Abstract
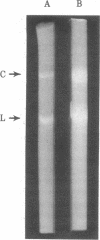
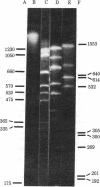
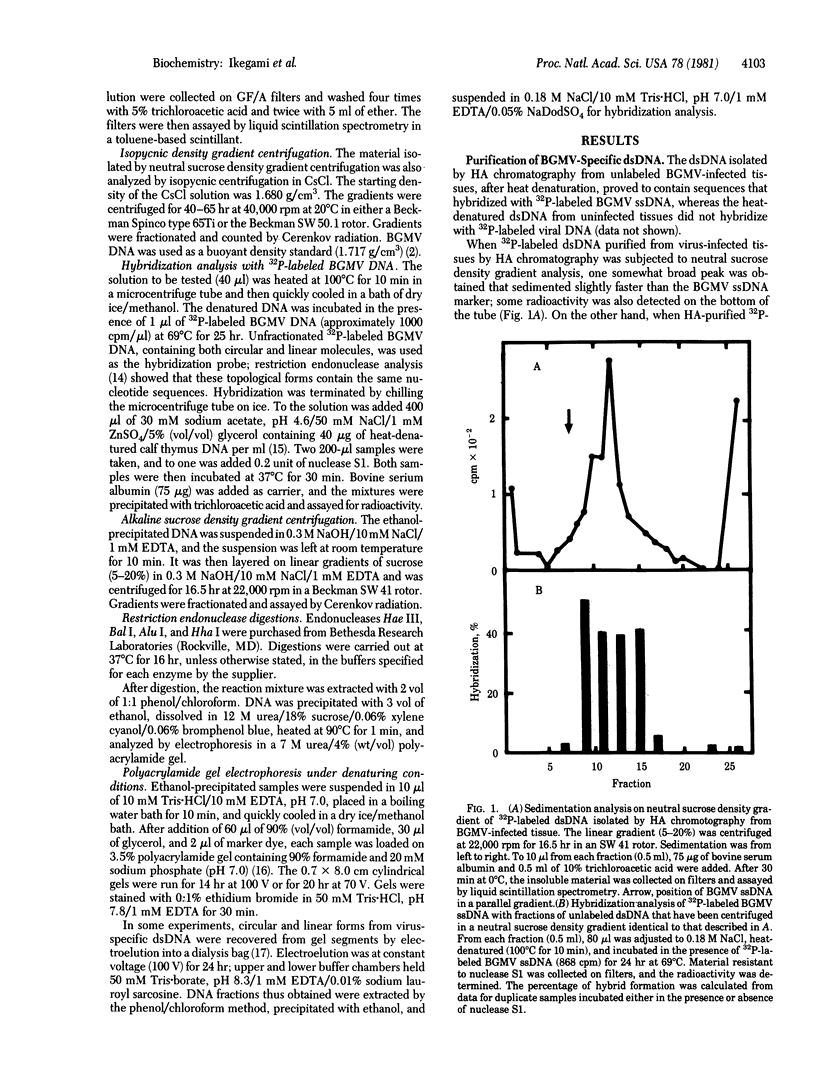
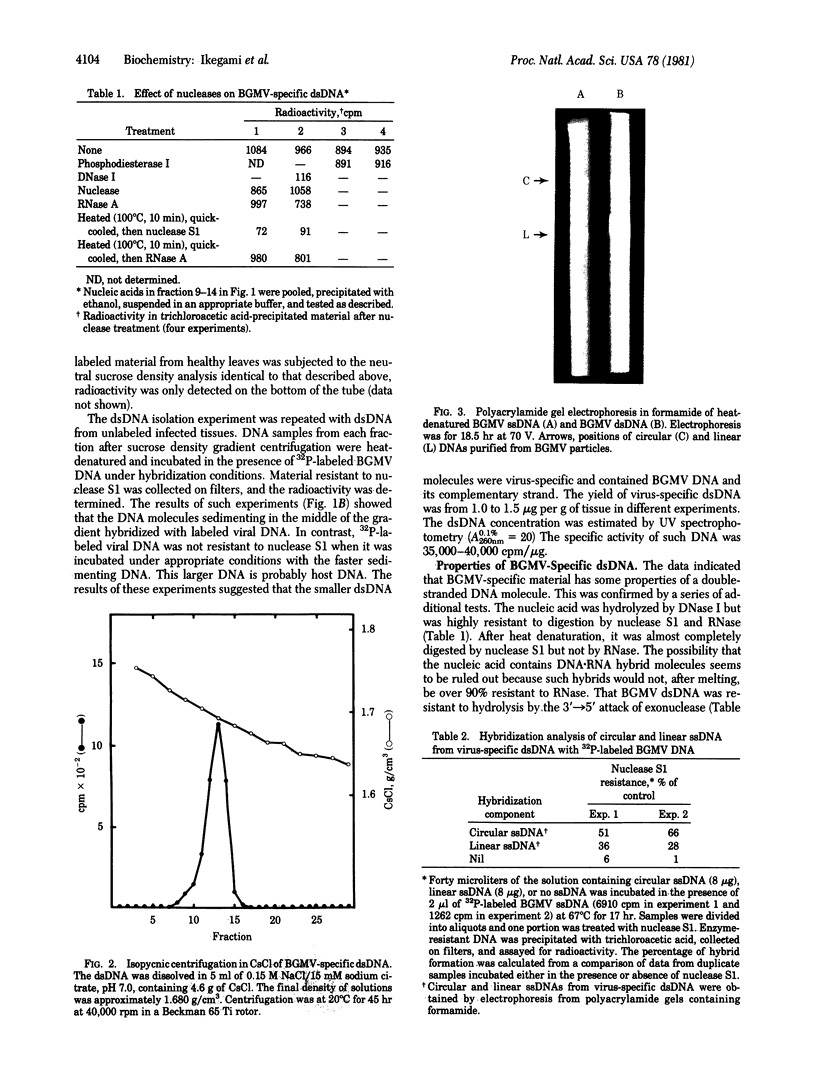
A double-stranded (ds) DNA which may be a replication intermediate was isolated from bean (Phaseolus vulgaris L. “Top Crop”) leaves systemically infected with bean golden mosaic virus, a whitefly-transmitted plant virus with a genome of circular single-stranded (ss) DNA. The isolation method used phenol/chloroform extraction, hydroxyapatite column chromatography, and rate-zonal centrifugation. The dsDNA had sequences complementary to those of viral DNA. The guanine-plus-cytosine content was 35%, and the sedimentation coefficient in alkaline sucrose density gradients was similar to that of viral ssDNA. Digestion of the dsDNA by Hha I endonuclease produced fragments that corresponded exactly in number and size with those produced by complete digestion of circular viral ssDNA by Hha I, when the fragments were denatured and analyzed on polyacrylamide gels. The dsDNA molecule was a circular structure with one discontinuity in one strand; hybridization results suggest that some of a the dsDNA has a discontinuity in the viral strand and some has a discontinuity in the nonviral strand. On the basis of these structures for the dsDNA, a preliminary model for replication of viral DNA is discussed.
Keywords: circular replication intermediate, geminivirus, whitefly-transmitted plant virus, endonucleases
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Sanger F., Coulson A. R. Nucleotide and amino acid sequences of gene G of omegaX174. J Mol Biol. 1976 Dec 15;108(3):519–533. doi: 10.1016/s0022-2836(76)80134-9. [DOI] [PubMed] [Google Scholar]
- Aoki S., Takebe I. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus ribonucleic acid. Virology. 1969 Nov;39(3):439–448. doi: 10.1016/0042-6822(69)90092-0. [DOI] [PubMed] [Google Scholar]
- BURTON A., SINSHEIMER R. L. PROCESS OF INFECTION WITH PHI-X174: EFFECT OF EXONUCLEASES ON THE REPLICATIVE FORM. Science. 1963 Nov 15;142(3594):962–963. doi: 10.1126/science.142.3594.962. [DOI] [PubMed] [Google Scholar]
- Birnie G. D. Separation of native and denatured DNA, RNA and hybrid on sodium iodide gradients. FEBS Lett. 1972 Oct 15;27(1):19–22. doi: 10.1016/0014-5793(72)80399-5. [DOI] [PubMed] [Google Scholar]
- Burton A., Sinsheimer R. L. The process of infection with bacteriophage phi-X174 VII. Ultracentrifugal analysis of the replicative form. J Mol Biol. 1965 Dec;14(2):327–347. doi: 10.1016/s0022-2836(65)80185-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Symons R. H. Determination of the sequence homology between the four RNA species of cucumber mosaic virus by hybridization analysis with complementary DNA. Nucleic Acids Res. 1977 Nov;4(11):3787–3802. doi: 10.1093/nar/4.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov G. G., Ivanov I. G. Hydroxyapatite column chromatography in procedures for isolation of purified DNA. Anal Biochem. 1974 Jun;59(2):555–563. doi: 10.1016/0003-2697(74)90309-1. [DOI] [PubMed] [Google Scholar]
- Matthews R. E. The classification and nomenclature of viruses. Summary of results of meetings of the International Committee on Taxonomy of Viruses in The Hague, September 1978. Intervirology. 1979;11(3):133–135. doi: 10.1159/000149025. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]