Groups Compared |
|||||
|---|---|---|---|---|---|
| Experiment | Animal Number | Preoperative Age, wk | Approximate Time of Investigation* | Gastric Bypass | Sham |
| Hormone assay | 18 | 10 | 200 | 9 | 9 |
| Food preference study for chow | 26 | 8 | 10 | 13 | 13 |
| Early 2- bottle preference test | 30 | 8 | 10 | 18 | 12 |
| Late 2-bottle preference test | 20 | 10 | 200 | 10 | 10 |
| Brief access test | 16 | 10 | 150 | 8 | 8 |
| Conditioned taste aversion to corn oil | 38 | 10 | 100 | 16 | 22 |
Time of investigation is days after surgery.
Surgery
After 1 wk of acclimatization, rats were randomized to gastric bypass or sham operation. Surgery was performed by one surgeon (M. Bueter) according to an established protocol as previously described (8, 9). Briefly, the gastric bypass procedure started with identification of the pylorus. From here, the duodenum and proximal jejunum was followed 20 cm aborally where the bowel was transected and closed creating the distal end of the future biliopancreatic limb and the proximal end of the future alimentary limb. Subsequently, the cecum was identified, and the ileum was followed orally and connected in side-to-side fashion to the distal end of the biliopancreatic limb, leaving a common channel of 25–30 cm. Creation of the gastric pouch started then with stomach transection close to the gastroesophageal junction with preservation of vagal fibers in the dorsal vagal trunk as previously described (8). The gastric remnant was closed, and the gastric pouch was anastomosed in end-to-side fashion to the proximal end of the alimentary limb with a length of ∼50 cm. Sham operations consisted of an anterior gastrotomy and a jejunotomy about 25 cm proximal to the cecum with subsequent closure. The complete gastric bypass procedure lasted ∼70 min, while sham operations took ∼30 min. After both operations the abdominal wall and the skin were closed in layers. All rats were given a 10-day period to completely recover from surgical trauma prior to further testing. Body weight and food intake were monitored daily during the complete observation period.
Hormone Assay
Rats were fasted for 12 h from the beginning of the light cycle about 200 days after surgery. At the onset of the dark cycle, animals were offered 5 g of standard chow, all of which was consumed within half an hour by the animals. Blood was then obtained by puncture of a sublingual vein under brief isoflurane anesthesia from sham-operated controls (n = 9) and gastric bypass rats (n = 9). Blood was collected into EDTA-rinsed tubes and immediately centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was stored at −80°C until further analysis. Concentrations of active GLP-1 and PYY were analyzed using a rat endocrine lincoplex kit (model RENDO-85K; Labodia, Yens, Switzerland).
Food Preference Study for Chow
A food preference test was conducted prior to surgery with 26 male Wistar rats aged 8 wk. Food was offered in three equal compartments that were filled with 30 g of the following three food choices: 60% fat diet (cat. no. D12492, energy content: 23.9 kJ/g; Research Diets), the same 60% fat diet with added Bisto (gravy-type flavor), and normal chow with 2% fat (14.7 kJ/g RM1 diet; Special Diet Services, UK). Bisto was added to one section of the high-fat chow for the rats to differentiate the taste and smell from the other high-fat chow in the next session. The three diet options thus contained three distinct flavors and two different calorie densities. Food intake was recorded after 24 h intervals over 2 days by weighing the food at the end of the dark cycle. Rats were then randomized to bypass (n = 13) or sham operations (n = 13) for baseline measurements; the effect of surgery on high-fat versus low-fat intake was tested ∼10 days after surgery in the same animals.
Two-Bottle Preference Test
Intralipid (Fresenius Kabi, UK) is a fat emulsion used for parenteral nutrition in malnourished patients. The emulsion consists of soy bean oil, egg phospholipids, glycerin, omega-6 essential fatty acids, α-linoleic acid, and linolenic acid. We diluted the standard 20% Intralipid solution with deionized water to provide seven concentrations (0.005%, 0.01%, 0.05%, 0.1%, 0.5%, 1%, 5%) for this study. All solutions were freshly prepared daily with deionized water and presented at room temperature.
Rats were presented with two preweighed bottles, one of which contained deionized water and the other of which contained Intralipid solution in ascending concentrations. Readings were recorded at the start of the light phase by reweighing the bottles. Positions and content of the bottles were changed 1 h after the start of the light phase, and bottles were then weighed 24 h thereafter. Rats were given access to the same concentration for 2 days and the positions of the bottles were switched each day to preclude the development of a side preference. To control for spillage during the manipulation of the bottles, two additional bottles were placed in cages without animals, and daily measurements were obtained. The average amount of spillage (0.69 ± 0.04 ml) was subtracted from measured volumes of distilled water and Intralipid intake before further analysis.
Each animal was tested for 14 days (7 × 2-day periods). Intralipid preference for each 24-h period was defined as: [Intake of Intralipid (in ml)/total fluid intake (ml)] × 100. Two-bottle preference tests were performed early after surgery (10 days) and late after surgery (200 days). In the early phase experiment 10 days after surgery, two groups of fat-taste naive rats aged 8 wk were used. Twelve sham-operated controls and 18 gastric bypass rats were subjected to the two-bottle preference test as described above. Preference (%) and Intralipid intake (ml), as well as caloric intake (Intralipid plus chow; kJ), were measured daily. In the late phase experiment 200 days after surgery, another group of 10 sham-operated controls and 10 gastric bypass rats were subjected to the same two-bottle preference test as described above.
Brief Access Test
Sixteen male Wistar rats that were naive to the taste of Intralipid were tested in a lickometer (model Davis MS-160; DiLog Instruments, Tallahassee, FL) after being randomized to sham or gastric bypass operation (each group, n = 8). The brief access test procedure was conducted by placing the rat in the test chamber of the apparatus. A motorized shutter opened allowing the rat access to a single sipper tube containing Intralipid. A small fan, positioned above the sample slot directed a current of air past the drinking spout to minimize potential olfactory cues from the Intralipid. Rats initiated a trial by licking the spout. Once initiated, each trial was 10 s, followed by a 7.5-s intertrial interval during which time the tube was changed via a motorized block for the next trial. A concentration-response function was derived in three test sessions of 30 min each during which rats were able to initiate as many trials as possible (29). The briefness of the test as suggested by its name allows the minimization of postingestive effects during a given trial as only small amounts are ingested and immediate responses are measured.
Before being tested for Intralipid, all rats underwent 4 days of water training (adapted from Ref. 15). Briefly, after 23 h of water deprivation, the rats were placed in the Davis Rig for 30 min during the lights-on phase. Rats had access to a single tube of water for 10 s to familiarize themselves with the shutter operation and 10-s trial structure. Each trial started upon the first lick of the spout after the shutter opened. After each presentation, the shutter closed for 7.5 s (intertrial interval) before being reopened for the next trial. Tubes were presented in randomized order (without replacement) within blocks. To allow euhydration, rats had unlimited access to water for 45 min from 1300 to 1345 (dark onset) before water deprivation started again for the next training day.
The same paradigm was used for the Intralipid tests. Bottles were filled with the same seven Intralipid concentrations as in the two-bottle preference tests; deionized water was used as control. All bottles were presented in randomized order (without replacement) in blocks of trials. Rats were first tested with water (and food) available ad libitum (except during the test session) and then on a 23-h restricted, water-access schedule for three daily sessions every other day in two subsequent weeks.
Conditioned Taste Aversion Against Corn Oil
Thirty-eight experimentally naive male Wistar rats aged 10 wk were randomized to gastric bypass (n = 16) or sham operation (n = 22). After 100 days following surgery, rats were individually housed for 1 wk with ad libitum access to food and water before they underwent the conditioned taste aversion experiment. Rats were slightly sedated by brief exposure to isoflurane before the oral gavage with corn oil or saline. The five groups included gastric bypass rats receiving saline (n = 8), gastric bypass rats receiving corn oil (n = 8), sham-operated rats receiving saline (n = 8), sham-operated rats receiving corn oil (n = 8), and sham-operated rats receiving intraperitoneal lithium chloride as a positive control (76.2 mg/kg body wt ip) (n = 6). At the beginning of the experiment, water was withdrawn for all animals at the start of the dark phase (day 0). To acclimate the rats to weighing and timing of distilled water presentation, the animals were presented with two water bottles (volume 100 ml) at the onset of the light phase from day 1 until day 4 for 30 min and 4 h later for another 45-min period. At the end of presentation, water bottles and rats were weighed. Individual water consumption from each bottle was measured for each rat every day. On day 5 each rat was given 30-min access to the conditioned stimulus (novel flavor of 0.3% solution of saccharin sodium salt hydrate) contained in both bottles at the onset of the light phase. All groups consumed similar amounts of saccharin solution during the 30-min access prior to the various treatments. Following the access to the saccharin, rats were weighed and received either an oral gavage of 1 ml corn oil, 1 ml sterile isotonic saline or an intraperitoneal injection of lithium chloride. The small volume of 1 ml for oral gavage was chosen to minimize potential side effects by the administered volume per se, considering the altered anatomy of the stomach in gastric bypass rats. Corn oil was consciously chosen since the fat content of 1 ml corn oil corresponds approximately with the amount of fat that is ingested with a standard small meal of a 60% high-fat diet. In other words, 1 ml corn oil contains ∼1 g fat, which is similar to the fat content of a normal, small meal of 2 g of a 60% high-fat diet. This analogy would not have been possible if we had used Intralipid as we would have had to give ∼20 ml of a 5% Intralipid solution to administer 1 g of fat. Rats were offered water 4 h after light onset for a 45-min period.
Intralipid
The same protocol was repeated on day 8 and day 11. On all other days, rats were given access to water for 30 min at the onset of the light phase and 4 h later for another 45 min as described above (washout period). On day 14 each rat was presented with two bottles, one of which contained water and the other 0.3% saccharin solution, and the respective consumption was measured for 30 min at light onset.
Statistical Analysis
Data are expressed as means ± SE. Preference, acceptance, food intake, and energy intake in the two-bottle preference tests were analyzed with a two-way group (between subjects) × concentration (within subjects) ANOVA. A one-way ANOVA followed by Bonferroni post hoc tests for each concentration was applied when there was a significant group × concentration interaction. In the brief access test, the mean number of licks at each concentration per trial was collapsed across the three test sessions. For each rat, the mean number of licks to water was subtracted from the mean number of licks at each concentration, yielding a licks-to-intralipid minus licks-to-water value. This measure has also been successfully used in previous studies (15, 31) to produce concentration-response curves that are relative to a water baseline. The lick response (adjusted for water) for each concentration of a stimulus was compared using ANOVAs. Otherwise, Student's t-test was used to test for significant differences between independent samples. The statistical rejection criterion of P < 0.05 was used for all analyses.
RESULTS
Food Preference in Humans
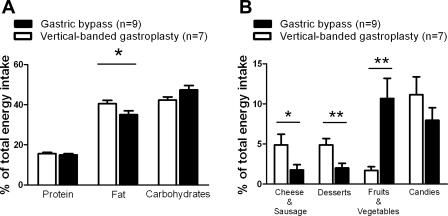
Six years after surgery, gastric bypass patients had reduced their body mass index by 26.5 ± 2.9%, while vertical gastroplasty patients had lowered their body mass index by 17.8 ± 2.5% (P = 0.042). However, the reported energy intake between the two groups remained similar at 6 yr after surgery (preoperative gastroplasty: 12,770 ± 1,484 kJ vs. bypass: 10,686 ± 919 kJ, P = 0.26 and postoperative gastroplasty: 11,952 ± 1,082 kJ vs. bypass: 9,726 ± 769 kJ, P = 0.11). While there was no difference in percentage of energy intake from fat between the two groups before surgery (gastroplasty: 33.6 ± 1.0% vs. bypass: 34.4 ± 1.4%, P = 0.26), gastric bypass patients consumed less energy from fat compared with vertical-banded gastroplasty patients 1 yr after surgery (gastroplasty: 35.2 ± 6.3% vs. bypass: 30.5 ± 5.5%, P = 0.001) (21) and 6 yr after surgery (gastroplasty: 40.5 ± 1.6% vs. 35.0 ± 1.9%, P = 0.046). Proportions of total energy intake from protein, fat, and carbohydrates 6 yr after surgery are shown in Fig. 1A. There was no difference in the proportion of calories from carbohydrates (P = 0.09) or proteins (P = 0.48) compared with vertical-banded gastroplasty patients. As shown in Fig. 1B, gastroplasty patients reported a higher proportion of their total energy intake from foods high in fat [e.g., cheese and sausages (P = 0.041) and desserts (P = 0.007)] compared with bypass patients, who instead reported a higher relative intake from fruits and vegetables (P = 0.004).
Fig. 1.
The proportion of total energy intake from protein, fat, and carbohydrates (A) and from various food groups (B) 6 years after laparoscopic gastric bypass (black columns) and laparoscopic vertical-banded gastroplasty (white columns). Data are shown as means ± SE (*P < 0.05, **P < 0.01).
Food Preference in Rats
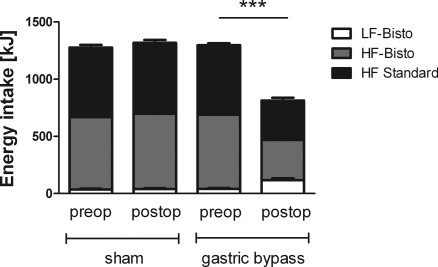
Mean preoperative body weight of rats used in this study was 385 ± 8 g. After a short period of postsurgical weight loss, body weight increased in sham-operated rats to 428 ± 12 g on postoperative day 10, and it increased further for the rest of the observation period. In contrast, gastric bypass animals lost 13.8 ± 3.0% of their preoperative weight by postoperative day 10 (319 ± 8 g); body weight then leveled off to ∼320 g. Figure 2 shows intake of the three types of diet before and after surgery. There was no difference in total 48-h energy intake before and after sham operation (1,277 ± 115 kJ vs. 1,318 ± 102 kJ, P = 0.35); gastric bypass rats significantly reduced their 48-h energy intake on day 10 after surgery (1,297 ± 92 kJ vs. 813 ± 202 kJ, P < 0.001). Sham-operated rats consumed similar proportions of the three food choices before and after surgery (high-fat: 609 ± 82 kJ before vs. 621 ± 89 kJ after surgery, P = 0.72; Bisto-flavored high-fat plus Bisto: 633 ± 91 kJ vs. 658 ± 93 kJ, P = 0.48; normal chow: 36 ± 27 kJ vs. 39 ± 26 kJ, P = 0.75). Gastric bypass rats significantly reduced their energy intake of the two high-fat diets (high-fat: 607.4 ± 62.1 kJ vs. 344 ± 89 kJ, P < 0.001; Bisto-flavored high-fat: 649 ± 105 kJ vs. 352 ± 108 kJ, P < 0.001), while they significantly increased their intake of the normal chow (normal chow: 41 ± 26 kJ vs. 117 ± 63 kJ, P < 0.001). The rats with gastric bypass increased their normal chow intake from 3.2 ± 2.1% to 14.0 ± 6.6% (P < 0.001) of total energy intake.
Fig. 2.
Energy (kJ) from 60% high-fat (HF) diet, of 60% HF diet + Bisto (gravy-type flavor) and normal chow (LF-Bistro) in gastric bypass rats (n = 13) and sham-operated rats (n = 13) before and 10 days after surgery. LF, low fat. Data are means ± SE (***P < 0.001: total energy intake preoperative vs. postoperative after gastric bypass).
Two-Bottle Preference Test
Body weight.
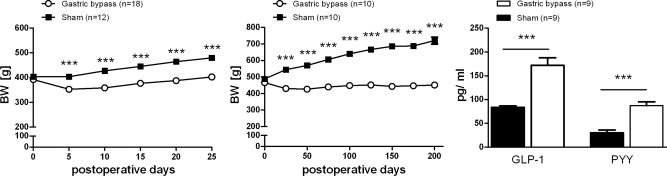
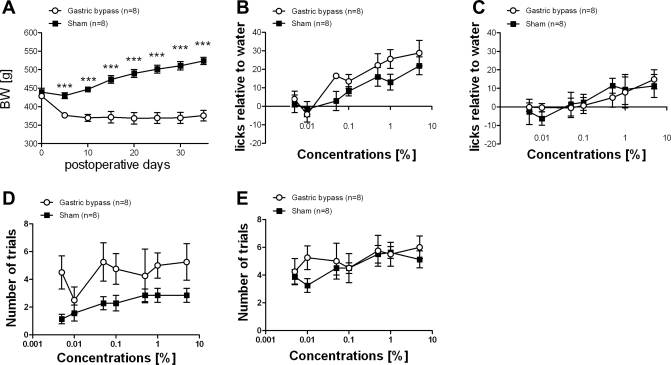
Average presurgical body weight of rats used for the early phase experiment was 368 ± 2 g. Ten days after surgery, sham-operated controls weighed significantly more compared with gastric bypass rats (sham: 404 ± 6 g vs. bypass: 353 ± 6 g, P < 0.001). Average presurgical body weight of rats used for the late phase experiment was 477 ± 4 g; 200 days after surgery sham-operated rats had a significantly higher body weight than gastric bypass rats (sham: 713 ± 11 g vs. bypass: 456 ± 14 g, P < 0.001). Body weight changes for both groups are shown in Fig. 3, A and B.
Fig. 3.
Body weight (BW) changes for the gastric bypass (n = 18, ○) and sham-operated rats ad libitum fed (n = 12, ■) used for the 2-bottle preference test in the early phase (left) and body weight changes for the gastric bypass (n = 10, ○) and sham-operated rats ad libitum fed (n = 10, ■) used for the 2-bottle preference test in the late phase (middle) after surgery. Right: PYY and GLP-1 level for the gastric bypass (n = 9, white columns) and sham-operated rats ad libitum fed (n = 10, black columns) 200 days after surgery. Data are means ± SE (***P < 0.001).
Postprandial plasma levels of PYY and active GLP-1.
Gastric bypass rats had significantly higher active GLP-1 and PYY levels compared with sham-operated controls measured 30 min after the 5-g test meal (Fig. 3C).
Preference.
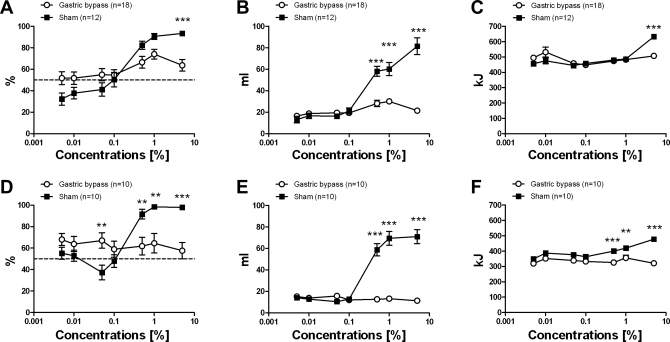
A two-way ANOVA revealed a significant main effect of Intralipid concentration [F(6,405) = 19.9; P < 0.001], but not of surgical group [F(1,405) = 0.28; P = 0.59]. However, the group × concentration interaction was also significant [F(6,405) = 6.04; P < 0.001]. Ten days after surgery, both sham-operated rats and gastric bypass rats showed a significant increase in preference (Intralipid vs. total intake) for Intralipid at concentrations above 0.1% (one-way ANOVA: sham: P < 0.001; bypass: P = 0.024). However, preference for the Intralipid solutions was found to be markedly less pronounced in gastric bypass rats (Fig. 4A).
Fig. 4.
Two-bottle preference test in sham-operated rats (n = 12, ■) and in gastric bypass rats (n = 18, ○) during the early phase experiment (postoperative day 10, A–C) and in gastric bypass (n = 10, ○) and sham-operated rats (n = 10, ■) during the late phase experiment (postoperative day 200, D–F). Seven Intralipid concentrations were used in ascending order: 0.005%, 0.01%, 0.05%, 0.1%, 0.5%, 1%, 5%. A and D, preference; B and E, Intralipid intake; C and F, total calorie intake. Data are means ± SE. When 2-way ANOVA revealed a significant group × concentration interaction, post hoc Bonferroni test was used for concentration-to-concentration analysis between the 2 groups (**P < 0.01, ***P < 0.001).
Observations were similar in the late phase of the weight stabilization study. The two-way ANOVA showed a significant main effect of Intralipid concentration [F(6,266) = 7.73; P < 0.001], but not of surgical group [F(1,266) = 2.80; P = 0.12], while the group × concentration interaction was significant [F(6,266) = 9.93; P < 0.001]. On postoperative day 200 sham-operated rats had a higher preference for Intralipid concentrations above 0.1%, while gastric bypass rats showed no preference for high concentrations of Intralipid (one-way ANOVA: sham: P < 0.001; bypass: P = 0.95) (Fig. 4D).
Intralipid intake.
There was a significant main effect of Intralipid concentration [F(6,405) = 44.30; P < 0.001] and of surgical group [F(21, 405) = 81.94; P < 0.001] on intralipid intake during the two-bottle tests. The group × concentration interaction was also significant [F(6,405) = 26.82; P < 0.001]. During the early weight stabilization phase both sham-operated rats and gastric bypass rats demonstrated significantly increased Intralipid intake (in ml) at concentrations above 0.1% (one-way ANOVA: sham: P < 0.001; bypass: P < 0.001) (Fig. 4B). However, the increase in Intralipid intake was found to be markedly less pronounced in gastric bypass rats. In the late phase of weight stabilization two-way ANOVA revealed a significant main effect of Intralipid concentration [F(6,266) = 36.31; P < 0.001] and of surgical group [F(1,266) = 158.89; P < 0.001]. The interaction was significant [F(6,266) = 41.60; P < 0.001]. Sham-operated rats showed a significantly increased Intralipid intake (in ml) for concentrations above 0.1% (one-way ANOVA: P < 0.001), while intake did not increase in gastric bypass rats as concentration was raised (one-way ANOVA: P = 0.51) (Fig. 4E).
Calorie intake.
Total calorie intake was the sum of calories consumed as food (14.74 kJ/g) and Intralipid (energy content of the standard 20% solution: 42.0 kJ/ml). There was a significant main effect of Intralipid concentration [F(6,405) = 11.83; P < 0.001], but not of surgical group [F(1,405) = 0.35; P = 0.56] in the two-way ANOVA. The group × concentration interaction was also significant [F(6,405) = 6.30; P < 0.001]. During the early weight stabilization phase sham-operated rats increased their calorie intake when exposed to the 5% Intralipid solution (one-way ANOVA: P < 0.001), although this was not demonstrated in the gastric bypass group (Fig. 4C). During the late weight stabilization phase, the main effects of Intralipid concentration [F(6,266) = 5.79; P < 0.001] and of surgical group [F(1,266) = 75.53; P < 0.001] were significant in the two-way ANOVA. The group × concentration interaction was also significant [F(6,266) = 5.91; P < 0.001]. Sham-operated rats increased their energy intake when exposed to the 0.5%, 1%, and 5% Intralipid solutions compared with gastric bypass rats (one-way ANOVA: P < 0.001), which showed no increase in energy intake even with the highest Intralipid concentration (5%) (one-way ANOVA: P = 0.48) (Fig. 4F).
Brief Access Test
Body weight.
Average presurgical body weight of rats used for the brief access test was 434 ± 6 g. From postoperative day 5 sham-operated controls weighed significantly more compared with gastric bypass rats (postop day 5: sham: 430 ± 8 g vs. bypass: 377 ± 7 g, P < 0.001). Body weight changes for both groups are shown in Fig. 5A.
Fig. 5.
A: body weight changes for the gastric bypass (n = 8, ○) and sham-operated rats fed ad libitum (n = 8, ■) and used for the brief access test performed around postoperative day 150. B and C: postoperative Intralipid concentration-response functions relative to a water baseline are shown without (B) and with 23-h water restriction (C). D and E: absolute number of initiated trials for each Intralipid concentration are shown without (D) and with (E) water restriction. Data are means ± SE (***P < 0.001).
Licking response.
Two-way ANOVA revealed no significant difference between the licking response of sham-operated and gastric bypass-operated rats after surgery with or without water restriction. When water (and food) was available ad libitum prior to the test, there was a significant main effect of Intralipid concentration [F(6,54) = 15.16; P < 0.001], but not of surgical group [F(1,54) = 1.52; P = 0.25] in the two-way ANOVA. The group × concentration interaction was also not significant [F(6,54) = 1.20; P = 0.32]. When water was restricted, there was a significant main effect of Intralipid concentration [F(6,60) = 5.16; P < 0.001], but not of surgical group [F(1,60) = 0.00; P = 0.99] in the two-way ANOVA. The group × concentration interaction was also not significant [F(6,60) = 0.61; P = 0.72]. The Intralipid concentration-response functions (i.e., the number of licks to Intralipid adjusted to water baseline) for the two test conditions are shown in Fig. 5, B and C. The lower values for both groups, while tested after water-restriction (Fig. 5C), are due to the high rates of licking to water, which placed a ceiling on the maximum possible licking achievable.
Number of trials.
Two-way ANOVA revealed no differences between gastric bypass rats and sham-operated controls in the absolute number of trials initiated to the Intralipid concentrations with or without water restriction. When water was available ad libitum prior to the test, there was a significant main effect of Intralipid concentration [F(6,54) = 5.85; P < 0.001], but not of surgical group [F(1,54) = 4.86; P = 0.055] in the two-way ANOVA. The group × concentration interaction was also not significant [F(6,54) = 2.04; P = 0.076]. When water was restricted, there was a significant main effect of Intralipid concentration [F(6,60) = 6.08; P < 0.001], but not of surgical group [F(1,60) = 0.41; P = 0.54] in the two-way ANOVA. The group × concentration interaction was also not significant [F(6,60) = 1.73; P = 0.13]. The number of initiated trials for each Intralipid concentration during the two test conditions is shown in Fig. 5, D and E.
Conditioned taste aversion for corn oil.
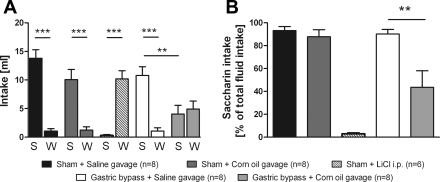
There was no difference in saccharin intake on the final test day between sham-operated rats that were previously exposed to gavage with sterile isotonic saline or with corn oil (P = 0.13); both groups showed a significantly higher saccharin intake compared with water intake. Saccharin intake of sham-operated rats was significantly reduced after intraperitoneal injection of the positive control lithium chloride compared with rats that received oral saline or corn oil gavage (P < 0.001). In contrast to sham-operated rats, gastric bypass rats reduced their saccharin intake significantly after corn oil gavage compared with saline gavage (P < 0.01). Gastric bypass rats preferred saccharin over water after saline gavage (saccharin: 10.8 ± 1.5 ml vs. water: 1.1 ± 0.6 ml, P < 0.001), but there was no preference after corn oil gavage (saccharin: 4.1 ± 1.5 ml vs. water: 4.9 ± 1.4 ml, P = 0.68). Saccharin and water intake for all groups are shown in Fig. 6A. Apart from the positive control group receiving lithium chloride, saccharin intake expressed as percentage of total fluid intake was significantly reduced in gastric bypass rats after corn oil gavage compared with all other groups (Fig. 6B, P < 0.001).
Fig. 6.
Conditioned taste aversion. A: saccharin (S) and water (W) intake in sham-operated rats after oral gavage with 1 ml sterile isotonic saline (n = 8), 1 ml of corn oil (n = 8), intraperitoneal injection of 76.2 mg/kg body wt LiCl (n = 6), and in gastric bypass rats after oral gavage with sterile isotonic saline (n = 8) and corn oil (n = 8). B: saccharin intake expressed as % total fluid intake in sham-operated rats after oral gavage with sterile isotonic saline (n = 8), corn oil (n = 8), intraperitoneal injection of 76.2 mg/kg body wt LiCl (n = 6), and in gastric bypass rats after oral gavage with sterile isotonic saline (n = 8) and corn oil (n = 8). Experiments were performed on postoperative day 100. Data are means ± SE (**P < 0.01, ***P < 0.001; saccharin vs. water).
DISCUSSION
Patients randomized to receive gastric bypass report a lower dietary fat intake compared with patients after vertical-banded gastroplasty 6 yr after surgery. Furthermore, the results of our rat experiments showed a shift away from solid high-fat to solid low-fat food after gastric bypass. We also demonstrated that gastric bypass rats have a reduced preference for liquid high-fat Intralipid relative to water during ad libitum access. In contrast, when postingestive effects were minimized during taste trials, gastric bypass rats and sham-operated rats did not differ in their concentration-dependent lick responsiveness to Intralipid during a brief access test. Postingestive factors may therefore contribute to the reduction in preference for high Intralipid concentrations in gastric bypass rats, and thus we examined a potential aversive effect of fat and its postingestive consequences. We confirmed previous findings demonstrating that gastric bypass leads to increased postprandial levels of GLP-1 and PYY compared with sham-operated control rats (17, 18). An oral gavage of a small volume of corn oil also induced a conditioned taste aversion in gastric bypass rats. Previous reports demonstrated that centrally administered GLP-1 can cause conditioned taste aversion in mice and rats (24, 33). Thus, exaggerated postprandial GLP-1 responses after gastric bypass cannot be excluded as a potential mediator of a fat preference after gastric bypass in rats.
In humans, a lower preference for high-fat foods following gastric bypass was found to be the single most pronounced factor distinguishing the two groups at 6 yr postoperatively. Our findings extend the previous reports of reduced short- and medium-term dietary fat intake after gastric bypass surgery (21). This may partly explain why gastric bypass patients find it easier to comply with general lifestyle advice to reduce dietary fat (4). This sets the stage for our rat model to pursue the physiological and endocrine mechanisms underlying the effects of gastric bypass surgery on food preference. We demonstrated that rats decrease their total energy intake from pelleted food by 37% 10 days after gastric bypass, and specifically decrease their preference for high-fat chow, while actually increasing low-fat chow consumption. The relative contribution of normal chow to energy intake increased fourfold, while the contribution of high-fat chow decreased by 11% after gastric bypass; this reflects the direction of the preference shift from high- toward low-fat chow. Similar to our finding, Shin et al. (25) also demonstrated a significantly decreased preference for high-fat diet of gastric bypass rats compared with obese sham-operated rats (sham: 95.1% vs. bypass: 63.3%, P < 0.01). Interestingly, these changes in food preference were observed 8 mo after gastric bypass surgery. Furthermore, Zheng et al. (37) found a significantly reduced preference for solid fat 7 wk after surgery compared with obese sham-operated rats; this difference further increased from postoperative weeks 8 to 20. The same authors also demonstrated a lower fat preference in gastric bypass rats than in sham-operated obese rats when liquid diets were provided (37).
Rats after gastric bypass, when given a choice over 48 h between distilled water and liquid Intralipid solutions, had a lower preference for the higher Intralipid concentrations compared with sham-operated rats. The latter showed a clear preference for high concentrations (>0.1%) of Intralipid. Sham-operated rats consumed up to 100 ml/day Intralipid, a volume equivalent to 20% of their body weight. After bypass, the reduced preference for ad libitum Intralipid was demonstrated soon after surgery and persisted for at least 200 days. This is in line with recent findings of others demonstrating decreased taste-related affective responding to high concentrations of sucrose (12, 35) and corn oil solutions (25) in gastric bypass rats compared with intact sham-operated controls as assessed by a brief access test and by long-term two-choice preference tests with both solid and liquid diets differing in energy from fat (37).
In contrast, we observed no difference in concentration-dependent lick responsiveness to Intralipid between bypass and control rats in a brief access test, which is specifically designed to reduce postingestive effects during a given trial (29, 30). If bypass rats found the taste of Intralipid in their mouths averse, then we would have noted decreases in licking to the stimulus when the rats were tested under water restriction. Instead, both gastric bypass and sham rats increased their licking as concentration was raised, and this was evident even when rats were tested nondeprived. In fact, although it just missed the statistical rejection criterion, when tested nondeprived, gastric bypass rats may have increased their appetitive behavior since the number of trials initiated showed a trend to be increased compared with sham-operated animals. Associations of particular Intralipid concentrations with possible postingestive effects, such as satiety or visceral malaise, appear to have been minimized in the brief access test (29, 30).
The fact that we did not demonstrate an attenuation of the licking response to a range of Intralipid concentrations in the brief access test after gastric bypass contrasts partly with the findings of Shin et al. (25) who demonstrated a significant decrease in licking to the highest concentration of corn oil tested (32%). However, Shin et al. (25), in fact, reported significantly increased responding to corn oil concentrations ranging from 0.03 to 4% in gastric bypass rats compared with sham-operated obese rats. The disparity between the outcomes could be due to some methodological differences between the two studies. Of course, we cannot rule out that the exact composition of the liquid fat stimulus used (i.e., Intralipid vs. corn oil) might play a role. Also, the technical aspects of the gastric bypass operation including surgical parameters, such as a different gastric pouch size or different limb lengths, could lead to differences in the gastric and/or intestinal transit time of ingested nutrients. Alternatively, variations in the design of the brief access test, such as whether a trial was started before the stimulus was ever sampled and the number of trials presented, might have contributed to the disparity in the outcomes across the two studies. Additionally, the strain of rat used could prove to be a critical factor; we used Wistar rats and Shin et al. (25) used Sprague-Dawley rats. Finally, operations were performed on rats weighing between 350 g and 500 g in our experiments, and a formal analysis of body composition (e.g., by CT scan) to confirm obesity was not conducted. Thus, we cannot exclude that observed differences between studies may also be due to different levels of total or abdominal obesity of rats used in different studies and that our model may not be representative of all cases of human obesity. Regardless of the root of the disparity, it is clear that unconditioned taste-driven motivational responses to preferred liquid fat stimuli, as assessed in a brief access test, are not universally decreased by gastric bypass surgery under all conditions.
Based on our findings in the brief access tests, we hypothesized that the reduced preference for high-fat food seen in rats after gastric bypass may in part be due to postingestive effects leading to the formation of conditioned taste aversions after ingestion of larger amounts of fat. We further investigated this possibility and found that when gastric bypass rats were treated with 1 ml of pure corn oil by gastric gavage soon after ingesting a novel saccharin solution, which they normally prefer, they subsequently showed a marked reduction in their saccharin preference compared with saline-gavaged controls or oil-gavaged sham-operated rats. As described above, corn oil gavage was chosen since the fat content of 1 ml corn oil corresponds approximately with the amount of fat that is ingested with a standard small meal of a 60% high-fat diet. However, it is important to note that it takes a rat a few minutes to eat a small meal, while the corn oil gavage took only a few seconds. Thus, the gavage itself may represent a different challenge to the rearranged gut of the gastric bypass rat compared with the spontaneous consumption of a high-fat meal. Further studies may therefore be necessary before our findings of aversion to a high-fat load can be extrapolated to a more physiological feeding situation.
However, the taste aversion was not complete or as strong as in the case of the positive control with lithium chloride, because gastric bypass rats continued to consume at least 50% (vs. ∼5% in LiCl-treated rats) of their fluid intake as saccharin solution. Consistent with this finding was the observation that in the two-bottle tests comparing ad libitum Intralipid and distilled water intake, Intralipid still made up ∼50% of total liquid intake of the gastric bypass rats. The LiCl group was included as a positive control in the taste aversion experiment, without having a priori knowledge of whether corn oil would serve as an effective unconditioned stimulus; hence we did not make an effort to match the aversive potency of the treatments. Accordingly, this can potentially explain the disparity in the magnitude of the aversion between the two groups.
Our own, unpublished results further indicate that peripheral administration of the GLP-1 receptor agonist exendin-4 also produces a conditioned taste aversion in unoperated rats (data not shown). As endogenous GLP-1 was raised postprandially in gastric bypass rats, we therefore cannot exclude the possibility that alterations in fat preference after gastric bypass may result in part from the induction of an aversive response mediated by increased levels of GLP-1, as central administration of GLP-1 has been previously shown to induce a conditioned taste aversion in mice and rats, respectively (24, 33). However, more studies are needed to establish a causal relationship between increased GLP-1 levels and the mediation of conditioned taste aversion.
Our data in rats after gastric bypass are consistent with our own and previous human findings demonstrating that gastric bypass patients display not only a reduced food intake (17–19), but also have a lower preference for food high in fat compared with patients after vertical-banded gastroplasty (6, 7, 14, 21). The anatomical rearrangement of the small bowel is not part of the vertical-banded gastroplasty, which is known not to induce substantial changes in postprandial gut hormone levels (36).
Gastric bypass leads to reduced hunger (5), increased satiation (5), and increased energy expenditure (9, 32), all of which are at least partly mediated by alterations in gastrointestinal and central neuroendocrine signaling (17, 18, 32). Indeed, we confirmed previous findings demonstrating that gastric bypass leads to increased postprandial levels of GLP-1 and PYY compared with sham-operated control rats (17, 18). In addition, GLP-1 or PYY may also influence fatty acid detection or perception, and there may be parallels with the recognition of sweet stimuli. Mice lacking the GLP-1 receptor show decreased behavioral responsiveness to sucrose. This receptor has been shown to be expressed on taste-afferent fibers, and GLP-1 is expressed in taste buds cells (11, 26).
Gastric bypass rats still ingested more calories from high-fat than from low-fat chow, while they also continued to drink Intralipid. This raises some interesting questions as it suggests that the physical properties of the fat stimulus might also play an important role for differences in preference. Alternatively, after gastric bypass, rats may develop aversions to Intralipid and other oils, e.g., corn oil, but not to a high-fat diet. Finally, if indeed lower fat preference is due to the formation of conditioned taste aversions after gastric bypass surgery, then the preoperative experience with the fat stimulus should attenuate this effect since taste novelty is a critical feature to effective conditioning. This might explain why rats still preferred the high-fat maintenance diet after gastric bypass surgery. Accordingly, rats might not develop an aversion to Intralipid if it is presented preoperatively. It would therefore be instructive to perform further experiments to determine the role of the learning processes in our gastric bypass animal model.
Perspectives and Significance
Gastric bypass patients show a lower preference for high-fat food compared with vertical-banded gastroplasty patients; findings for pre- vs. postoperative comparisons within gastric bypass patients are mixed (6, 7). However, gastric bypass rats exhibit a clearly reduced preference for high concentrations of Intralipid solution. Furthermore, rats decrease their total energy intake from pelleted food after gastric bypass, and specifically decrease their preference for high-fat chow, while actually increasing low-fat chow consumption. Postingestive effects and conditioned taste aversion may partly explain our findings, which may be mediated by gut hormones such as GLP-1. By elucidating the mechanisms by which obesity surgery reduces the consumption of high-fat foods, new surgical and nonsurgical therapies could be developed that mimic these mechanisms to offer safe and effective weight loss.
GRANTS
M. Bueter was supported by the Deutsche Forschungsgemeinschaft (DFG). T. A. Lutz and C. W. le Roux were supported by the Swiss National Research Foundation. N. Theis was supported by the Centre for Integrative Human Physiology, University of Zurich. T. Olbers and M. Werling were supported by the Research Council of the Western Region of Sweden (Västra Götaland Region). S. R. Bloom and C. W. le Roux were supported by a Department of Health Clinician Scientist Award. Imperial College London receives support from the National Institute for Health Research Biomedical Research Centre Funding Scheme.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, O'Malley W. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg 19: 845–849, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Allison DB, Saunders SE. Obesity in North America. An overview. Med Clin North Am 84: 305–332, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner I, Pacheco-Lopez G, Ruttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol 22: 557–563, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc 97: S63–S69, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brolin RL, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg 220: 782–790, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown EK, Settle EA, Van Rij AM. Food intake patterns of gastric bypass patients. J Am Diet Assoc 80: 437–443, 1982 [PubMed] [Google Scholar]
- 8. Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, le Roux CW. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 20: 616–622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, Sharkey KA, Lutz TA, le Roux CW. Gastric bypass increases energy expenditure in rats. Gastroenterology 138: 1845–1853, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 95: 666–670, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Feng XH, Liu XM, Zhou LH, Wang J, Liu GD. Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem 110: 151–154, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967–G979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes 5: 457–464, 1981 [PubMed] [Google Scholar]
- 15. Jiang E, Blonde G, Garcea M, Spector AC. Greater superficial petrosal nerve transection in rats does not change unconditioned licking responses to putatively sweet taste stimuli. Chem Senses 33: 709–723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaplan LM. Pharmacological therapies for obesity. Gastroenterol Clin North Am 34: 91–104, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365, 2005 [DOI] [PubMed] [Google Scholar]
- 18. le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lindroos AK, Lissner L, Sjostrom L. Validity and reproducibility of a self-administered dietary questionnaire in obese and non-obese subjects. Eur J Clin Nutr 47: 461–481, 1993 [PubMed] [Google Scholar]
- 21. Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 244: 715–722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olbers T, Fagevik-Olsen M, Maleckas A, Lonroth H. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic vertical banded gastroplasty for obesity. Br J Surg 92: 557–562, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Scruggs DM, Buffington C, Cowan GS., Jr Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg 4: 24–28, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106: 455–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Smith JC. The history of the “Davis Rig”. Appetite 36: 93–98, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr Opin Neurobiol 19: 370–377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096–1109, 1996 [PubMed] [Google Scholar]
- 32. Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 17: 1839–1847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thiele TE, Van DG, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Tichansky DS, Boughter JD, Jr, Madan AK. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2: 440–444, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Tichansky DS, Rebecca GA, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc 25: 1176–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Valverde I, Puente J, Martin-Duce A, Molina L, Lozano O, Sancho V, Malaisse WJ, Villanueva-Penacarrillo ML. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg 15: 387–397, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]