Abstract
Sweat Na+ concentration ([Na+]) varies greatly among individuals and is particularly high in cystic fibrosis (CF). The purpose of this study was to determine whether excess sweat [Na+] differentially impacts thirst drive and other physiological responses during progressive dehydration via exercise in the heat. Healthy subjects with high-sweat [Na+] (SS) (91.0 ± 17.3 mmol/l), Controls with average sweat [Na+] (43.7 ± 9.9 mmol/l), and physically active CF patients with very high sweat [Na+] (132.6 ± 6.4 mmol/l) cycled in the heat without drinking until 3% dehydration. Serum osmolality increased less (P < 0.05) in CF (6.1 ± 4.3 mosmol/kgH2O) and SS (8.4 ± 3.0 mosmol/kgH2O) compared with Control (14.8 ± 3.5 mosmol/kgH2O). Relative change in plasma volume was greater (P < 0.05) in CF (−19.3 ± 4.5%) and SS (−18.8 ± 3.1%) compared with Control (−14.3 ± 2.3%). Thirst during exercise and changes in plasma levels of vasopressin, angiotensin II, and aldosterone relative to percent dehydration were not different among groups. However, ad libitum fluid replacement was 40% less, and serum NaCl concentration was lower for CF compared with SS and Control during recovery. Despite large variability in sweat electrolyte loss, thirst appears to be appropriately maintained during exercise in the heat as a linear function of dehydration, with relative contributions from hyperosmotic and hypovolemic stimuli dependent upon the magnitude of salt lost in sweat. CF exhibit lower ad libitum fluid restoration following dehydration, which may reflect physiological cues directed at preservation of salt balance over volume restoration.
Keywords: plasma volume, salt, osmolality, thermoregulation, eccrine
individuals with the autosomal recessive disease cystic fibrosis (CF) typically excrete sweat with a three to five times higher Na+ concentration ([Na+]) compared with sweat from healthy individuals (11, 39). High [Na+] can be measured in the sweat from some apparently healthy (non-CF) individuals as well, with values approaching that of CF (36). For both CF and healthy individuals who excrete exceptionally salty sweat secondary to inherent ductal ion channel differences (9), it is unclear how the loss of proportionately less free water (FW) from plasma to sweat (30) may impact physiological responses directed at body water regulation during exercise-induced dehydration, including apparent thirst drive. Plasma water loss invokes a rise in blood osmolality, which serves as a strong physiological signal for thirst and prompts drinking behavior aimed at restoration of fluid balance (15, 21, 35, 44). An attenuation of FW loss may result in a diminished hyperosmolality-dependent sensitivity of thirst and promote involuntary dehydration (15) in individuals with high-sweat [Na+]. For instance, children with CF have been observed to drink less during exercise presumably because of a decreased hyperosmotic trigger attenuating the thirst drive (6, 20, 27). However, this has not been examined directly in the laboratory.
In addition to a rise in blood osmolality, loss of plasma water via normal sweating invokes a shift in fluid from the intracellular compartment (ICF) to the vascular space of the extracellular compartment (ECF) to maintain circulating blood volume (17, 25, 26). Because ECF-to-ICF fluid shifts are driven by the osmotic gradient determined primarily by blood NaCl concentration ([NaCl]), dehydration in the absence of hyperosmolality is generally accompanied by an exaggerated hypovolemia (14, 17, 22). Suggestion of blunted thirst for CF patients secondary to a diminished hyperosmotic trigger is contrary to reports in humans and animals following other forms of isoosmotic fluid depletion (i.e., bleeding, diuretics) which indicate that thirst is maintained from hypovolemic stimulus (3, 13, 14, 17, 22). Whether dehydration in individuals with high-salt losses is accompanied by an attenuated hyperosmolality and exacerbated hypovolemia, and if the net effect preserves thirst sensitivity during prolonged sweating, is not clear and has important practical implications.
Excreting sweat that is nearly isotonic to plasma, individuals with CF are at potentially greater risk for the development of dehydration and hyponatremia during prolonged exercise in the heat (43). Improved management of respiratory and digestive sequelae over the past decade has facilitated increased participation in sports for those with CF, and, with higher levels of aerobic fitness associated with increased life expectancy (28, 46), regular physical exercise is encouraged for patients. To maximize safety and performance during sports and other physical activity in the heat, electrolyte and fluid losses during prolonged sweating need to be clearly defined for optimal fluid replacement guidance. Although CF responses to thermally induced sweating during exercise is reported (6, 20, 32, 33), methodological limitations such as uncontrolled volume of fluid ingestion during exercise (6, 32, 33) and extremely disparate fitness levels for CF and non-CF groups (33) were major limitations.
Therefore, the purpose of the present study was to examine thirst with progressive dehydration in trained subjects representing the physiological extremes of Na+ losses during prolonged exercise in the heat. We hypothesized that high sweat Na+ losses (in both healthy and CF) would attenuate the increase in blood osmolality but concomitantly elicit greater relative plasma volume loss. With these two mechanisms serving as dual regulators of thirst, the decreased osmotic trigger in exceptionally “salty sweaters” could be compensated for with a greater hypovolemic signal to maintain thirst drive. However, based on previous observations in CF children, we hypothesized that fluid replacement immediately following exercise would be lower in CF subjects compared with healthy individuals across a range of sweat [Na+].
MATERIALS AND METHODS
Preliminary screening and subject selection.
Subjects were recreationally active young adults (aged 18–40 yr) not known to have CF. During preliminary sweat collection sessions (i.e., 30–60 min of cycling or running at self-selected pace until 1.5–2 ml of sweat was obtained), eight exceptionally salty sweaters (SS) with sweat [Na+] >70 mmol/l and eight individuals with typical sweat [Na+] (<60 mmol/l) (Control) were identified. The cut off of >70 mmol/l used for selecting SS is ∼ 2 SD higher than the mean recently reported for regional sweat [Na+] collected under similar conditions and with a similar technique (23).
In addition, six young adults with CF were recruited through the Emory University Cystic Fibrosis Center and the local community to participate as volunteers. All CF had sweat Cl− concentration ([Cl−]) values from previous diagnostic pilocarpine testing of >75 mmol/l. One CF subject was ΔF508/R1162X, one was ΔF508/1717–1G→A, and the remaining four were homozygous for ΔF508 mutations. All CF were in stable clinical status with a forced expiratory volume/1 s >75% predicted, performed aerobic exercise for a minimum of 4 h/wk, and were cleared by their physician for participation. Informed written consent was obtained from both CF and non-CF subjects, and the protocol was approved by the Institutional Review Boards at the Georgia Institute of Technology and Emory University.
Study design and subject characteristics.
Responses to progressive dehydration induced by prolonged exercise in the heat in Control, SS, and CF were compared using a cross-sectional design. Matching of each SS subject with a Control subject was based on the criteria that Control had lower sweat [Na+] by at least ∼50% compared with matched SS in addition to similar age, gender, anthropometry, training history, and aerobic capacity. Subject characteristics are presented in Table 1. Sample size among the groups was not equally balanced because of difficulty in recruiting CF subjects and a subject drop-out in SS. Compared with Control and SS, CF were younger, had lower aerobic capacity, and lower weekly training volume as expected given the nature of the disease. Paired Control and SS were tested in the same month, and between the months of December through May, to control for natural heat acclimation (a well-documented modifier of sweat composition) (1, 19, 26, 42). CF subjects were not necessarily tested within the same month as Control and SS; however, this was not a major study limitation, since CF sweat composition does not change with heat acclimation (33). All female subjects were tested in the early follicular phase of the menstrual cycle to avoid estrogen and progesterone influences on osmotic thirst and arginine vasopressin (AVP) responses during the luteal phase (47). Comparison of collected sweat among groups was performed at the same relative dehydration (0.5–3.0%) and exercise intensity to minimize potential effects of these known modifiers on sweat electrolytes.
Table 1.
Mean ± SD physical characteristics, exercise training volume, and aerobic fitness and heart rate
| Control (n = 8) | SS (n = 7) | CF (n = 6) | |
|---|---|---|---|
| Gender | 2 F, 6 M | 2 F, 5 M | 2 F, 4 M |
| Age, yr | 30.5 ± 5.7 | 31.6 ± 6.5 | 22.2 ± 4.5* |
| Weight, kg | 68.8 ± 11.8 | 74.4 ± 14.7 | 64.0 ± 13.8 |
| Body fat, % | 14.5 ± 6.8 | 17.3 ± 8.1 | 15.3 ± 5.6 |
| Training volume, h/wk | 11.1 ± 4.9 | 12.0 ± 4.0 | 5.1 ± 1.6* |
| V̇o2max, ml · kg−1 · min−1 | 52.8 ± 5.7 | 50.1 ± 6.5 | 39.9 ± 4.5* |
| HRmax, beats/min | 184.9 ± 7.1 | 181.4 ± 6.2 | 190.0 ± 4.8 |
Mean ± SD physical characteristics, exercise training volume, and aerobic fitness [maximal oxygen uptake (V̇o2max)] and heart rate during maximal exercise (HRmax) of Control, noncyctic fibrosis salty sweaters (SS), and cystic fibrosis (CF) subjects;
n, no. of subjects. F, females; M, males.
Significantly less than Control and SS, P < 0.05.
Initial testing session: Aerobic capacity assessment and familiarization.
In the first test session, a graded, incremental cycling test was conducted in the heat (32–33°C and 35% relative humidity) to determine maximal oxygen uptake (V̇o2max). Collection of expired gases (Parvo Medics, Salt Lake City, UT) to determine oxygen consumption (V̇o2) and respiratory exchange ratio (RER), heart rate (HR), and rating of perceived exertion (RPE) (7) were recorded during each test stage (consisting of 25- to 50-watt increments every 2 min) until volitional exhaustion. V̇o2max was considered achieved at test termination based on attainment of at least two of the following criteria: a plateau in V̇o2 during the last two stages (increase <2.1 ml·kg−1·min−1), a HR within 10 beats/min of age-predicted maximum HR, a RER ≥1.10, or a minute ventilation >115 l/min.
Following the V̇o2max test, subjects performed a 30-min familiarization ride in the heat (32–33°C and 35% relative humidity) at 50% V̇o2max to validate the work loads for the next test session. Nude dry body weight was obtained before and after the 30-min ride to determine individual whole body sweat rates (SW). During the familiarization ride, a regional sweat sample was collected from the right scapula to confirm group placement.
Body composition was assessed using dual-energy X-ray absorptiometry with a Lunar Prodigy whole body scanner (GE Medical Systems, Madison, WI). At the completion of the initial testing session, subjects were instructed in recording food and beverages for the three days before their next test session.
Dehydration protocol via prolonged cycling in heat.
Subjects abstained from caffeine at least 12 h before and alcohol at least 32 h before reporting to the laboratory for testing. Twenty-four hour food logs indicated that subjects complied with instructions to consume a standardized breakfast meal (bagel, toast, or English muffin with cream cheese, butter, and/or peanut butter and orange juice) on the morning of testing. There was no difference (P > 0.05) among groups in Na+ intake relative to body weight (12.7 ± 8.0 mg/kg) for the morning of testing, and for the average of three days before testing (60.0 ± 24.8 mg/kg).
To minimize variation in preexercise hydration, subjects followed a euhydration protocol. Subjects ingested 12 ml water/kg body wt the evening before and also the morning of testing. No physical exercise was performed 24 h before testing. Euhydration was confirmed with urine specific gravity (USG) values <1.021 (4) 1 h before and immediately before beginning the exercise protocol and measurement of serum osmolality <290 mosmol/kgH2O (37). All subjects began the protocol well-hydrated with no difference (P < 0.05) among groups in initial mean ± SD serum osmolality (Osmopre) (Control 279.9 ± 2.8, SS 282.8 ± 2.5, and CF 284.0 ± 3.2 mosmol/kgH2O) or USG (Control 1.006 ± 0.002, SS 1.006 ± 0.001, and CF 1.009 ± 0.004).
The experimental protocol consisted of prolonged cycling in a heated environmental chamber (32–33°C and 35% relative humidity). Cycling was performed at 50% V̇o2max, in 20-min bouts, separated by 5-min rest periods (in the chamber) and continued until 3% body weight loss. To estimate whole body fluid loss, nude body weight was obtained preexercise and every 20 min during cycling. No fluids were ingested during exercise.
Physiological measurements.
V̇o2 and RER were obtained 5 min before the end of every 20-min exercise stage by open-circuit spirometry using a Parvo Medics TrueOne 2400 Metabolic Measurement System (Parvo Medics). HR was measured via telemetry (Polar Electro, Woodbury, NY) and recorded every 5 min. Core temperature was monitored within the gastrointestinal tract using an ingestible temperature sensor (CoreTemp; HTI Technologies, Palmetto, FL) and recorded every 20 min. RPE according to a 15-point Borg Scale (7) was recorded at the end of each 20-min stage. Rating of perceived thirst, obtained before and every 20 min during cycling, and every 10 min during the postexercise recovery period, was assessed using a nine-point thirst scale with verbal anchors ranging from one (“not thirsty at all”) to nine (“very, very thirsty”) (21).
As an additional measure of thirst, volume of beverage voluntarily ingested following exercise was recorded at 10-min intervals during a 60-min recovery period in a thermoneutral room (22°C). Rating of perceived thirst was also collected at these intervals. A carbohydrate-electrolyte replacement beverage with 20 mM Na+ (Gatorade; Pepsico, Purchase, NY) was provided to subjects in their preferred flavor (out of orange, lemon-lime, and fruit punch) with standardized instructions for ad libitum drinking. The drinking container was refilled when between half and three-fourths empty. Subjects were not aware of the larger container from which their drink was refilled nor the monitoring of their drinking behavior. Because of CF subject complaints (e.g., headache, cognitive difficulty, confusion) during rehydration, presumably from substantial sweat NaCl losses, CF were also provided ad libitum salty foods (e.g., potato chips, salty crackers) beginning at 40 min postexercise. Unfortunately, this necessary departure from protocol precludes comparisons between CF and non-CF subjects past the 40-min postexercise time point.
Regional sweat analysis.
Sweat was collected with the modified Brisson (8) method using a collection pouch constructed with impermeable Parafilm (7 × 8 cm) (American Can, Greenwich, CT) and Opsite wound dressing (10 × 14 cm) (Smith & Nephew, Largo, FL). While whole body washdown may be the preferred method for examining whole body losses of sweat electrolytes (5, 38), our examination of sweat over progressive dehydration (not just postexercise) made the whole body washdown method extremely difficult and impractical. Furthermore, an additional purpose of the study protocol was to compare sweat electrolytes with sweat duct ion channel abundance determined from skin biopsy performed at the regional sweat collection site (9). Because the sweat collected at the scapula correlates well with whole body sweat concentrations for [Na+] and [Cl−] (34), and the concern for biopsy wound and scarring at this location is less than for other regional sites (i.e., forehead, forearm, foot), the scapula was chosen for this protocol. The skin of the scapula was cleaned with alcohol, deionized water, and sterile gauze and air-dried before application of the collection pouch. Sweat was aspirated from the collection pouch every 20 min during cycling. Sweat [Na+], [Cl−], and K+ ([K+]) concentration were measured in triplicate using a chemistry analyzer (Nova 5; Nova Biomedical, Waltham, MA). Frequent removal of accumulated sweat minimized electrolyte leaching from the epidermal layer into the sweat sample. Stable sweat [K+] values throughout the collection time points provided evidence that this potential source of error was minimal (48).
Blood and urine analysis.
A forearm vein was cannulated with subjects in a supine position, and, following 12 min in a sitting position, a resting blood sample was drawn. Blood samples were also drawn every 20 min during the cycling protocol and following the 60-min recovery period. For most CF subjects, additional recovery samples were drawn at 40, 90, and 120 min. The catheter was kept patent with a sodium heparin lockflush solution. Following removal of an ∼1.5-ml waste sample, venous blood was drawn in an EDTA-treated test tube and immediately analyzed for hemoglobin (Hb) (HemaCue) and hematocrit (Hct) (microhematocrit centrifugation). At all collection times, a venous sample was also drawn in a serum separator tube and allowed 30–60 min to clot before processing. Blood was centrifuged at 3,000 rpm for 10 min, and plasma and serum were stored at −20°C. Plasma hormone assays were performed by an outside laboratory (Yerkes Biomarkers Core Laboratory; Emory University, Atlanta, GA). Plasma samples corresponding to baseline (0%), 1.5%, and 3.0% dehydration were analyzed using commercially available radioimmunoassay kits for aldosterone (ALDO) (Diagnostic Systems Laboratories, Beckman Coulter, Webster, TX) and angiotensin II (ANG II) (American Laboratory Products, Windham, NH) and a commercially available enzyme immunoassay kit for AVP (Assay Designs, Ann Arbor, MI). Serum osmolality was determined via the freezing-point depression method (MicroOsmette Precision Systems, Natick, MA). Serum [Na+], [K+], and [Cl−] were also measured with the Nova5 chemistry analyzer. Insufficient blood sampling for one CF subject and one SS subject reduced our serum and plasma measures (n = 19).
Urine was collected pre- and postexercise to assess volume, specific gravity (via refractometry), and electrolyte concentration ([electrolytes]). There was no urine output during exercise except for one SS subject. In this case, the urine output during exercise was included in the postexercise urine volume. Urine [Na+], [K+], and [Cl−] were also measured with the Nova5 chemistry analyzer.
Calculations.
Total body water (TBW) loss was estimated from body weight loss. Total sweat loss was calculated from net change in body weight. Electrolyte losses in sweat and urine were calculated by multiplying the volume lost by the [electrolytes] of each (2). Cation loss was calculated as [([Na+]mmol/l + [K+]mmol/l)sweat × volume (liters)sweat] + [([Na+]mmol/l + [K+]mmol/l)urine × volume (liters)urine]. FW loss was calculated as TBW loss − (cation loss × 2)/serum Osmopre. The ratio of FW loss to TBW loss describes the nature of the dehydration challenge (isotonic vs. hypotonic) and determines how the TBW loss is shared by the different body fluid compartments (17, 30). The relative change in plasma volume (%ΔPV) was calculated from changes in Hb and Hct (10).
Statistical analysis.
A mixed-model analysis with the between factor of group and repeated factor of dehydration level or time was used to assess differences among groups for physiological responses across body weight losses of 0–3.0%, or across time of recovery from exercise. Because of the unequal n values, a homogeneity of variance test was run on the groups and found to be tenable. When a significant interaction effect was determined (group × level or time), post hoc analysis (Tukey) was performed to identify between-group differences. One-way ANOVA was used to assess differences among groups for variables not expressed over progressive dehydration or time such as subject characteristics, pretest measures, and change relative to baseline for variables measured postexercise. When a significant main effect for group was observed, post hoc analysis (Tukey) was performed to identify between-group differences. Association of sweat [Na+] with serum osmolality, serum [Na+], %ΔPV, hormone responses, and ad libitum drinking and association of serum [electrolytes] with ad libitum drinking were analyzed with Pearson product-moment correlation. The number of subjects in each group (minimum of 6) provided sufficient statistical power (β = 0.20) to detect a thirst rating difference equal to two times (1.0) the typical within-subject SD of 0.5, based on intrasubject variablility in thirst ratings determined in a previous study (18). Statistical testing was conducted using SPSS (version 17.0; Chicago, IL). An α level of 0.05 was used to indicate statistical significance. All values are presented as means ± SD.
RESULTS
Sweating characteristics and responses.
Sweating-related characteristics are presented in Table 2. Control, SS, and CF had similar exercise time, TBW loss, and percent body weight loss (3% dehydration) achieved at the termination of exercise. Work rate was lower for CF because of lower maximum oxygen uptake. There was no difference among groups in SR expressed relative to body weight (kg) or as absolute values (0.9 ± 0.2 l/h for Control, 1.0 ± 0.3 l/h for SS, and 0.7 ± 0.2 l/h for CF). SR was not constant during the dehydration protocol, as expected, but subtle changes were not different among groups (P = 0.93). Sweat electrolytes (Table 2) reflect the average for all values obtained throughout the exercise protocol. As an expected outcome based on the subject recruitment procedure, sweat [Na+] and [Cl−] were significantly higher for CF and SS compared with Control (P < 0.001) and also higher for CF compared with SS (P < 0.001). Consistent with previous reports (36, 40), CF sweat [K+] was also significantly higher compared with both Control (P = 0.04) and SS (P = 0.02); moreover, Control sweat [K+] did not differ from SS (P = 0.94). There was no significant relationship observed for SR and sweat [Na+] (r = −0.28, P = 0.22). Sweat [Na+] and [Cl−] increased with progressive dehydration as expected (25), and the change relative to time and to dehydration was not different (P < 0.05) among groups. Higher calculated total sweat [Na+] was significantly and inversely associated with FW loss (r = −0.99, P < 0.001). Calculated FW loss relative to kilogram of body weight (Table 2) was significantly lower than Control for SS (P < 0.001) and CF (P < 0.001). FW loss in CF was also significantly less than SS (P < 0.001). This was also observed when FW loss was expressed as a percentage of ΔTBW (data not shown).
Table 2.
Mean ± SD values for Control, SS, and CF subjects for exercise time, percent dehydration, TBW loss, free water loss relative to body weight, average whole body SR relative to body weight, and regional sweat [Na+], [Cl−], and [K+]
| Control (n = 8) | SS (n = 7) | CF (n = 6) | |
|---|---|---|---|
| Exercise time, min | 122.5 ± 16.7 | 125.7 ± 27.6 | 130.0 ± 24.5 |
| Dehydration, % | 3.1 ± 0.1 | 3.0 ± 0.2 | 2.9 ± 0.2 |
| TBW loss, ml/kg | 33.8 ± 3.3 | 32.1 ± 2.8 | 30.4 ± 2.8 |
| Free water loss, ml/kg | 21.7 ± 1.8 | 12.2 ± 2.9* | 1.1 ± 2.5*# |
| Sweat rate, ml · kg−1 · h−1 | 12.8 ± 1.9 | 12.9 ± 3.5 | 11.0 ± 1.7 |
| Sweat [Na+], mmol/l | 43.7 ± 9.9 | 91.0 ± 17.3* | 132.6 ± 6.4*# |
| Sweat [Cl−], mmol/ | 41.9 ± 6.9 | 84.1 ± 18.0* | 127.0 ± 12.1*# |
| Sweat [K+], mmol/l | 4.7 ± 0.5 | 4.4 ± 0.3 | 7.4 ± 3.3*# |
Mean ± SD values for Control, SS, and CF subjects for exercise time, percent dehydration, total body water (TBW) loss, free water loss relative to body weight, average whole body sweat rate (SR) relative to body wt, and regional sweat sodium (Na+), chloride (Cl−), and potassium (K+) concentration (indicated by brackets).
Significantly different from Control and
CF significantly different from SS, P < 0.05.
Blood responses.
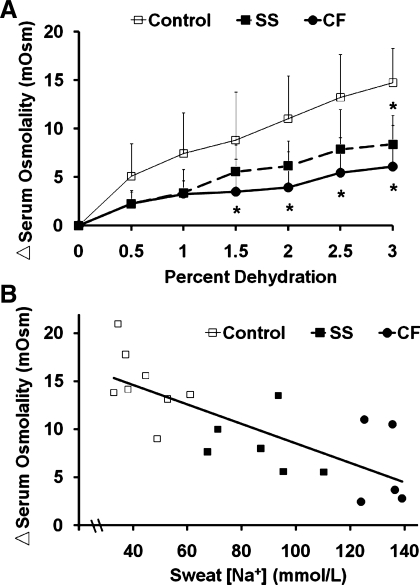
Over progressive dehydration (0–3% body wt loss), change in serum osmolality (Fig. 1A) was significantly different by group (P = 0.034). The net increase in serum osmolality from preexercise (0% dehydration) to postexercise (3% dehydration) (Fig. 1A) was significantly lower for CF (P = 0.002) and SS (P = 0.012) compared with Control. Higher-sweat [Na+] was significantly associated (r = −0.76, P < 0.001) with an attenuated rise in serum osmolality (Fig. 1B). Examining absolute values, baseline serum osmolality tended (P = 0.06) to be lower for Control (279.9 ± 2.4 mosmol/kgH2O) compared with CF (284.1 ± 3.2 mosmol/kgH2O) but not SS (282.9 ± 2.5 mosmol/kg). By the exercise termination point (3% dehydration), serum osmolality increased to 294.8 ± 4.1 mosmol/kgH2O in Control compared with 290.2 ± 2.4 mosmol/kgH2O for CF and 291.2 ± 4.9 mosmol/kgH2O for SS (P = 0.14).
Fig. 1.
A: increase in serum osmolality from baseline over dehydration (%body wt loss) via exercise in the heat was significantly affected by group (P = 0.034) with attenuated rise observed for cystic fibrosis (CF) subjects [sweat Na+ concentration ([Na+]) = 132.6 ± 6.4 mmol/l] compared with Controls (mean ± SD sweat [Na+] = 43.7 ± 9.9 mmol/l) and non-CF salty sweaters (SS, [Na+] = 91.0 ± 17.3 mmol/l). B: scatter plot indicating relationship (r = −0.76, P < 0.001) between individual subjects' change from baseline in serum osmolality observed at 3% dehydration and regional sweat [Na+]. *Less than Control, P < 0.05.
Increase in serum [Na+] over progressive dehydration was significantly affected by group (P = 0.004). Baseline serum [Na+] was not significantly different (P > 0.05) between Control (142.1 ± 0.9) and SS (141.6 ± 1.1), but CF was higher than Control (143.2 ± 0.8 mmol/l, P = 0.027). At exercise termination (3% dehydration), serum [Na+] was significantly lower for CF (146.1 ± 1.2, P = 0.003) and tended to be lower for SS (147.9 ± 1.7, P = 0.071) compared with Control (149.7 ± 1.5 mmol/l). Importantly, change in serum [Na+] from pre- to postexercise was less for CF (2.9 ± 1.7) compared with SS (6.3 ± 1.0, P = 0.001) and Control (7.5 ± 1.0, P < 0.001) and less (P = 0.05) for SS compared with Control. Increase in serum [Cl−] over progressive dehydration was also significantly affected by group (P = 0.002). Absolute values for serum [Cl−] were not significantly different (P < 0.05) from Control (107.2 ± 2.0) at baseline for SS (106.2 ± 1.3) and CF (107.6 ± 1.3); however, at exercise termination (3% dehydration), CF (106.7 ± 1.5) had significantly lower serum [Cl−] than Control (112.0 ± 1.5, P = 0.001) and SS (109.1 ± 2.4 mmol/l, P = 0.04). Furthermore, change in serum [Cl−] from pre- to postexercise was less for CF (−0.8 ± 0.7) compared with SS (2.9 ± 1.5, P < 0.001) and Control (4.9 ± 1.0 mmol/l, P < 0.001) and less (P = 0.017) for SS compared with Control. Serum [K+] was similar (P = 0.77) among groups at baseline (Control 4.3 ± 0.3, SS 4.2 ± 0.2, and CF 4.3 ± 0.3 mmol/l) and not different (P = 0.27) after 3% dehydration (5.1 ± 0.3, 5.0 ± 0.3, 5.3 ± 0.2 mmol/l for Control, SS, and CF, respectively).
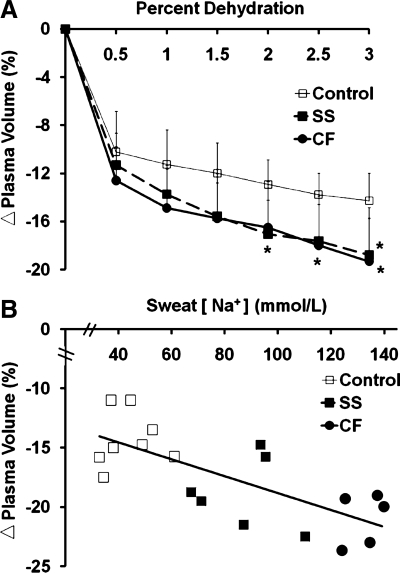
Percent reduction in plasma volume (PV) across progressive dehydration during exercise (Fig. 2A) was significantly affected by group (P = 0.05). Pre- to postexercise %ΔPV reduction was greater in CF (P = 0.03) and SS (P = 0.05) compared with Control. Higher sweat [Na+] was significantly related (r = 0.53, P = 0.02) to greater postexercise %ΔPV (Fig. 2B).
Fig. 2.
A: percent change (Δ) in plasma volume from baseline with percent dehydration (%body wt loss) via exercise in the heat was significantly affected by group (P = 0.05), with a greater drop observed for CF subjects and non-CF SS than Controls. B: scatter plot indicating relationship (r = 0.53, P = 0.02) between individual subjects' relative change in plasma volume observed at 3% dehydration and regional sweat [Na+]. *Less than Control, P ≤ 0.05.
Change from baseline to postexercise varied greatly within each group for plasma AVP concentration ([AVP]), and there were no between-group differences determined (Control 12.5 ± 6.2, range 3.5–18.6; SS 25.5 ± 18.5, range 5.83–44.0; CF 12.9 ± 6.8, range 5.4–21.7 pg/ml, P = 0.38). Also for plasma ALDO concentration, change from baseline to postexercise varied greatly within each group, and there were no between-group differences determined (Control 601.9 ± 264.9, range 317.5–1,003.3; SS 710.1 ± 372.0, range 308.3–1,309.9; CF 796.8 ± 444.8, range 317.8–1,278.9 pg/ml, P = 0.62). Finally, plasma ANG II concentration ([ANG II], pg/ml) also demonstrated high within-group variability for change from baseline to postexercise, and there were no between-group differences determined (Control 31.3 ± 25.6, range 4.4–76.1; SS 41.5 ± 23.3, range 16.4–76.1; and CF 45.5 ± 28.0, range 22.0–78.6 pg/ml, P = 0.87).
Physiological responses.
As designed, relative exercise intensity (%V̇o2max) was similar among groups (51.9 ± 2.5% for Control, 51.2 ± 2.6% for SS, and 52.6 ± 2.8% for CF). Mean HR across the dehydration protocol were higher for CF compared with Control and SS (P = 0.01), but mean percent peak HR was not different (P = 0.22) among groups (72.5 ± 4.4% for Control, 72.8 ± 5.6% for SS, and 77.6 ± 7.1% for CF). There was no difference among groups in core temperature relative to dehydration level (P = 0.29). Final core temperature was 38.4 ± 0.3, 38.1 ± 0.2, and 38.1 ± 0.4°C for Control, SS, and CF, respectively (P = 0.21), and increased similarly among groups (net change of 1.5 ± 0.5, 1.4 ± 0.6, and 1.0 ± 0.2°C for Control, SS, and CF, respectively, P = 0.16). There was no difference (P = 0.67) in RPE rating among groups relative to dehydration, and increases in RPE (2.1 ± 1.7 for Control, 2.8 ± 2.2 for SS, and 2.9 ± 1.4 for CF) were similar over time (P = 0.70).
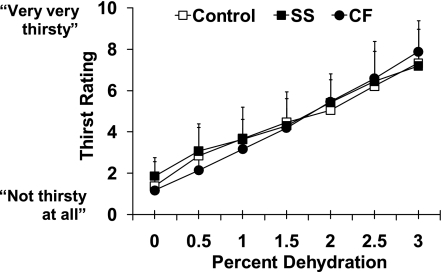
Thirst responses.
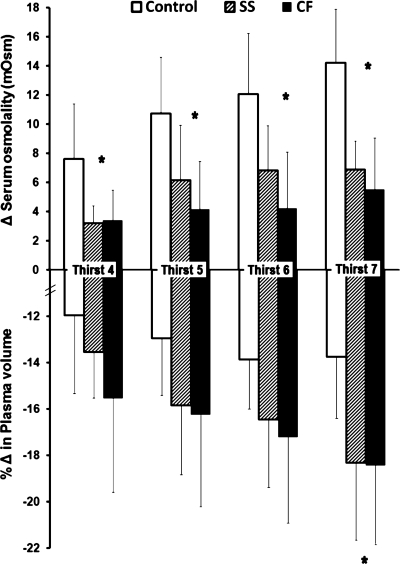
Thirst ratings (units on the 9-point Likert scale) increased as expected over progressive dehydration (P < 0.001), but this was not different by group (P = 0.310) (Fig. 3). There was no difference among groups in thirst rating at baseline (P = 0.40) or in net change in thirst from baseline to exercise termination (3% dehydration) (P = 0.30). Thirst rating at exercise termination tended to be higher in subjects with greater increases in plasma [AVP] (r = 0.41, P = 0.08, n = 16). The percent reduction in body weight (%dehydration) observed at specific scale ratings such as “somewhat thirsty” or “very thirsty” was not significantly affected by group (P = 0.35). As an example, the %dehydration level eliciting a thirst rating of “four” (somewhat thirsty) was 1.2 ± 0.8% for Control, 1.1 ± 0.9% for SS, and 1.4 ± 0.2% for CF (P = 0.80). Finally, to permit examination of responses in the context of perceived thirst during exercise, serum osmolality and relative PV change at an absolute thirst rating of four to seven are presented in Fig. 4.
Fig. 3.
Mean ± SD thirst rating for Controls (mean ± SD sweat [Na+] = 43.7 ± 9.9 mmol/l), non-CF SS ([Na+] = 91.0 ± 17.3 mmol/l), and CF subjects (sweat [Na+] = 132.6 ± 6.4 mmol/l) increased as expected over progressive dehydration (%body wt loss) (P < 0.001) via exercise in the heat, but this was not affected by group (P = 0.310).
Fig. 4.
Attenuated rise in serum osmolality for CF subjects (P = 0.004) and non-CF SS (P = 0.018) but greater percent change (Δ) in plasma volume from baseline (P = 0.012 for CF, 0.015 for SS) compared with Controls across reported thirst levels of “4” through “7” during exercise. Groups were at similar levels of dehydration across these reported thirst levels. *SS and CF different from Control (P < 0.05).
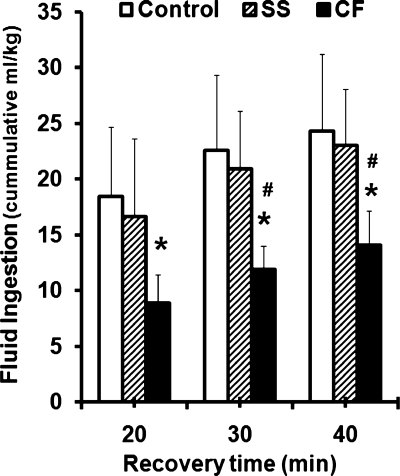
During recovery, thirst ratings obtained at 10-min intervals beginning before ad libitum drinking (10 min postexercise) to 40 min postexercise were not different among groups (P = 0.554), with scores ranging from seven (very thirsty) to one to two (not thirsty), respectively, over these recovery time points. Mean ± SD thirst ratings were 7.8 ± 2.0, 2.3 ± 1.7, 1.5 ± 1.2, and 1.1 ± 1.0 for Control; 7.4 ± 2.6, 3.7 ± 2.2, 2.7 ± 2.0, and 2.1 ± 1.6 for SS; and 7.3 ± 1.6, 3.3 ± 2.1, 2.3 ± 1.5, and 1.3 ± 1.0 for CF. The volume of beverage voluntarily ingested following exercise was recorded as an additional measure of thirst. There was no difference between Control and SS in the volume of fluid ingested ad libitum (normalized by body wt) (P = 0.901) during the 40 min following exercise. However, the volume consumed by CF (normalized by body wt) was 40% lower compared with Control (P = 0.007) and SS (P = 0.021) (Fig. 5) despite similar reported thirst at the end of exercise (3% dehydration) (Fig. 3). Absolute fluid volume ingested by CF (925.0 ± 375 ml) was also significantly lower, by 737.5 and 766.4 ml, compared with Control and SS (P < 0.05), respectively. Regression analysis indicated that subjects with higher serum [Cl−] (r = 0.645, P = 0.003) and [Na+] (r = 0.491, P = 0.033) at exercise termination (3% dehydration) demonstrated greater ad libitum drinking volume relative to body wt (40 min postexercise), which was also related to lower average [Na+] (r = −0.49, P = 0.024), and [Cl−] (r = −0.474, P = 0.030) losses in sweat. The hypotonic sports drink provided 0.46 mg of Na+ ingested per milliliter consumed, which equates to 761 ± 226 mg for Control, 774 ± 191 mg for SS, and 471 ± 171 mg for CF in the 40 min following exercise.
Fig. 5.
Mean ± SD cumulative volume of carbohydrate-electrolyte fluid ingested ad libitum during recovery (normalized by body wt) following 3% exercise-induced dehydration was 40% less for CF subjects (sweat [Na+] = 132.6 ± 6.4 mmol/l) compared with Controls (P = .007) (mean ± SD sweat [Na+] = 43.7 ± 9.9 mmol/l) and non-CF SS ([Na+] = 91.0 ± 17.3 mmol/l) (P = 0.021). *CF < Control, P < 0.05. #CF < SS, P < 0.05.
Postexercise blood responses.
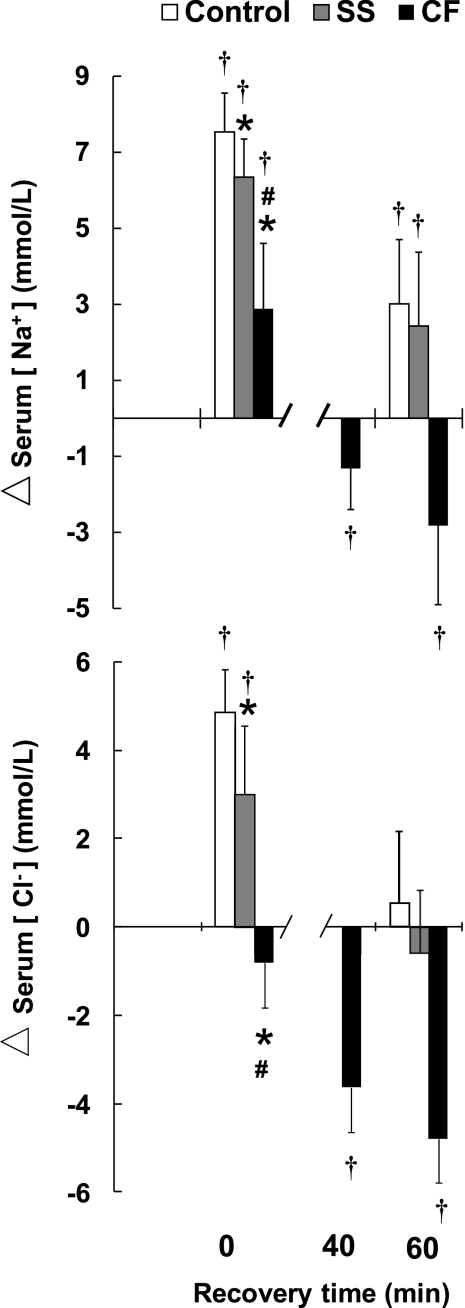
Net change in serum [electrolytes] from baseline resting (preexercise) values are presented in Fig. 6. Compared with baseline, serum [Na+] and [Cl−] at the start of recovery (3% dehydration) were higher for Control (P < 0.001) and SS (P < 0.001). For CF at the start of recovery, serum [Na+] was higher than baseline (P = 0.02), but serum [Cl−] tended to be lower than baseline (P = 0.06). By 40 min postexercise, serum [Na+] (P = 0.05) and [Cl−] (P < 0.001) were below baseline for CF and were not measured at this time point for Control and SS. Because of CF subject complaints, salty foods were offered to CF subjects starting immediately after their 40-min postexercise blood collection. By 60 min postexercise, serum [Na+] (P = 0.039) and serum [Cl−] (P = 0.001) were still below baseline for CF despite an ad libitum NaCl intake of 6.0 ± 3.7 mg/kg body wt and a 40% lower fluid intake (Fig. 5). For Control and SS at 60 min postexercise, serum [Na+] was still above baseline (Control, P = 0.001; SS, P = 0.028), but serum [Cl−] was not (Control, P = 0.372; SS, P = 0.356). CF were monitored for an additional hour in recovery, and, despite ad libitum salty foods along with fluids, serum [Na+] and [Cl−] remained below preexercise values at 2 h (by 2.6 ± 1.2 mmol/l for Na+ and 4.2 ± 1.6 mmol/l for Cl−). Because the provision of salty foods could have influenced CF responses, between-group comparisons for recovery serum [electrolytes] (Fig. 6) do not include CF.
Fig. 6.
Mean ± SD change (Δ) relative to baseline in serum [Na+] and serum Cl− concentration ([Cl−]) after 3% dehydration (time point 0), with ad libitum fluid ingestion during recovery for Control, non-CF SS, and CF subjects. At 40 min postexercise, an additional blood sampling was performed for CF followed by provision of ad libitum salty foods (see text). Between-group statistical comparisons after the 40-min time point do not include CF. *Significantly less than Control, P < 0.01 for CF and P = 0.05 for SS. #CF significantly less than SS, P < 0.01. †Significant change from baseline, P < 0.05.
DISCUSSION
The present study provided a novel comparison of thirst responses to prolonged exercise in the heat among groups at the extremes of sweat Na+ loss. The most salient finding was that perceived thirst was not differentially affected by high-sweat [Na+] during exercise. With progressive dehydration, subjects with excessive sweat Na+ loss (CF and SS) had an attenuated rise in osmolality (Fig. 1) but a greater loss of PV relative to dehydration (Fig. 2). Because both rise in blood osmolality and fall in PV serve as potent stimulators of thirst in humans (3, 12, 17, 22, 44), similar perceived thirst (Fig. 3) is logical and was consistent with our hypothesis.
As hypothesized, osmolality increased less in subjects with exceptionally salty sweat (CF and SS) compared with Controls with “typical” sweat [Na+] (Fig. 1). Furthermore, these salty sweaters did not maintain PV with dehydration as well as Control (Fig. 3A) such that sweat [Na+] was directly proportional to %PV loss (Fig. 2B). This observation is consistent with a lower osmotic-driven shift of fluid from the ICF space to the ECF space and is consistent with previous observations by Nose and coworkers (30) who reported a strong positive correlation between dehydration-induced change in blood osmolality and change in ICF volume, and between sweat [Na+] and dehydration-induced change in ECF volume. The changes in ICF and ECF that undoubtedly occurred in our subjects cannot be reported here because the calculations require measurement of initial total PV. However, given that the relationships observed for sweat [Na+] with FW loss relative to body weight (r = −0.978) and change in blood osmolality with FW loss (r = 0.719) are remarkably similar to those reported by Nose and coworkers (30) for subjects exercising to 2.3% body weight loss, it is plausible that a smaller shift from the ICF to ECF compartment occurred in SS and CF compared with Control because of the diminishing effect of high-sweat [Na+] on osmotic pressure to maintain fluid in vessels with dehydration.
Because the reduction in ECF volume incurred with PV loss also serves as a signal for thirst (3, 13, 41), although believed to be a secondary stimulus following hyperosmolality (13), the approximately one-third greater loss in PV than Control for SS and CF (Fig. 2) likely compensated for their reduced hyperosmotic stimulus for thirst and permitted thirst sensitivity to be maintained. Examining blood measures that correspond to thirst ratings four through seven during exercise illustrates this point (Fig. 4). Although all groups were at similar levels of dehydration across these reported thirst levels, rise in serum osmolality for Control was significantly steeper than for SS (P = 0.018) and also for CF (P = 0.004), whose attenuated rise never exceeded the putative 5–6 mosmol increase in serum osmolality threshold to trigger thirst. For both SS and CF, another stimulus, hypovolemia, may have largely contributed to the triggering of thirst in the absence of a strong hyperosmotic signal. Indeed, PV was reduced more drastically in CF (P = 0.012) and SS (0.015) across reported thirst levels of four through seven during exercise compared with Controls (Fig. 4), so it is probable that hypovolemia was in fact providing a compensatory stimulus to trigger thirst in these two groups. From this, we suggest that, for all individuals, thirst is a linear function of dehydration during exercise, with relative contributions by hyperosmolality and hypovolemia dependent upon magnitude of salt lost in sweat.
The tendency for postexercise thirst rating (at 3% dehydration) to be higher in subjects with greater dehydration-induced increase in plasma [AVP] is consistent with the well-documented dipsogenic effect of this hormone (21, 35, 47). A primary driver of hyperosmotic thirst, AVP is released from the posterior pituitary gland in response to cell shrinking (from loss of ICF), mediated through volume-depleted activation of osmoreceptor cells in the preoptic anterior hypothalamus (3). Because we anticipated that dehydration-induced relative hypernatremia and hyperosmolality, and thus secondary dehydration of the ICF compartment, would be less in CF and SS, we predicted that plasma AVP response to dehydration in these groups would be blunted as well. Furthermore, we anticipated that the dipsogenic hormone most sensitive to PV loss, ANG II (13), might demonstrate a more pronounced rise in SS and CF because of greater relative PV loss at similar levels of dehydration. Surprisingly, however, changes in plasma [AVP] and plasma [ANG II] were not different among groups. The absence of differential effect of sweat [Na+] on dipsogenic hormone response may be explained by the significant cross talk that is known to exist between the hyperosmotic and hypovolemic thirst pathways (3, 13).
The drive to drink in humans may be blunted in healthy individuals under certain physiological and environmental stresses (i.e., athletic competition, cold) (15, 16). In CF, involuntarily dehydration during exercise has been suggested because of an impaired hyperosmotic trigger for thirst (6, 20). To our knowledge, these are the first observed physiological differences reported in CF on drinking behavior in response to dehydration and electrolyte imbalance. Although thirst relative to dehydration was similar for CF despite attenuated hyperosmolality, when presented with fluids during recovery, CF drank 40% less fluid ad libitum compared with non-CF, which is inconsistent with their similarly high perceived thirst ratings (to SS and Control) throughout exercise but mirrors the “involuntary dehydration” noted by other investigators (6, 20) for CF children during ad libitum fluid replacement following exercise. This suggests that volitional fluid intake for CF is influenced by factors in addition to TBW loss, particularly the unique challenge to replace significant NaCl loss with prolonged sweat excretion.
Volitional drinking in CF may have been more strongly influenced by the hypotonicity of the recovery beverage provided ([Na+] = 20 mM). Unique to CF, postexercise serum [Cl−] was reduced, and postexercise serum [Na+] was increased minimally. Within only 30 min of ad libitum sports drink ingestion, mean serum [Cl−] and [Na+] for CF was reduced to ∼4 and 1 mmol/l, respectively, below preexercise values (Fig. 6), and, in addition to some CF subject complaints, strong salt cravings were reported by all CF subjects. Despite provision of salty foods for CF beginning after the 40-min postexercise time point, serum [Na+] and [Cl−] continued to fall until 90 min into recovery and remained below preexercise values at 2 h. It is likely that the relatively rapid decrease in serum [NaCl] below baseline may have served to blunt the appetite for the hypotonic beverage provided. Consistent with this, drinking is known to be influenced by salt needs (17), with input provided not only by osmoreceptors but also possibly via specialized salt sensors with Na+-sensitive channels in cells of the circumventricular organs (29). Analysis of drinking patterns in laboratory rats with experimentally induced hypertonic vs. hypotonic hypovolemia suggests that avoidance of salt imbalance is given preference over the stimulation of fluid intake for blood volume restoration (31, 41, 45). Similar to that demonstrated repeatedly in animal models, the reduced ad libitum fluid ingestion in CF (Fig. 5) may reflect physiological cues directed at preservation of salt balance (serum [Na+] and [Cl−]; Fig. 6) as priority over volume restoration. Further investigation to tease out cause-effect and mechanisms underlying this observed association is warranted.
Illustrating the potential importance of a strategy aimed at preservation of salt balance over volume restoration, the two CF subjects who drank the least in recovery had the best restoration of serum [Na+] and [Cl−]. Although no CF subject developed hyponatremia (serum [Na+] <135 mmol/l), it is tempting to speculate upon the rate of decline in CF serum [Na+] and [Cl−] that would have been measured without provision of ad libitum salty snacks along with the hypotonic fluid replacement beginning at 40 min postexercise.
Perspectives and Significance
In summary, despite smaller FW loss and attenuated serum hyperosmolality with dehydration, thirst perception during exercise was not diminished in subjects with excess sweat [Na+] loss. The greater relative PV loss with exercise-induced dehydration observed in our healthy and CF salty sweaters likely served as compensatory input to the thirst drive in the absence of a strong hyperosmotic signal. Hence, despite large variability in sweat electrolyte loss in humans, thirst perception appears to be appropriately maintained. Sweat Na+ losses observed in SS and CF provide empirical support for models (24) and recommendations (37) for consuming electrolytes during prolonged exercise to guard against the potential for hyponatremia in salty sweaters. Involuntary dehydration reported to occur in CF, and observed via the blunted appetite for hypotonic fluids in the recovery phase of this study, may reflect physiological cues directing behavioral responses to preserve salt balance over volume restoration. It is clear that, for CF, fluid replacement following prolonged exercise should not consist solely of hypotonic fluids, and drinking beyond appetite for fluids should not be encouraged. Further research is needed to identify optimal fluid and electrolyte replacement strategies for this population who can obtain health-related benefits from moderate-to-vigorous exercise.
GRANTS
This study was supported, in part, by the Carl V. Gisolfi Memorial Fund from the American College of Sports Medicine (to M. B. Brown), a traineeship grant from the Cystic Fibrosis Foundation (to M. B. Brown), and Grants UL1 RR-025008, KL2 RR-025009, or TL1 025010 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
DISCLOSURES
None.
ACKNOWLEDGMENTS
We thank the subjects for cheerful participation and Dr. Michael Schechter, the Emory Cystic Fibrosis (CF) Center, and Dr. Lawrence McKean for assistance in CF subject recruitment. Andrew Phelps, Mateo Garcia, Denise Butler, and Andrew Crane provided assistance with data collection, and Jennifer Frediani performed dietary analyses. Dr. Teresa Snow provided assistance with statistical analyses. Dr. Ed Balog provided mentoring in laboratory skills and assistance with manuscript editing. We also thank Smith and Nephew for donation of the Opsite Wound Dressings used in this study.
Current address for M. B. Brown: Indiana University School of Health and Rehabilitation Sciences, Indianapolis, IN.
REFERENCES
- 1. Allan JR, Wilson CG. Influence of acclimatization on sweat sodium concentration. J Appl Physiol 30: 708–712, 1971 [DOI] [PubMed] [Google Scholar]
- 2. Amatruda TTJ, Welt LG. Secretion of electrolytes in thermal sweat. J Appl Physiol 5: 759–772, 1953 [Google Scholar]
- 3. Antunes-Rodrigues J, De Castro M, Elias LLK, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev 84: 169–208, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong L, Maresh C, Castellani J, Bergeron M, Kenefick R, La Gasse K, Riebe D. Urinary indices of hydration status. Int J Sport Nutr Exerc Metab 4: 265–279, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Baker LB, Stofan JR, Hamilton AA, Horswill CA. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise. J Appl Physiol 107: 887–895, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Bar-Or O, Blimkie C, Hay J, MacDougall J, Ward D, Wilson W. Voluntary dehydration and heat intolerance in cystic fibrosis. Lancet 339: 696–699, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Borg G. Perceived exertion: a note on “history” and methods. Med Sci Sports Exerc 19: 398–403, 1987 [PubMed] [Google Scholar]
- 8. Brisson GR. A simple and disposable sweat collector. Eur J Appl Physiol 63: 269–272, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Brown MB, Haack KKV, Pollack BP, Millard-Stafford M, McCarty NA. Low abundance of sweat duct Cl- channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise. Am J Physiol Regul Integr Comp Physiol 300: R605–R615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974 [DOI] [PubMed] [Google Scholar]
- 11. Emrich HM, Stoll E, Friolet B, Colombo JP, Richterich R, Rossi E. Sweat composition in relation to rate of sweating in patients with cystic fibrosis of the pancreas. Pediatr Res 2: 464–478, 1968 [DOI] [PubMed] [Google Scholar]
- 12. Engell D, Maller O, Sawka MN, Francesconi N, Drolet L, Young AJ. Thirst and fluid intake following graded hypohyration levels in humans. Physiol Behav 40: 229–236, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Fitzsimons JT. Drinking by rats depleted of body fluid without increase in osmotic pressure. J Physiol 159: 297–309, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenleaf JE. Problem: thirst, drinking behavior, and involuntary dehydration. Med Sci Sports Exerc 24: 645–656, 1992 [PubMed] [Google Scholar]
- 16. Greenleaf JE, Sargent F. Voluntary dehydration in man. J Appl Physiol 20: 719–724, 1965 [DOI] [PubMed] [Google Scholar]
- 17. Johnson A. The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exerc 39: 1388–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kenefick R, St. Pierre A, Riel N, Cheuvront S, Castellani J. Effect of increased plasma osmolality on cold-induced thirst attenuation. Eur J Appl Physiol 104: 1013–1019, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kirby CR, Convertino VA. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J Appl Physiol 61: 967–970, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Kriemler S, Wilk B, Schurer W, Wilson W, Bar-Or O. Preventing dehydration in children with cystic fibrosis who exercise in the heat. Med Sci Sports Exerc 31: 774–779, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Maresh CM, Gabaree-Boulant CL, Armstrong LE, Judelson DA, Hoffman JR, Castellani JW, Kenefick RW, Bergeron MF, Casa DJ. Effect of hydration status on thirst, drinking, and related hormonal responses during low-intensity exercise in the heat. J Appl Physiol 97: 39–44, 2004 [DOI] [PubMed] [Google Scholar]
- 22. McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. Physiology 19: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Montain S, Cheuvront SN, Lukaski H. Sweat mineral element responses during 7 hr of exercise-heat stress. Int J Sport Nutr Exerc Metab 17: 574–582, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Montain SJ, Cheuvront SN, Sawka MN. Exercise associated hyponatremia: quantitative analysis to understand the aetiology. Br J Sports Med 40: 98–106, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan R, Patterson M, Nimmo M. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol Scand 182: 37–43, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen B, Strange S, Christensen N, Warberg J, Salin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch-Eur J Physiol 434: 49–56, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Nixon PA. Role of exercise in the evaluation and management of pulmonary disease in children and youth. Med Sci Sports Exerc 28: 414–420, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Nixon PA, Orenstein DM. The prognostic value of exercise testing in patients with cystic fibrosis (Abstract). N Engl J Med 327: 1785, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Noda M. The subfornical organ, a specialized sodium channel, and the sensing of sodium levels in the brain. Neuroscientist 12: 80–91, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Nose H, Mack GW, Shi X, Nadel ER. Shift in body fluid compartments after dehydration in humans. J Appl Physiol 65: 318–324, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Nose H, Yawata T, Morimoto T. Osmotic factors in restitution from thermal dehydration in rats. Am J Physiol Regul Integr Comp Physiol 249: R166–R171, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Orenstein D, Henke K, Costill D, Doershuk C, Lemon P, Stern R. Exercise and heat stress in cystic fibrosis patients. Ped Res 17: 267–269, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Orenstein DM, Henke KG, Green CG. Heat acclimation in cystic fibrosis. J Appl Physiol 57: 408–412, 1984 [DOI] [PubMed] [Google Scholar]
- 34. Patterson MJ. Variations in regional sweat composition in normal human males. Exp Physiol 85: 869–875, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ. Osmotic thirst and vasopressin release in humans: a double-blind crossover study. Am J Physiol Regul Integr Comp Physiol 248: R645–R650, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Sato K, Sato F. Na+, K+, H+, Cl−, and Ca2+ concentrations in cystic fibrosis eccrine sweat in vivo and in vitro. J Lab Clin Med 115: 504–511, 1990 [PubMed] [Google Scholar]
- 37. Sawka M, Burke L, Eichner E, Maughan R, Montain S, Stachenfeld N. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Shirreffs SM, Maughan RJ. Whole body sweat collection in humans: an improved method with preliminary data on electrolyte content. J Appl Physiol 82: 336–341, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Shwachman H, Mahmoodian A. Pilocarpine iontophoresis sweat testing results of seven years' experience. Mod Probl Pediat 10: 158–182, 1966 [PubMed] [Google Scholar]
- 40. Shwachman H, Mahmoodian A, Neff RK. The sweat test: sodium and chloride values (Abstract). J Pediatr 98: 576, 1981 [DOI] [PubMed] [Google Scholar]
- 41. Skott O. Body sodium and volume homeostasis. Am J Physiol Regul Integr Comp Physiol 285: R14–R18, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Smiles KA, Robinson S. Sodium ion conservation during acclimatization of men to work in the heat. J Appl Physiol 31: 63–69, 1971 [DOI] [PubMed] [Google Scholar]
- 43. Smith HR, Dhatt GS. Cystic fibrosis presenting as hyponatraemic heat exhaustion. Br Med J 310: 579–580, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stachenfeld NS. Acute effects of sodium ingestion on thirst and cardiovascular function. Curr Sports Med Rep 7: S7–S13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stricker E, Callahan J, Huang W, Sved A. Osmoregulation in water-deprived rates drinking hypertonic saline: effect of area postrema lesions. Am J Physiol Regul Integr Comp Physiol 280: R831–R842, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Turchetta A, Salerno T, Lucidi V, Libera F, Cutrera R, Bush A. Usefulness of a program of hospital-supervised physical training in patients with cystic fibrosis. Pediatr Pulmonol 38: 115–118, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Volkes T, Weiss N, Schreiber J, Gaskill M, Robertson G. Osmoregulation of thirst and vasopressin during normal menstrual cycle. Am J Physiol Regul Integr Comp Physiol 232: R461–R647, 1977 [DOI] [PubMed] [Google Scholar]
- 48. Weschler LB. Sweat electrolyte concentrations obtained from within occlusive coverings are falsely high because sweat itself leaches skin electrolytes. J Appl Physiol 105: 1376–1377, 2008 [DOI] [PubMed] [Google Scholar]