Abstract
Female growth-restricted offspring are normotensive in adulthood. However, ovariectomy induces a marked increase in mean arterial pressure (MAP) that is abolished by renin angiotensin system (RAS) blockade, suggesting RAS involvement in the etiology of hypertension induced by ovariectomy in adult female growth-restricted offspring. Blockade of the RAS also abolishes hypertension in adult male growth-restricted offspring. Moreover, sensitivity to acute ANG II is enhanced in male growth-restricted offspring. Thus, we hypothesized that an enhanced sensitivity to acute ANG II may contribute to hypertension induced by ovariectomy in female growth-restricted offspring. Female offspring were subjected to ovariectomy (OVX) or sham ovariectomy (intact) at 10 wk of age. Cardio-renal hemodynamic parameters were determined before and after an acute infusion of ANG II (100 ng·kg−1·min−1 for 30 min) at 16 wk of age in female offspring pretreated with enalapril (40 mg·kg−1·day−1 for 7 days). Acute ANG II induced a significant increase in MAP in intact growth-restricted offspring (155 ± 2 mmHg, P < 0.05) relative to intact control (145 ± 4 mmHg). Ovariectomy augmented the pressor response to ANG II in growth-restricted offspring (163 ± 2 mmHg, P < 0.05), with no effect in control (142 ± 2 mmHg). Acute pressor responses to phenylephrine did not differ in growth-restricted offspring relative to control, intact, or ovariectomized. Furthermore, renal hemodynamic responses to acute ANG II were significantly enhanced only in ovariectomized female growth-restricted offspring. Thus, these data suggest that enhanced responsiveness to acute ANG II is programmed by intrauterine growth restriction and that sensitivity to acute ANG II is modulated by ovarian hormones in female growth-restricted offspring.
Keywords: developmental programming, angiotensin II, intrauterine growth restriction, blood pressure, estrogen
the developmental origins of health and disease (DOHaD) hypothesis proposes that adverse influences during critical periods of early development can lead to long-term health risk and disease (1, 2). Numerous experimental studies provide compelling evidence to suggest a critical role for the kidney in the developmental programming of hypertension (3, 10). Moreover, sex differences in blood pressure and cardiovascular risk are often observed in these experimental models despite the method of developmental insult, indicating that sex steroids may play a pivotal role (12, 31). In experimental models of DOHaD induced by uteroplacental insufficiency, female growth-restricted offspring are not hypertensive in adulthood (1, 26). However, we report that hypertension can be induced by ovariectomy in adult female growth-restricted offspring, suggesting that estrogen is protective against the adverse programming effects of uteroplacental insufficiency (32).
It is well established that ANG II is a critical mediator of blood pressure and body fluid homeostasis (17). Numerous studies indicate that modulation of the intrarenal renin angiotensin system (RAS) by sex steroids contributes to sexual dimorphism in experimental models of hypertension (38, 53). Importantly, sex-specific changes in the peripheral and renal RAS are associated with sex differences in hypertension programmed by diverse methods of in utero insult (15, 25). Our studies indicate that hypertension induced by ovariectomy in female growth-restricted offspring is abolished by inhibition of the RAS; estrogen replacement therapy also normalizes blood pressure in ovariectomized female growth-restricted offspring (32). Therefore, modulation of the RAS by sex hormones may serve as a mechanism that contributes to sex differences in this model of programmed hypertension.
We recently reported that sensitivity to acute ANG II is enhanced in adult male growth-restricted offspring and that hyperresponsiveness to ANG II is testosterone dependent (31). Prenatal exposure to nicotine also results in enhanced sensitivity to acute ANG II in adult male offspring; however, sensitivity to acute ANG II is not enhanced in female offspring in this model, suggesting that the programming of sensitivity to ANG II in response to prenatal exposure to nicotine is sex-specific (52). Yet, fetal exposure to maternal low protein induces renal and cardiovascular sensitivity to acute ANG II in both male and female offspring (24). Whether sensitivity to acute ANG II is sex-specific in the model of programming induced by uteroplacental insufficiency and, whether ovariectomy will augment or enhance sensitivity to acute ANG II is unknown. Thus, the aims of this study were to determine whether an enhanced responsiveness to acute ANG II is observed in adult female growth-restricted offspring, to determine whether responsiveness to acute ANG II is enhanced by ovariectomy and to determine whether an enhanced pressor response to another vasoactive agent, such as phenylephrine, is observed in female growth-restricted offspring.
MATERIALS AND METHODS
Animals.
All experimental procedures were in accordance with National Institutes of Health guidelines with the approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with food and water available ad libitum. Timed pregnant Sprague-Dawley (SD) rats were purchased from Harlan (Indianapolis, IN). At day 14 of gestation, rats destined for reduced uterine perfusion were clipped as described in Reduced uterine perfusion in the pregnant rat. All dams were allowed to deliver at term with offspring's birth weight recorded within 12 h of delivery. At this time, the number of pups in the control and reduced uterine perfusion litters were culled to 8 pups per dam to ensure equal nutrient access for all offspring. The ratio of male to female pups remained equivalent after culling when possible. Animals were weighed twice weekly. Pups were weaned at 3 wk of age. Offspring from 13 control pregnant and 14 reduced uterine perfusion pregnant litters were randomly assigned into six groups. Thirteen intact female control, and 13 female ovariectomized control from control pregnant dams and 12 intact female growth-restricted, and 13 female ovariectomized growth-restricted from reduced uterine perfusion pregnant rats were utilized for determination of systemic and renal hemodynamic parameters. The pressor response to phenylephrine (Phe) was studied in a subset of 7 intact female control, 6 intact female growth-restricted, 7 ovariectomized control, and 7 ovariectomized growth-restricted offspring, as previously described (31). All experimental end points were performed in offspring at 16 wk of age.
Reduced uterine perfusion in the pregnant rat.
As previously described, reduced uteroplacental perfusion was utilized for induction of intrauterine growth restriction (IUGR) (1). Briefly, at day 14 of gestation, rats were anesthetized with 2% isoflurane and a silver clip (0.203-mm ID) was placed around the lower abdominal aorta above the iliac bifurcation. Because compensation of blood flow occurs through an adaptive increase in ovarian blood flow, a silver clip was slipped around each branch of the ovarian arteries (0.100-mm ID). The sham procedure involved visualization of the uterine horn only.
Ovariectomy in female offspring.
Ovariectomy (OVX) was performed at 10 wk of age in animals anesthetized with 2% isoflurane. The skin was prepared for aseptic surgery followed by a dorsal incision. The dorsal musculature was incised, and the ovaries were visualized. In the ovariectomized group, the ovarian vessels were tied off, and the ovaries were removed (OVX group). The sham operation for the sham-ovariectomized group involved a dorsal midline incision followed by visualization of the ovaries. However, in the sham ovariectomized group, the ovaries were not tied off and removed. The incision was closed in two layers, muscular and skin.
Measurement of estradiol levels.
Serum estradiol levels were measured to assess the effect of ovariectomy on estradiol levels using a commercially available kit (UltraSensitive E2 RIA DSL-4800), as previously described (32).
Drug administration.
The angiotensin-converting enzyme (ACE) inhibitor, enalapril (40 mg·kg−1·day−1) (Sigma Aldrich, St. Louis, MO) was administered in the drinking water from 15 to 16 wk of age at a dose previously shown to block the endogenous production of ANG II in the rat (31). The untreated group received vehicle (tap water ad libitum). Water consumption was monitored daily for the duration of the treatment period. At 16 wk of age, ANG II (100 ng·kg−1·min−1 in 0.9% saline solution; Sigma Aldrich) was administered acutely during the measurement of renal and systemic hemodynamics. In a subset of animals, the dose response to acute ANG I (0.025, 0.25, 2.5, and 25 mg/kg body wt in 0.9% saline solution) was utilized to ensure complete blockade of endogenous RAS. In addition, the dose response to Phe (1, 10, and 100 μg·kg−1·min−1 in 0.9% saline solution; Sigma Aldrich) was administered acutely to determine whether an enhanced pressor response in female growth-restricted offspring was observed in response to another vasoactive agent. For these studies, doses were administered in random order delivered at 50 μl per min, and blood pressure values were allowed to return to baseline between each dose.
Measurement of systemic and renal hemodynamics.
As previously described (1), offspring under isoflurane anesthesia were surgically instrumented with flexible polyethylene (PE) catheters (PE-50 tubing) in the right jugular vein for infusion and in the right carotid artery for measurement of arterial pressure and collection of blood; the bladder was also instrumented with a flexible catheter (PE-90 tubing) for collection of urine. All catheters were tunneled to the nape of the neck and exteriorized. Renal function and arterial pressure measurements were performed in the conscious state after a 24-h recovery phase. Mean arterial pressure (MAP) was monitored in conscious, chronically instrumented rats via connection of the arterial catheter to a pressure transducer and a data acquisition system with a computer for continuous recording [PowerLab 16/30; with software, Lab Chart Pro V7; both from ADInstruments (Colorado Springs, CO)]. Glomerular filtration rate (GFR) and effective renal plasma flow (eRPF) were calculated from radioactivity of [I125]-iothalamate and concentration of para-aminohippuric acid, respectively, in plasma and urine. Renal vascular resistance (RVR) and filtration fraction (FF) were calculated: RVR = (MAP/eRPF) × (1 − hematocrit) and FF = (GFR/eRPF), respectively. Data were collected before and after (20-min clearance) the acute infusion of ANG II for comparison between adult female control and adult female growth-restricted offspring.
Tissue morphology.
Thoracic aortas were collected from intact and ovariectomized female control and female growth-restricted offspring randomly assigned from different dams into eight groups: intact untreated control and intact untreated growth-restricted; ovariectomized untreated control and ovariectomized untreated growth-restricted; intact treated control and intact treated growth-restricted; and ovariectomized treated control and ovariectomized treated growth-restricted. The treated group, as described under drug administration, received enalapril. After collection, vessels were placed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (4-μm thickness) and then stained with hematoxylin and eosin (Suripath Medical Industries, Leica, IL). Aortic medial wall thickness was determined by software analysis (NIKON, NIS Elements, version 3). Measurement of aortic wall thickness was obtained from four sections from each rat by an examiner blinded to sample identity.
Statistics.
Data are presented as means ± SE. Responses to angiotensin were compared using two-way repeated-measures ANOVA (GraphPad Prism 5.0; GraphPad Software, San Diego, CA). Post hoc testing was performed using Student-Newman-Keuls multiple-comparisons test where appropriate. Plasma estradiol concentration, body and kidney weights, and water consumption were analyzed with one-way ANOVA (GraphPad Prism 5). Significant differences were identified with the Bonferroni's post hoc test for multiple intragroup comparisons. Differences were reported as significant only when P < 0.05.
RESULTS
Birth weight, body weight, kidney weight, and water consumption.
Birth weight was significantly reduced in female growth-restricted compared with female control offspring (5.6 ± 0.1 vs. 6.4 ± .1 g; IUGR vs. control, respectively). By 16 wk of age, body weight did not differ upon comparison of intact female growth-restricted to intact female control offspring (Table 1). However, ovariectomy at 10 wk of age led to a marked increase in body weight in female control offspring by 16 wk of age (P < 0.05) compared with intact control and growth-restricted offspring (Table 1). Ovariectomy did not increase body weight in female growth-restricted offspring relative to intact animals at 16 wk of age (Table 1). In addition, body weight was not significantly different upon comparison of ovariectomized control to ovariectomized growth-restricted rats (Table 1). Kidney weight did not differ upon comparison of female control to female growth-restricted offspring, intact or ovariectomized; kidney to body weight ratio also did not differ (Table 1). Water consumption was monitored daily during the administration of the ACE inhibitor, enalapril, and the average daily volume did not differ among the different groups (Table 1).
Table 1.
Body and kidney weights and water consumption in intact and ovariectomized female control and growth-restricted offspring at 16 wk of age
| Parameter | Control Intact | IUGR Intact | Control OVX | IUGR OVX |
|---|---|---|---|---|
| Body weight, g | 260 ± 5 | 259 ± 6 | 307 ± 12*† | 290 ± 16 |
| Total kidney weight, g | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.2 |
| Kidney/body weight ratio | 0.70 ± 0.05 | 0.65 ± 0.03 | 0.61 ± 0.04 | 0.71 ± 0.06 |
| Water consumption, ml/day | 30.5 ± 0.4 | 30.4 ± 0.3 | 30.3 ± 0.3 | 30.2 ± 0.2 |
All data are expressed as means ± SE. Body and kidney weights and water consumption in intact and ovariectomized (OVX) female control and growth-restricted offspring at 16 wk of age.
P < 0.05 vs. intact female control;
P < 0.05 vs. intact female growth-restricted.
Pressor responses to acute ANG II.
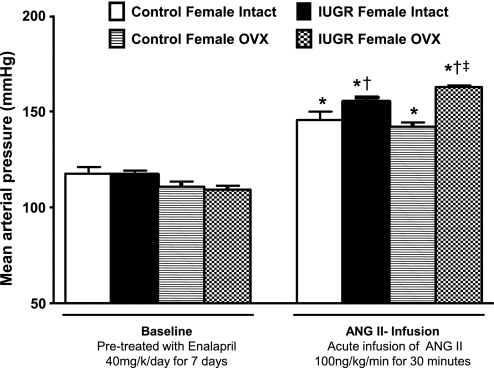
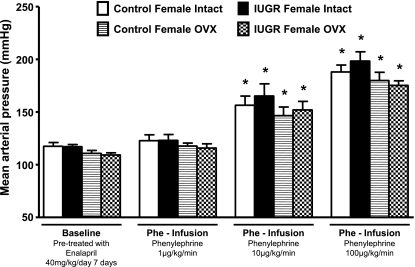
MAP did not differ upon comparison of all groups—control or growth-restricted, intact or ovariectomized—as measured under conditions of chronic ACE inhibition with enalapril (baseline) (Fig. 1). Blood pressure was increased in intact control and intact growth-restricted offspring in response to an acute infusion of ANG II (Fig. 1); however, the pressor response to acute ANG II was greater in the intact growth-restricted offspring (increase in blood pressure of 34 ± 2 mmHg, P < 0.05) compared with the pressor response to acute ANG II in intact control offspring (increase of 21 ± 5 mmHg) (Fig. 1). Ovariectomy did not alter the pressor response to acute ANG II in control offspring (increase of 25 ± 3 mmHg); however, the pressor response to acute ANG II was enhanced in ovariectomized growth-restricted offspring (increase of 52 ± 3 mmHg, P < 0.05) relative to intact growth-restricted (Fig. 1). Thus, the pressor response to acute ANG II was potentiated in female growth-restricted offspring relative to their control counterparts, suggesting that programming led to a differential pressor response to acute infusion of ANG II in female growth-restricted offspring (Fig. 1). However, in response to another vasoactive factor, Phe, blood pressure was increased in a dose-dependent manner in both control and growth-restricted offspring, intact or ovariectomized; yet, at each dose, the pressor response to acute Phe was increased to a similar extent in all groups (Fig. 2). Thus, these data indicate that the enhanced pressor response to acute ANG II observed in female growth-restricted offspring is not generalized to all vasoconstrictors.
Fig. 1.
Mean arterial pressure (MAP) under chronic angiotensin-converting enzyme (ACE) inhibition (baseline) with enalapril (40 mg·kg−1·day−1 for 7 days), and following acute infusion of ANG II (100 ng·kg−1·min−1) in intact and ovariectomized (OVX) control and growth-restricted, conscious chronically instrumented animals at 16 wk of age. *P < 0.05 vs. enalapril-treated (baseline) counterparts; †P <0.05 vs. control ANG II counterparts; ‡P < 0.05 vs. IUGR intact ANG II. All data are presented as means ± SE.
Fig. 2.
MAP under chronic ACE inhibition (baseline) with enalapril (40 mg·kg−1·day−1 for 7 days), and following acute infusion of phenylephrine (Phe; 1, 10, or 100 μg·kg−1·min−1) in intact and OVX control and growth-restricted, conscious chronically instrumented animals at 16 wk of age. *P < 0.05 vs. enalapril-treated (baseline) counterparts. All data are presented as means ± SE.
Renal hemodynamic responses to acute ANG II.
Following chronic blockade of the endogenous RAS with enalapril (baseline), GFR, GFR-adjusted per kidney weight, eRPF, RVR, and filtration fraction (Table 2) did not differ upon comparison of intact female growth-restricted offspring to intact female control offspring. Ovariectomy did not alter GFR and GFR adjusted per kidney weight in control or growth-restricted offspring; however, ovariectomy reduced eRPF in growth-restricted offspring relative to intact animals (P < 0.05) (Table 2). Ovariectomy also had no effect on RVR or FF (Table 2).
Table 2.
Renal hemodynamic parameters under chronic ACE inhibition (baseline) with enalapril and following acute ANG II infusion in intact and OVX control and growth-restricted offspring at 16 wk of age
| Control Intact | IUGR Intact | Control OVX | IUGR OVX | |
|---|---|---|---|---|
| Baseline (enalapril-treated) | ||||
| GFR, ml/min | 2.9 ± 0.3 | 2.7 ± 0.1 | 3.1 ± 0.4 | 3.0 ± 0.1 |
| GFR/KW, ml·min−1·g kidney−1 | 1.7 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.2 | 1.5 ± 0.1 |
| eRPF, ml/min | 12.6 ± 2.0 | 11.1 ± 0.5 | 10.3 ± 0.6 | 7.9 ± 0.7*‡ |
| RVR, mmHg·ml−1·min−1 | 6.3 ± 0.1 | 6.3 ± 0.3 | 6.4 ± 0.7 | 8.1 ± 0.6 |
| Filtration fraction | 0.14 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.02 | 0.19 ± 0.01 |
| Acute ANG II | ||||
| GFR, ml/min | 1.1 ± 0.1† | 1.2 ± 0.2† | 1.1 ± 0.2† | 0.2 ± 0.1*†‡ |
| GFR/KW, ml·min−1·g kidney−1 | 0.61 ± 0.06† | 0.70 ± 0.1† | 0.60 ± 0.1† | 0.11 ± 0.02*†‡ |
| eRPF, ml/min | 7.0 ± 0.5† | 5.4 ± 0.5† | 6.7 ± 0.4† | 1.9 ± 0.1*†‡ |
| RVR, mmHg·ml−1·min−1 | 12.1 ± 1.0 | 16.9 ± 1.5† | 11.9 ± 0.9 | 49.2 ± 4.3*†‡ |
| Filtration | 0.18 ± 0.02 | 0.27 ± 0.04† | 0.18 ± 0.03 | 0.12 ± 0.03‡ |
| Fraction |
All data are presented as means ± SE. Enalapril-treated rats received 40 mg·kg−1·day−1 for 7 days.ANG II was infused at 100 ng·kg−1·min−1.
P < 0.05 vs. control OVX counterpart;
P <0.05 vs. enalapril-treated (baseline) counterpart;
P < 0.05 vs. intact control.
In response to an acute infusion of ANG II GFR, GFR adjusted per kidney weight, and eRPF (Table 2) were decreased in intact control and intact growth-restricted offspring relative to intact, enalapril-treated (baseline) animals (P < 0.05). However, the reduction in GFR, GFR per kidney weight, and eRPF in response to acute ANG II were potentiated in ovariectomized growth-restricted offspring (P < 0.05) relative to other ANG II groups. Acute infusion of ANG II had no effect on RVR in control offspring, intact or ovariectomized (Table 2). However, RVR was elevated in response to acute ANG II in growth-restricted offspring (P < 0.05); and importantly, the increase in RVR in response to acute ANG II was potentiated in ovariectomized growth-restricted offspring relative to intact growth-restricted (P < 0.05) (Table 2). Thus, these data suggest that ovariectomy potentiates the renal hemodynamic responses to acute ANG II in growth-restricted female offspring (Table 2).
FF was increased in intact female growth-restricted offspring in response to acute ANG II relative to intact animals at baseline (enalapril treated) (P < 0.05; Table 2). However, ovariectomy abolished this increase (P < 0.05, OVX IUGR ANG II vs. intact IUGR ANG II) (Table 2).
Vessel morphology.
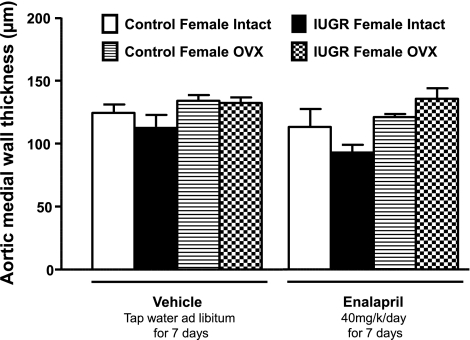
Aortic medial wall thickness did not differ in intact untreated female growth-restricted offspring relative to intact untreated female control offspring at 16 wk of age (Fig. 3). Aortic medial wall thickness at 16 wk of age was not altered by ovariectomy or treatment with the ACE inhibitor, enalapril, for 1 wk (Fig. 3).
Fig. 3.
Aortic medial wall thickness in intact and ovariectomized control and growth-restricted animals at 16 wk of age, treated and untreated with ACE inhibitor enalapril (40 mg·kg−1·day−1 for 7 days). No statistically significant differences were identified.
Estradiol levels.
Serum estradiol levels did not differ between control and growth-restricted adult female offspring (26.5 ± 1.1 vs. 24.3 ± 0.3 ng/dl; control vs. IUGR, respectively). Ovariectomy significantly decreased serum estradiol levels compared with intact animals in both control and growth-restricted offspring (6.5 ± 0.9 and 6.2 ± 0.6 ng/dl; control vs. IUGR, respectively; P < 0.05 vs. intact animals).
DISCUSSION
The major findings presented in this study are as follows: 1) the pressor response to acute ANG II is greater in intact female growth-restricted offspring relative to intact female control offspring; 2) ovariectomy further potentiates the pressor response to acute ANG II in female growth-restricted offspring with no effect in female control; 3) acute ANG II reduces GFR and GFR normalized to kidney weight in intact control, intact growth-restricted, ovariectomized control, and ovariectomized growth-restricted offspring relative to enalapril-treated animals (baseline), but ovariectomized growth-restricted offspring demonstrate a further reduction in GFR and GFR normalized to kidney weight; 4) pressor responses to the vasoconstrictor, phenylephrine do not differ among groups; and 5) remodeling of vessel morphology may not contribute to enhanced ANG II sensitivity regardless of estrogen status.
Sex differences are observed in the blood pressure response to chronic ANG II with male mice (54) and rats (41, 49), exhibiting a greater response to ANG II-induced hypertension in the presence of a functional RAS. Castration attenuates ANG II-induced hypertension in male mice; whereas ovariectomy augments the development of ANG II-induced hypertension in female mice, indicating a key role for sex hormones in mediating sex differences in ANG II hypertension (54). Sex differences are also observed in cardiovascular and renal sensitivity to acute ANG II (43), yet blockade of the endogenous RAS in rats on normal salt intake abolishes this sex difference (42). In the current study, female SD rats demonstrated systemic and renal sensitivity to acute ANG II following chronic RAS blockade, and this pressor response was augmented in female growth-restricted offspring. We previously reported that the pressor response to acute ANG II is also enhanced in male growth-restricted offspring (34). Thus, findings from these studies indicate that enhanced sensitivity to ANG II may be programmed in response to poor fetal growth. However, male growth-restricted offspring are hypertensive in adulthood; whereas female growth-restricted offspring are not (1), suggesting that placental insufficiency leads to differential programming of blood pressure and sensitivity to ANG II.
Hypertension can be induced in female growth-restricted offspring by ovariectomy, and this effect is attenuated by estradiol replacement, indicating a key role for ovarian hormones in female growth-restricted offspring (32). Blockade of the RAS also attenuates ovariectomy-induced hypertension in female growth-restricted offspring (32), suggesting that modulation of the RAS by ovarian hormones may contribute to the long-term regulation of blood pressure in female growth-restricted offspring. In the current study, the pressor response to acute ANG II was enhanced by ovariectomy in female growth-restricted offspring with no effect in female control offspring, providing further support for modulation of the RAS via ovarian hormones status in this model of developmental programming of hypertension.
Numerous studies suggest that regulation of the RAS by estrogen serves as a potential mechanism in mediating the sex-specific differences in blood pressure in many experimental models of hypertension (6, 20, 21, 35),. In normotensive rat strains such as the SD rat, a reduction in renal ACE mRNA expression and activity is induced by estrogen replacement in ovariectomized female rats (11) Moreover, vascular angiotensin type 1 (AT1) receptor mRNA expression is elevated in ovariectomized female Wistar-Kyoto rats relative to intact; importantly, restoration of estrogen downregulates AT1 receptor gene expression to intact levels, suggesting a key role for estrogen in the gene regulation of the vascular AT1 receptor (30). Changes in expression of the RAS mediated via alterations in estrogen status may play a functional role in blood pressure control (5, 18). Hypertension induced by ovariectomy in the Dahl salt-sensitive rat is associated with an increase in renal AT1R protein expression (18), and estrogen replacement is associated with a reduction in circulating levels of ANG II and blunting of the pressor response to ANG II in transgenic hypertensive rats (5). Thus, changes in RAS via ovarian hormones status may contribute to sex differences in blood pressure and ANG II sensitivity.
Blood pressure is increased in female growth-restricted offspring, as early as 4–6 wk of age relative to control (1). However, after puberty, female growth-restricted offspring normalize their blood pressure to levels comparable to female control (1). Sex-specific differences in the RAS are observed in other animal models of prenatal insult with the circulating and renal RAS implicated to play a key role in the developmental programming of hypertension and adult disease (14, 22, 25, 31, 32, 45). However, no significant differences in plasma renin activity or plasma renin substrate are observed in intact female control relative to intact female growth-restricted offspring (32). In addition, despite the increase in blood pressure, ovariectomy has no effect on systemic components of the RAS in female growth-restricted offspring (32). Hypertension in adult male growth-restricted offspring is also not associated with inappropriate systemic activation of the RAS (32). Furthermore, enhanced sensitivity to acute ANG II in male growth-restricted rats is not associated with an increase in intrarenal AT1 receptor density or mRNA expression (15). Renal AT1 receptor mRNA expression is not elevated in normotensive female rats in the model of placental insufficiency induced by bilateral uterine ligation in the rat (26). Whether renal expression of AT1 receptors is altered by programming in female growth-restricted offspring in this study is not yet known. Furthermore, whether ovariectomy will enhance renal AT1 receptor expression in control and/or growth-restricted rats is also not known. Future studies will determine the programming effects of IUGR on renal AT1 receptor expression in female rats and whether loss of ovarian hormones alters renal AT1 receptors expression in female control or growth-restricted offspring. However, hypertension induced by ovariectomy in female growth-restricted offspring is associated with a marked decrease in renal ACE2 mRNA expression (32), suggesting that modulation of the renal ACE2 pathway by ovarian hormones may contribute to the development of hypertension in female growth-restricted offspring. Therefore, an enhanced pressor response to acute ANG II is programmed in response to placental insufficiency in growth-restricted rat offspring. Moreover, hypersensitivity to ANG II may serve as a potential mechanism involved in the age-related sexual dimorphism in blood pressure observed in growth-restricted offspring with ovarian hormones providing a protective effect against programmed cardiovascular risk in female growth-restricted offspring.
Unlike the blood pressure response to acute ANG II, GFR was decreased to a similar degree in response to acute ANG II in intact female growth-restricted and intact female control offspring. However, male growth-restricted offspring exhibit an enhanced decrease in GFR in response to acute ANG II upon comparison to male control offspring (14). The enhanced reduction in GFR observed in male growth-restricted offspring is testosterone dependent (33); in the current study, the decrease in GFR was further enhanced by ovariectomy in female growth-restricted offspring in response to acute ANG II, with no further effect on GFR mediated via ovariectomy in female control offspring. Under basal conditions in humans and rodents, GFR decreases with age due to changes in structure and function (4, 27), with female rats demonstrating a delay in age-related decreases in renal function (4, 23, 29) Whether renoprotection in females is due to the presence of estrogen or a reduction in androgen is not clear (4, 23, 29). However, the renal RAS plays a critical role in modulating glomerular filtration and sodium excretion (13, 16), and differential regulation of the RAS has been reported in females compared with males (7, 8, 37, 39, 41, 53, 55).
Ovariectomy also significantly enhanced the increase in RVR observed in response to acute ANG II in female growth-restricted offspring. However, RVR was not increased in female control offspring, nor was an increase in RVR in response to acute ANG II observed in the previous study in male control or male growth-restricted offspring. ANG II acts acutely to increase blood pressure via promotion of vasoconstriction and an increase in vascular resistance. Vascular responsiveness to acute ANG II demonstrates a sexual dimorphism (44) with pressor responses to acute ANG II attenuated by chronic estrogen replacement (5). Thus, in the model of IUGR programmed by placental insufficiency, testosterone may enhance renal vascular sensitivity to acute ANG II in male growth-restricted offspring, while ovarian hormones may play a renoprotective role in female growth-restricted offspring. Therefore, these studies suggest that developmental programming of the RAS may be sex-specific in this model of IUGR with loss of ovarian hormones, leading to differential alterations in the intrarenal RAS and a further enhancement in ANG II sensitivity in the adult female growth-restricted rats.
Changes in vessel morphology, which include thickening of the vessel wall, can be associated with alterations in renal hemodynamics (9, 40, 47, 50) and increased response to vasoconstrictors (6, 28, 48). However, enhanced sensitivity to acute ANG II in intact female growth-restricted offspring was not associated with changes in vessel morphology. Thus, as in male growth-restricted offspring (31), the enhanced response to acute ANG II in female growth-restricted offspring may not be due to programming of vascular structure. In addition, ovariectomy did not alter vessel structure, indicating that the protective effect of estradiol in female growth-restricted offspring may not be due to remodeling of the vascular wall.
In summary, these studies suggest that female growth-restricted offspring exhibit an enhanced sensitivity to acute ANG II. Male growth-restricted offspring are hypertensive in adulthood; female growth-restricted offspring are normotensive in adulthood. Thus, males and females demonstrate differential programming of cardiovascular risk in response to adverse influences during fetal life. In addition, ovariectomy potentiates sensitivity to acute ANG II in female growth-restricted offspring, indicating that modulation of the RAS by ovarian hormones may contribute to sex differences in IUGR-induced hypertension.
Perspectives and Significance
Adverse influences during critical periods of early development can program hypertension in later life. Sexual dimorphism in the adult responses to developmental insult is observed in numerous experimental models, with female rats demonstrating greater resilience to cardiovascular programming following exposure to fetal insults of dietary manipulation (36, 51), placental insufficiency (1, 26), as well as early life exposure to hypoxia (19, 36, 51). Regulation of the RAS by estrogen is well established in experimental models of hypertension (5, 7, 37, 46), yet the exact mechanisms mediating protection of female offspring in response to developmental programming and, in particular, the role of ovarian hormones in conjugation with other regulatory systems remains unclear. Thus, further investigation is needed to clarify whether similar pathways mediate differential programming of cardiovascular risk in male and female offspring. Understanding the exact mechanisms involved in mediating the sexual dimorphism of the early origins of hypertension may unveil new preventive strategies to diminish later cardiovascular risk in individuals exposed to in utero insult.
DISCLOSURES
The authors have no relationships that could be perceived as real or apparent conflicts of interest.
ACKNOWLEDGMENTS
Dr. Alexander is supported by the National Institutes of Health grants HL074927 and HL51971. We would like to thank the Department of Physiology Histology core, specifically, Dr. Christine Maric and Mrs. Stephanie Peters for their excellent assistance with histological evaluation of vessel morphology.
REFERENCES
- 1. Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health 58: 114–115, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 298: F235–F247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol 5: 384–396, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol Regul Integr Comp Physiol 273: R1908–R1915, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2 Lewis rat. Hypertension 42: 781–786, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2) Lewis strain. Am J Physiol Renal Physiol 299: F35–F42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean SA, Tan J, O'Brien ER, Leenen FH. 17β-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 288: R759–R766, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Dilley RJ, Kanellakis P, Oddie CJ, Bobik A. Vascular hypertrophy in renal hypertensive spontaneously hypertensive rats. Hypertension 24: 8–15, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Dotsch J, Plank C, Amann K, Ingelfinger J. The implications of fetal programming of glomerular number and renal function. J Mol Med 87: 841–848, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33: 323–328, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol 295: R1941–R1952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Granger JP, Schnackenberg CG. Renal mechanisms of angiotensin II-induced hypertension. Semin Nephrol 20: 417–425, 2000 [PubMed] [Google Scholar]
- 14. Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 5 Suppl. A: S121–S132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996 [PubMed] [Google Scholar]
- 17. Hall JE, Guyton AC, Mizelle HL. Role of the renin-angiotensin system in control of sodium excretion and arterial pressure. Acta Physiol Scand Suppl 591: 48–62, 1990 [PubMed] [Google Scholar]
- 18. Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension 42: 1157–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol 289: H674–H682, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension 35: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans 27: 88–93, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med 5: 147–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMullen S, Osgerby JC, Thurston LM, Gadd TS, Wood PJ, Wathes DC, Michael AE. Alterations in placental 11 beta-hydroxysteroid dehydrogenase (11 betaHSD) activities and fetal cortisol:cortisone ratios induced by nutritional restriction prior to conception and at defined stages of gestation in ewes. Reproduction 127: 717–725, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 31 Suppl: S40–S46, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol 587: 2635–2646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muhlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology 45: 243–253, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci 17: 105–109, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol Renal Fluid Electrolyte Physiol 254: F223–F231, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97: 2197–2201, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: insight from animal models of nutritional manipulation. Hypertension 52: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth-restricted offspring. Hypertension 50: 679–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oparil S. Arthur C. Corcoran Memorial Lecture. Hormones and vasoprotection. Hypertension 33: 170–176, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295: H10–H20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Rorive GL, Carlier PJ, Foidart JM. Hyperplasia of rat arteries smooth muscle cells associated with development and reversal of renal hypertension. Clin Sci (Lond) 59 Suppl 6: 335S–338S, 1980 [DOI] [PubMed] [Google Scholar]
- 41. Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 51: 1170–1176, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899–R906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider S, Mohnen S, Schiltenwolf M. [“Are rich people healthier?”–Representative epidemiological data on socioeconomic group-specific disease prevalences among adults in Germany]. Dtsch Med Wochenschr 131: 1998–2003, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 53: 404–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Skov K, Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 181: 397–405, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens 20: 1571–1579, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 83: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Wiener J, Loud AV, Giacomelli F, Anversa P. Morphometric analysis of hypertension-induced hypertrophy of rat thoracic aorta. Am J Pathol 88: 619–634, 1977 [PMC free article] [PubMed] [Google Scholar]
- 51. Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension 51: 1239–1247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]