Abstract
Exertional dyspnea limits exercise in some mitochondrial myopathy (MM) patients, but the clinical features of this syndrome are poorly defined, and its underlying mechanism is unknown. We evaluated ventilation and arterial blood gases during cycle exercise and recovery in five MM patients with exertional dyspnea and genetically defined mitochondrial defects, and in four control subjects (C). Patient ventilation was normal at rest. During exercise, MM patients had low V̇o2peak (28 ± 9% of predicted) and exaggerated systemic O2 delivery relative to O2 utilization (i.e., a hyperkinetic circulation). High perceived breathing effort in patients was associated with exaggerated ventilation relative to metabolic rate with high V̇e/V̇o2peak, (MM = 104 ± 18; C = 42 ± 8, P ≤ 0.001), and V̇e/V̇co2peak, (MM = 54 ± 9; C = 34 ± 7, P ≤ 0.01); a steeper slope of increase in ΔV̇e/ΔV̇co2 (MM = 50.0 ± 6.9; C = 32.2 ± 6.6, P ≤ 0.01); and elevated peak respiratory exchange ratio (RER), (MM = 1.95 ± 0.31, C = 1.25 ± 0.03, P ≤ 0.01). Arterial lactate was higher in MM patients, and evidence for ventilatory compensation to metabolic acidosis included lower PaCO2 and standard bicarbonate. However, during 5 min of recovery, despite a further fall in arterial pH and lactate elevation, ventilation in MM rapidly normalized. These data indicate that exertional dyspnea in MM is attributable to mitochondrial defects that severely impair muscle oxidative phosphorylation and result in a hyperkinetic circulation in exercise. Exaggerated exercise ventilation is indicated by markedly elevated V̇e/V̇o2, V̇e/V̇co2, and RER. While lactic acidosis likely contributes to exercise hyperventilation, the fact that ventilation normalizes during recovery from exercise despite increasing metabolic acidosis strongly indicates that additional, exercise-specific mechanisms are responsible for this distinctive pattern of exercise ventilation.
Keywords: hyperventilation, lactic acidosis, metaboreflex, exercise, oxidative phosphorylation
exertional dyspnea was first described as a symptom that limited exercise in mitochondrial myopathy (MM) in patients with a familial disorder studied by Linderholm and coworkers in the 1960s (36, 38), a disease now recognized to be attributable to a mutation in the iron-sulfur cluster scaffold (ISCU) gene (40, 42) and associated with deficiency of multiple iron-sulfur proteins, including succinate dehydrogenase and aconitase (20, 21). Exertional dyspnea has since been described as a dominant feature of exercise intolerance in other mitochondrial myopathies that severely restrict muscle oxidative phosphorylation (3a, 22). Moreover, mitochondrial disease has been suggested to be an underrecognized cause of unexplained exertional dyspnea (16, 25), although, these reports lacked genetic confirmation and, in most cases, did not include biochemical assessment to indicate the presence and severity of the putative mitochondrial defect (16). To better define the physiological manifestations of exertional dyspnea in mitochondrial myopathy, we have evaluated two patients with ISCU myopathy, the disease originally described by Linderholm et al. (38), and three patients with exertional dyspnea with mitochondrial myopathy attributable to heteroplasmic mitochondrial DNA mutations.
Our previous studies have suggested that the severity of impaired muscle oxidative metabolism is a key variable in MM patients who experience dyspnea as a symptom that limits physical exertion (51). A consequence of severely impaired muscle oxidative phosphorylation is activation of glycolysis, resulting in marked increases in blood lactate in relation to exercise intensity (51, 53). Lactic acidosis is considered an important mediator of the compensatory hyperventilation that normally occurs at high-intensity exercise by stimulation of carotid chemoreceptors (55), and thus would be presumed to be an important contributor to the ventilatory response to exercise in MM patients. Exercise ventilation is also regulated by feedforward mechanisms via central command (15) and by feedback reflexes mediated by activation of metaboreceptors or mechanoreceptors (ergoreflexes) in working muscle (2, 13, 23, 31). Peak increases in blood lactate and maximal decreases in pH occur during the first minutes of recovery from exercise when the influence of central command ceases and ergoreflexes are rapidly withdrawn. To differentiate the contribution of acidosis from other regulators of exercise ventilation, we assessed ventilation in these patients both during incremental cycle exercise and during recovery from exercise to better define the role of lactic acidosis in this syndrome.
MATERIALS AND METHODS
Subjects.
We studied five genetically, biochemically, and physiologically defined MM patients and four age- and sex-matched, healthy control subjects (C). The diagnosis of mitochondrial myopathy was established by biochemical and genetic analysis, and patients were evaluated by prior exercise testing (Table 1). Three patients (1-MM, 2-MM, and 3-MM) had heteroplasmic mitochondrial DNA mutations in which the mutation was in high abundance (>90%) in skeletal muscle and resulted in deficiency of respiratory chain complexes containing mitochondrially encoded subunits affected by the mutation. In patient 1-MM with a 3243A>G mutation, enzymatic deficiency was most pronounced in complex I (27). In patient 2-MM with a cytochrome b mutation, the enzymatic block exclusively affected complex III (52). In patient 3-MM, a novel tRNAtrp mutation was associated with deficiency of multiple respiratory chain complexes, especially complex IV (52). Two patients had a mitochondrial myopathy with deficiency of multiple iron-sulfur cluster containing enzymes, particularly the tricarboxylic acid (TCA) cycle enzymes succinate dehydrogenase and aconitase, due to a mutation in the ISCU gene (20, 21, 40). The main clinical feature in all patients was severe exercise intolerance with prominent exertional dyspnea provoked by low-intensity exercise that had been present since childhood. Patients did not have muscle weakness and had no clinical evidence of impaired cardiac or pulmonary function. All patients showed a low peak rate of muscle oxidative phosphorylation with a severely restricted capacity to increase oxygen extraction during exercise, as indicated by a low peak systemic arteriovenous oxygen difference (a-vO2 diff., ranging from 3.4 to 5.7 ml O2/dl, Table 1, compared with normal subjects, who have peak a-vO2 difference of ∼15 ml O2/dl). Correspondingly, oxygen transport during exercise increased in great excess relative to the increase in oxygen uptake, as indicated by exaggerated increases in cardiac output relative to the increase in oxygen uptake (ΔQ/ΔV̇o2 ranging from 16 to 42, Table 1) compared with a normal ΔQ/ΔV̇o2 of ∼5 in healthy control subjects (21, 22, 51).
Table 1.
Genetic and physiological characteristics of mitochondrial myopathy patients
| Patient | Nature of Mutation | Genome | Muscle Mutation Load, % | Reference of DNA Mutation | Peak a-vO2 diff., ml O2/dl | ΔQ/ΔV̇o2 |
|---|---|---|---|---|---|---|
| 1-MM | tRNA A3243G | mtDNA | 92 | 4.3 | 21.0 | |
| 2-MM | G14846A Cytochrome b | mtDNA | 98 | (3a) | 3.4 | 42.1 |
| 3-MM | tRNAtrp T5543C | mtDNA | 95 | (3b) | 5.7 | 16.5 |
| 4-MM | ISCU | nDNA | N/A | (40) | 3.9 | 36.0 |
| 5-MM | ISCU | nDNA | N/A | (40) | 5.6 | 20.0 |
| Control Subjects | Control Subjects | |||||
| 13.9 ± 1.4‡ | 5.4 ± 0.6* | |||||
| (n = 3) | (n = 3) | |||||
Values for control subjects are expressed as means ± SD. MM, mitochondrial myopathy patients; ISCU, iron-sulfur scaffold protein; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; a-vO2 diff., systemic arteriovenous oxygen difference; ΔQ/ΔV̇o2, level of increase in cardiac output relative to increase in oxygen utilization. *Significantly different from MM patients, P ≤ 0.05, ‡P ≤ 0.001.
The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital, Dallas. All experiments were performed in accordance with the Declaration of Helsinki. Written informed consent was given by all subjects.
Pulmonary function testing.
All subjects underwent standard measurements of spirometry, lung volume, and diffusing capacity, as determined by whole body plethysmography (model V62W; Viasys Healthcare, Yorba Linda CA), according to American Thoracic Society guidelines (3). Maximum inspiratory pressure was also measured. Predicted values were based on established norms (7, 9, 10, 18, 33–35).
Hypoxic ventilatory response and hypercapnic ventilatory response.
To evaluate respiratory chemosensitivity, hypoxic ventilatory response (HVR) and hypercapnic ventilatory response (HCVR) were measured using a modification of the established progressive isocapnic hypoxia and hypercapnic technique (58, 59), as described previously (17).
Incremental exercise testing.
All subjects performed the exercise test twice on an electromagnetically braked cycle ergometer (MedGraphics, 2000), with the first test to familiarize subjects with the testing protocol. A radial artery catheter was placed for arterial blood sampling during the second test. Subjects were seated on the ergometer for resting data collection for 3 min, followed by 3 to 4 min of constant submaximal steady-state workload cycling after which the workload was increased every 60 s until the subject could no longer maintain pedal rate of 50 rpm. Workload increments were determined for each subject based on prior testing (MM, 5–10 W; C, 20–30 W). Subjects were encouraged to continue to exhaustion and remained seated on the ergometer for 5 min of resting (no cycling) recovery data collection. Throughout the testing, heart rate (HR) was monitored continuously by 12-lead ECG, blood pressure by an automated monitor (SunTech Tango, Morrisville, NC). Subjects' ratings of exercise effort were obtained at the end of each minute using Borg 6–20 scale for rating of overall perceived exertion (RPE) and 0–10 scale for rating of perceived breathlessness (RPB) (8). Arterial blood was collected at rest, every minute during submaximal exercise, at peak workload, and repeatedly during 5 min of recovery for measurement of lactate, pH, PaO2, PaCO2 and standard bicarbonate (HCO3−). Whole blood samples were placed on ice, and analyzed immediately after the exercise test using a Yellow Springs Instruments analyzer (2300 STAT+) for lactate and an Instrumentation Laboratories analyzer (GEM 3000) for pH, blood gases, and HCO3−. In control subject 2-C, only venous blood samples were collected.
Cardiac output (Q) and V̇o2 were measured during a separate incremental maximal cycle exercise test in all patients and three control subjects using acetylene rebreathing and standard gas exchange, in which systemic arteriovenous oxygen difference (a-vO2 diff.) was calculated from the Fick equation: V̇o2 = Q × a-vO2 diff., as previously described (51).
Ventilatory and gas exchange measurements.
O2 uptake (V̇o2), carbon dioxide production (V̇co2), respiratory exchange ratio (RER, V̇co2/V̇o2), expired minute ventilation (V̇e, BTPS), and respiratory frequency were recorded on a custom-built computerized breath-by-breath system and averaged over 20-s intervals, which we used for trend analyses. Additionally, expired gas was collected for 3 min at rest, for 60 s at submaximal workloads, and during peak exercise in 200-liter PVC bags (Harvard Apparatus, Holliston, MA). The fractions of O2, CO2, and N2 in each bag were analyzed by mass spectrometry (Perkin-Elmer 1100, Waltham, MA), and V̇o2 and V̇co2 were calculated using standard equations over the period of expired gas collection. V̇e, BTPS was measured with a Tissot spirometer. Tidal volume (Vt) was calculated as V̇e/respiratory frequency, Rf. Peak exercise values reported are obtained from the last minute of exercise (Table 4).
Table 4.
Peak exercise response
| Subject | V̇o2peak, %Pred | V̇o2peak, ml·kg−1·min−1 | Workloadpeak, %Pred | HRpeak, %Pred | HRpeak, bpm | Vtpeak, liter | Rfpeak, breaths/min |
|---|---|---|---|---|---|---|---|
| 1-MM | 21 | 9.0 | 16 | 96 | 188 | 1.0 | 47 |
| 2-MM | 19 | 5.2 | 21 | 83 | 143 | 1.6 | 25 |
| 3-MM | 41 | 9.3 | 56 | 93 | 152 | 1.7 | 42 |
| 4-MM | 30 | 10.8 | 36 | 90 | 169 | 3.4 | 29 |
| 5-MM | 30 | 10.6 | 42 | 99 | 184 | 2.0 | 54 |
| Mean (SD) | 28 ± 9‡ | 9.0 ± 2.3‡ | 34 ± 16† | 92 ± 6 | 167 ± 20 | 1.9 ± 0.9 | 39 ± 12 |
| 1-C | 83 | 25.6 | 97 | 97 | 186 | 2.0 | 59 |
| 2-C | 86 | 34.7 | 90 | 99 | 193 | 1.8 | 45 |
| 3-C | 84 | 23.7 | 108 | 93 | 176 | 2.4 | 45 |
| 4-C | 134 | 32.0 | 160 | 95 | 159 | 2.9 | 41 |
| Mean (SD) | 96 ± 25 | 29.0 ± 5.2 | 114 ± 32 | 96 ± 3 | 179 ± 15 | 2.3 ± 0.5 | 48 ± 8 |
Values are expressed individually and as group means ± SD. MM, mitochondrial myopathy patients; C, control subjects; V̇o2peak, peak oxygen uptake; V̇o2peak, %Pred is adjusted for differences in body weight (Pred body weight); %Pred, percent of predicted value based on height, gender, and age; HRpeak, peak heart rate; bpm, beats per minute; Vtpeak, peak tidal volume; Rfpeak, peak respiratory frequency; Expired gas data at peak exercise are obtained from bags.
Significantly different from control group, P ≤ 0.01;
P ≤ 0.001.
Exercise ventilation relative to V̇co2 was examined by evaluating two separate slopes of the V̇e-V̇co2 relationship (ΔV̇e/ΔV̇co2): 1) using data from rest to the ventilatory compensation point for exercise-induced metabolic acidosis (49), and 2) using all data from rest to peak exercise (4). The ventilatory compensation point (VCP) was determined by fitting the V̇e vs. V̇co2 data from rest to peak exercise into two linear segments. The intersection of the two segments is the VCP (6).
Simple breathing mechanics were measured to characterize differences among subjects in tidal flow-volume patterns, expiratory flow limitation, and lung volume at rest and during exercise (5).
Statistical analysis.
Statistical analysis was carried out using SigmaStat software version 3.5 (Systat Software, Chicago, IL). One-way ANOVA with repeated measures was applied for the changes over time in each group. Differences between MM patient and control group were evaluated with an unpaired t-test. For comparisons in which data were not normally distributed, the Mann-Whitney rank sum test was applied. The level of statistical significance was set at P ≤ 0.05. Data are expressed as single values and/or means ± SD.
RESULTS
Pulmonary function and chemosensitivity.
A summary of physical characteristics of the MM patients and control group is presented in Table 2. Pulmonary function values are shown in Table 3. There were no statistically significant differences in subject characteristics or pulmonary function values between the patients and the control group.
Table 2.
Physical characteristics
| Subject | Sex | Age, yr | Height, cm | Weight, kg |
|---|---|---|---|---|
| 1-MM | female | 19 | 154 | 48 |
| 2-MM | female | 57 | 170 | 58 |
| 3-MM | male | 60 | 170 | 76 |
| 4-MM | male | 37 | 183 | 80 |
| 5-MM | male | 38 | 180 | 79 |
| Mean (SD) | 42 ± 17 | 171 ± 11 | 68 ± 14 | |
| 1-C | female | 24 | 174 | 79 |
| 2-C | female | 20 | 178 | 65 |
| 3-C | male | 35 | 180 | 104 |
| 4-C | male | 56 | 178 | 86 |
| Mean (SD) | 34 ± 16 | 178 ± 3 | 83 ± 16 |
Values are expressed individually as group means ± SD. MM, mitochondrial myopathy patients; C, control subjects.
Table 3.
Pulmonary function testing
| Subject | FVC, %Pred | FEV1, %Pred | TLC, %Pred | DLCO, %Pred | MIP, %Pred | MVV, %Pred |
|---|---|---|---|---|---|---|
| 1-MM | 75 | 82 | 74 | 100 | 55 | 93 |
| 2-MM | 105 | 86 | 105 | 129 | 77 | 77 |
| 3-MM | 90 | 93 | 88 | 130 | 78 | 86 |
| 4-MM | 125 | 118 | 108 | 101 | 97 | 112 |
| 5-MM | 76 | 75 | 85 | 139 | 85 | 80 |
| Mean (SD) | 94 ± 21 | 91 ± 17 | 92 ± 14 | 120 ± 18 | 78 ± 15 | 90 ± 14 |
| 1-C | 110 | 108 | 101 | 91 | 112 | 113 |
| 2-C | 97 | 101 | 85 | 108 | 93 | 111 |
| 3-C | 84 | 74 | 83 | 75 | 86 | 62 |
| 4-C | 101 | 97 | 104 | 119 | 124 | 104 |
| Mean (SD) | 98 ± 11 | 95 ± 15 | 93 ± 11 | 98 ± 19 | 104 ± 17 | 98 ± 24 |
Values are expressed individually and as group means ± SD. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; TLC, total lung capacity; DLCO, carbon monoxide diffusing capacity; MIP, maximum inspiratory pressure; MVV, maximal voluntary ventilation; %Pred, percent of predicted value based on height, sex, and age; MM, mitochondrial myopathy; C, control subjects.
The HVR (slope of ΔV̇e l·min−1/ΔSaO2%) was higher in MM patients than in our controls (MM = 0.85 ± 0.13, C = 0.28 ± 0.02, mean ± SD, P ≤ 0.05) but within the range of normal for data collected previously by our laboratory (17). The HCVR (slope of ΔV̇e l·min−1/ΔPetCO2, l·min−1·Torr−1; PetCO2, end-tidal Pco2) for patients was not significantly different from control subjects (MM = 2.18 ± 1.34, range 0.72–4.0; C = 0.95 ± 0.30) and within the range of normal for data collected previously by our laboratory (17) or reported elsewhere (24).
Rest.
Resting V̇e was not significantly different between the two groups except for a higher RER and lower PaCO2 in control subjects (mean data, Table 5), indicating that control subjects were hyperventilating at rest. MM patients showed a tendency for elevated arterial lactate levels and significantly lower pH values compared with controls.
Table 5.
Ventilatory and blood variables during incremental exercise and recovery
| Rest |
Ventilatory Compensation Point |
Peak Exercise |
Recovery - min 2 |
Recovery - min 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MM | C | MM | C | MM | C | MM | C | MM | C | |
| Workload, W | 25 ± 17* | 113 ± 15 | 58 ± 31‡ | 210 ± 35 | ||||||
| V̇o2, l/min | 0.36 ± 0.10 | 0.32 ± 0.05 | 0.58 ± 0.18‡ | 1.57 ± 0.17 | 0.67 ± 0.26‡ | 2.55 ± 0.38 | 0.56 ± 0.23 | 0.77 ± 0.15 | 0.49 ± 0.19 | 0.59 ± 0.14 |
| V̇co2, l/min | 0.29 ± 0.08 | 0.29 ± 0.08 | 0.76 ± 0.21† | 1.47 ± 0.34 | 1.27 ± 0.49‡ | 3.17 ± 0.44 | 0.69 ± 0.28 | 1.11 ± 0.31 | 0.48 ± 0.18 | 0.73 ± 0.13 |
| V̇e, l/min | 10.87 ± 2.84 | 10.91 ± 1.87 | 25.32 ± 6.62* | 37.40 ± 6.28 | 70.12 ± 30.92 | 106.91 ± 17.34 | 42.86 ± 21.05 | 41.69 ± 16.29 | 26.33 ± 10.31 | 27.82 ± 8.51 |
| V̇e/MVV, % | 9 ± 3 | 8 ± 3 | 21 ± 3 | 29 ± 7 | 55 ± 16* | 82 ± 17 | 33 ± 9 | 33 ± 17 | 22 ± 8 | 22 ± 10 |
| V̇e/V̇o2 | 31 ± 6 | 34 ± 5 | 45 ± 5‡ | 24 ± 2 | 104 ± 18‡ | 42 ± 8 | 76 ± 15 | 53 ± 14 | 55 ± 12 | 47 ± 11 |
| V̇e/V̇co2 | 38 ± 8 | 39 ± 9 | 33 ± 2‡ | 26 ± 2 | 54 ± 9† | 34 ± 7 | 58 ± 6‡ | 37 ± 5 | 52 ± 4† | 37 ± 5 |
| RER | 0.80 ± 0.03* | 0.96 ± 0.11 | 1.35 ± 0.18† | 0.93 ± 0.12 | 1.95 ± 0.31† | 1.25 ± 0.03 | 1.30 ± 0.16 | 1.42 ± 0.19 | 1.06 ± 0.15 | 1.27 ± 0.17 |
| RPB | 4 ± 2 | 3 ± 1 | 10 ± 0* | 7 ± 1 | 7 ± 2 | 4 ± 1 | 3 ± 1 | 2 ± 1 | ||
| Lactate, mmol/l | 2.80 ± 1.38 | 0.83 ± 0.25 | 5.63 ± 0.96† | 2.44 ± 0.52 | 13.36 ± 2.26* | 8.67 ± 0.47 | 15.22 ± 2.43* | 11.03 ± 1.11 | 14.48 ± 1.87 | 11.73 ± 1.42 |
| pH | 7.42 ± 0.02* | 7.48 ± 0.03 | 7.40 ± 0.02 | 7.40 ± 0.01 | 7.32 ± 0.03 | 7.35 ± 0.05 | 7.22 ± 0.04 | 7.28 ± 0.04 | 7.19 ± 0.05 | 7.24 ± 0.03 |
| PaO2, mmHg | 101 ± 6 | 119 ± 16 | 126 ± 6‡ | 94 ± 4 | 137 ± 8* | 109 ± 14 | 131 ± 6 | 122 ± 7 | 125 ± 5 | 126 ± 5 |
| PaCO2, mmHg | 37 ± 4 | 32 ± 6 | 34 ± 3* | 40 ± 2 | 24 ± 1† | 35 ± 5 | 24 ± 3* | 31 ± 1 | 27 ± 4 | 31 ± 3 |
| Std HCO3−, mmol/l | 24 ± 2 | 25 ± 2 | 21 ± 2* | 25 ± 2 | 13 ± 1† | 19 ± 2 | 10 ± 2* | 15 ± 2 | 11 ± 2 | 13 ± 2 |
Values are group means ± SD. V̇o2, oxygen uptake; V̇co2, carbon dioxide production; V̇e, expired minute ventilation; V̇e/MVV, %, ratio of minute ventilation and maximal voluntary ventilation; V̇e/V̇o2, ventilatory equivalent for O2; V̇e/V̇co2, ventilatory equivalent for CO2; RER, respiratory exchange ratio, V̇co2/V̇o2; RPB, rating for perceived breathing effort; PaO2, PaCO2, arterial blood gases; Std HCO3−, standard bicarbonate.
Significantly different from control group, P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
Exercise.
Peak oxidative capacity was markedly lower in MM patients than in control subjects expressed as % of predicted values (28), (Table 4). There were no significant differences in HRpeak, HR% of predicted values (26), peak Vt, or peak respiratory frequency.
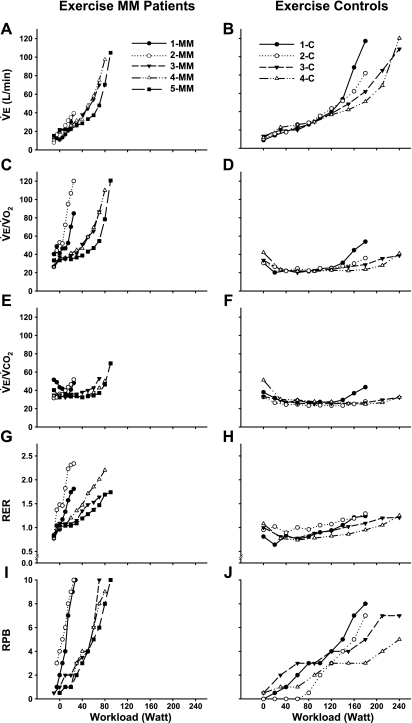
During incremental exercise (Table 5, Fig. 1 and 2), V̇e increased disproportionately in relation to workload and metabolic rate in MM patients (Fig. 1, A and B). At the VCP, workload, V̇o2, V̇co2, and V̇e were low in MM patients (Table 5), whereas the ventilatory equivalents for O2 and CO2, and RER were increased compared with controls. MM patients had higher arterial lactate levels and PaO2, and lower PaCO2 and HCO3− at the VCP.
Fig. 1.
Ventilatory and perceptional response during incremental exercise as function of workload in mitochondrial myopathy patients (MM; A, C, E, G, I) and control subjects (C; B, D, F, H, J). V̇e, expired minute ventilation; V̇e/V̇o2, ventilatory equivalent for O2; V̇e/V̇co2, ventilatory equivalent for CO2; RER, respiratory exchange ratio, V̇co2/V̇o2; RPB, rating for perceived breathing effort. Starting values are resting values for both groups in some MM patients followed by unloaded cycling.
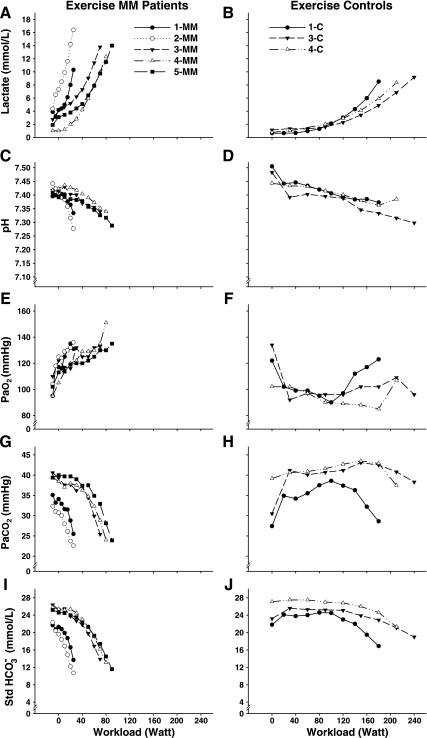
Fig. 2.
Arterial blood lactate (A, B), pH (C, D), blood gases (PaO2; E, F, and PaCO2; G, H), and standard bicarbonate (Std HCO3−; I and J) response during incremental exercise as a function of workload. Starting values are resting values for both groups, in some MM patients followed by unloaded cycling.
At peak exercise work capacity, V̇o2, V̇co2, and V̇e were lower in MM patients (Table 5). The ventilatory equivalents for O2 and CO2, and RER were markedly elevated, and perceived breathing effort (RPB) was significantly higher in MM patients at peak exercise. There were no differences in patients and controls for perceived overall exertion at peak exercise (RPE, MM = 19 ± 1, C = 18 ± 2). MM patients showed increased arterial lactate concentrations and decreased PaCO2 and HCO3−, with no significant difference in pH compared with controls. PaO2 values were increased at peak exercise.
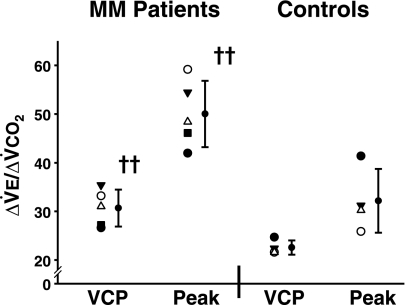
The exercise ventilatory response as indicated by the slope of ΔV̇e/ΔV̇co2 (Fig. 3) was increased in MM patients from rest to exercise at the VCP (MM = 30.7 ± 3.8, C = 22.6 ± 1.5, P ≤ 0.01), as well as for the complete slope from rest to peak exercise (MM = 50.0 ± 6.9, C = 32.2 ± 6.6, P ≤ 0.01) compared with controls. In control subjects, values for both V̇e-V̇co2 slopes were in the normal range (41, 49).
Fig. 3.
Exercise ventilatory response. VCP, slope ΔV̇e/ΔV̇co2 from rest to the ventilatory compensation point; Peak, V̇e/V̇co2 slope from rest to peak exercise. ††Significantly different from the control group, P ≤ 0.01.
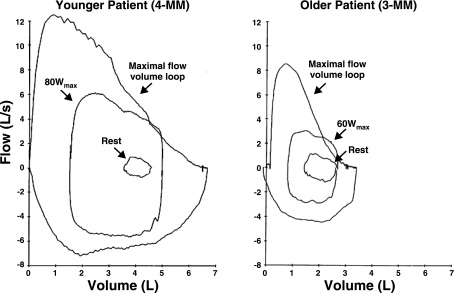
During peak exercise, the V̇e/maximum volume ventilation ratio was lower in MM patients (55%) compared with control subjects (82%). Maximal and tidal flow-volume loops measured at rest and during exercise for MM patients and controls were typical for their age and sex (Figs. 4 and 5), with no evidence of mechanical ventilatory limitation (i.e., inspiratory or expiratory flow limitation or hyperinflation) in patients.
Fig. 4.
Maximal and tidal flow-volume loops typical for two age ranges measured at rest and during exercise. Younger MM patient, 37 yr; older MM patient, 60 yr.
Recovery.
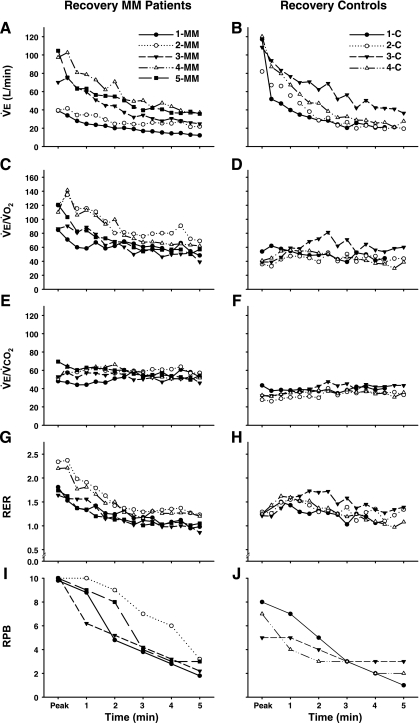
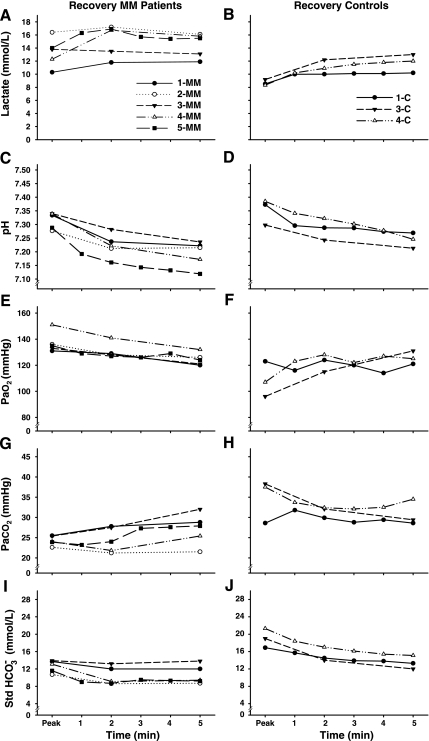
Individual data for 5 min of postexercise recovery are shown in Figs. 5 and 6, and mean data are shown in Table 5. During recovery, at a time when V̇e was rapidly falling (Fig. 5, A and B), blood lactate continued to increase and was elevated in MM patients compared with controls at minute 2 (Fig. 6, A and B; Table 5). pH progressively fell (P ≤ 0.001) after exercise with no significant difference between the patients and controls at 5 min of recovery (Fig. 6, C and D; Table 5). The ventilatory equivalent for O2 rapidly decreased (P ≤ 0.001, Fig. 5C) in patients, whereas V̇e/V̇o2 increased in control subjects (V̇e/V̇o2peak vs. minute 1, P ≤ 0.05, Fig. 5D). V̇e/V̇o2 was not different from controls at minute 5 of recovery (Table 5). The ventilatory equivalent for CO2 remained at levels corresponding to peak exercise in both MM patients and controls during the entire recovery period (Fig. 5, E and F; Table 5).
Fig. 5.
Ventilatory and perceptional response during recovery as function of recovery time in MM patients and C subjects. V̇e, expired minute ventilation (A, B); V̇e/V̇o2, ventilatory equivalent for O2 (C and D); V̇e/V̇co2, ventilatory equivalent for CO2 (E and F); RER, respiratory exchange ratio, V̇co2/V̇o2 (G and H); and RPB (I and J). Starting values are peak exercise values for both groups.
Fig. 6.
Arterial blood lactate, pH, blood gases (PaO2, PaCO2), and standard bicarbonate (Std HCO3−) response during recovery as a function of recovery time. Starting values are peak exercise values for both groups.
RER rapidly decreased in patients. In contrast, RER increased in controls during early recovery (MM, RERpeak vs. minute 2, P ≤ 0.01; C, RERpeak vs. min 1, P ≤ 0.001; Fig. 5, G and H). At 2 min of recovery, RER did not differ between patients and controls. PaCO2 remained lower during early recovery in MM patients compared with controls (Fig. 6, G and H; Table 5). In MM patients PaCO2 showed a tendency to increase (P = 0.089) and PaO2 decreased (P ≤ 0.001) throughout the recovery period in contrast to controls (Fig. 6, E and F). HCO3− was lower in MM patients during early recovery (Fig. 6, I and J; Table 5) compared with controls.
DISCUSSION
This is the first physiological study of the regulation of ventilation during exercise and recovery in patients with genetically and biochemically defined skeletal muscle mitochondrial defects in whom exercise is limited by symptoms of exertional dyspnea.
The main findings of our study are 1) exertional dyspnea is a feature of skeletal muscle mitochondrial defects that severely impair muscle oxidative phosphorylation and result in a hyperkinetic circulatory response to exercise, irrespective of the specific site of the metabolic block within the respiratory chain or TCA cycle; 2) exertional dyspnea in these patients is marked by exaggerated ventilation relative to metabolic rate, as indicated by high V̇e/V̇o2, V̇e/V̇co2, and RER and by an increased slope of ΔV̇e/ΔV̇co2 with no apparent signs of pulmonary insufficiency; 3) high arterial lactate concentrations in association with low PaCO2, low HCO3−, and markedly elevated V̇co2/V̇o2, imply that ventilatory compensation to metabolic acidosis contributes to this exaggerated ventilation during exercise; and 4) normalization of ventilation during recovery from exercise despite further increases in arterial lactate and decreases in pH indicates that severely impaired oxidative phosphorylation in working muscle stimulates ventilation by mechanisms that are specific to exercise and independent of metabolic acidosis.
Each of our patients had a severe limitation in muscle oxidative phosphorylation in which peak oxygen uptake during exercise was low and oxygen extraction from blood was severely restricted, as indicated by a systemic arteriovenous O2 difference that remained at or below resting levels during maximal effort exercise (Table 1). Associated was a hyperkinetic circulation during exercise in which the increase in oxygen transport (Δcardiac output) relative to the increase in oxygen utilization (ΔV̇o2) was 3–8 times normal.
In our patients, ventilation at rest was normal. In particular, there was no evidence of significant weakness of the muscles of respiration by the criteria of maximal inspiratory pressure and maximal voluntary ventilation. HVR and HCVR trended higher in MM patients but were generally consistent with values reported for healthy controls in our laboratory (mean, range; HVR, 0.71, 0.26–1.35; HCVR, 1.41, 0.88–2.13), (17). There is a considerable variation in normal ventilatory responses to hypoxia (57) and hypercapnia (mean, range; 2.69, −0.32−5.70), (24). Responses of all of our patients fell within these normal ranges to support the conclusion that there were no consistent alterations in respiratory chemosensitivity in our MM patients.
During exercise, patients developed dyspnea that was marked by greatly increased ventilation relative to workload and metabolic rate, as indicated by elevated peak V̇e/V̇o2, and V̇e/V̇co2, and an abnormally steep slope of increase in ΔV̇e/ΔV̇co2. Exaggerated ventilation also was indicated by steep increases in V̇co2/V̇o2, with values during peak exercise that ranged from 1.6 to 2.3. Although ventilation as a percentage of maximal voluntary ventilation (V̇e/MVV) at peak exercise was lower in patients than controls, values for both groups were in the normal range for maximal exercise in healthy subjects (5), and ventilatory flow volume loops during peak exercise were normal in patients, indicating no evidence of mechanical restriction of ventilation attributable to muscle weakness. Flow volume loops are definitive in the evaluation of ventilatory limitations during exercise and confirm that breathing mechanics are normal in our patients. Furthermore, ventilation relative to V̇o2 and V̇co2, and V̇co2 relative to V̇o2 increased throughout incremental exercise (Fig. 1), implying that dyspnea was attributable to progressive increases in ventilatory drive. These results define a pattern of gas exchange consistent with marked hyperventilation in MM patients who experience prominent exertional dyspnea that is linked to severely restricted muscle oxidative phosphorylation (12, 51) and is associated with a correspondingly exaggerated circulatory response to exercise.
Exertional dyspnea is not an invariant feature of mitochondrial myopathy (12, 51), so the presence of muscle mitochondrial enzyme deficiencies and the associated histological features of a mitochondrial abnormality are not sufficient to invoke the mechanism of mitochondrial myopathy-related exertional dyspnea that we describe. Our previous studies have indicated that peak levels of V̇e/V̇o2 during exercise in patients with MM correlate with the degree of impaired muscle oxidative phosphorylation and that patients with exercise hyperventilation of the magnitude seen in our patients with exertional dyspnea (i.e., with peak V̇e/V̇o2 ≥80) typically have profoundly limited muscle oxidative metabolism, as indicated by peak levels of a-vO2 difference that remain at or below resting levels (∼5 ml O2/dl), (51). Previous reports of MM patients with exertional dyspnea showed a lower magnitude of the ventilatory response to exercise than we observed. In the study of Hooper et al. (25), two of the three reported patients with exertional dyspnea had peak V̇e/V̇o2 of less than 46, i.e., levels that we consider within normal limits; and the mean peak RER, V̇co2/V̇o2 (as calculated from values for peak V̇e/V̇o2 and V̇e/V̇co2), in patients described by Flaherty et al. (16) as having exertional dyspnea that was attributed to an underlying mitochondrial defect had a mean peak RER of 1.03 (i.e., low normal), in contrast to an RER of ≥1.6 in the patients that we describe in this study and have previously reported (22).
Severely impaired muscle oxidative phosphorylation stimulates anaerobic glycolysis and accounts for dramatic increases in arterial lactate levels and steep declines in arterial pH in relation to workload in our patients (Fig. 2, A–D), (43). Thus, an attractive hypothesis to explain exaggerated ventilation during exercise in our patients is ventilatory compensation to rapid increases in lactic acidosis mediated by activation of carotid bodies (55, 56). Steep decreases in PaCO2 and HCO3− levels in MM patients during exercise are consistent with ventilatory compensation to acute metabolic acidosis to prevent continuing declines in arterial pH. Accordingly, despite markedly greater increases in arterial lactate, arterial pH with peak exercise in MM patients was comparable to control subjects.
CO2 produced in skeletal muscle metabolism is also considered a key regulator of ventilation during exercise in healthy humans (54), but it is not likely to be a contributor to exaggerated ventilation in mitochondrial myopathy patients. Metabolic CO2 produced in the decarboxylation of pyruvate and of TCA cycle intermediates is decreased in proportion to impaired muscle oxidative phosphorylation in mitochondrial defects (50). Alternatively, nonmetabolic CO2 could be increased as a result of bicarbonate buffering of increased H+ due to accelerated anaerobic metabolism in patients (54), although it has also been argued that such buffering of H+ does not increase CO2 production in the muscle over that which is produced by aerobic metabolism (44). Direct measurement of Pco2 in effluent venous blood from working muscle of patients with mitochondrial myopathies reveals low Pco2, despite high levels of lactate production, implying that this is not a major metabolic source of CO2 (50). This observation in combination with a steep decline in PaCO2 (Fig. 2G) indicates that high V̇co2 relative to V̇o2 during exercise in MM patients is a consequence rather than a cause of hyperventilation.
While increased lactic acidosis is an essential feature of exercise in patients with severe mitochondrial myopathy (22, 51), and acidosis is an established mechanism of stimulating ventilation (55), the question may be asked whether lactic acidosis is sufficient to account for the increased ventilatory drive during exercise in MM patients or whether other regulatory mechanisms may be operative. Central command by which ventilation is activated by a feedforward mechanism in parallel with the activation of cortical and spinal motor neurons is presumed to be an important regulator of exercise ventilation (14, 32). Both MM patients and control subjects performed maximal effort cycle exercise, and there were no differences in ratings of overall perceived exertion or maximal heart rate. Thus, we consider exaggerated central command in patients to be an unlikely mechanism for increased ventilatory drive. However, since thin-fiber muscle afferents may provide a somatosensory feedback signal that modulates central command (2), the level of contribution from central command to exaggerated exercise ventilation in patients is uncertain (30, 60).
Medullary respiratory centers may be activated by peripheral neuronal reflexes originating in skeletal muscle through activation of mechanoreceptors and metaboreceptors in active muscle. The role of reflex activation of the circulation, the exercise pressor reflex, mediated by stimulation of predominantly mechanically sensitive group III neural fibers, and predominantly metabolically sensitive group IV fibers, is well established (47). A central feature of normal circulatory regulation in exercise is a close matching of oxygen delivery to skeletal muscle oxygen utilization. Our previous studies indicate that mitochondrial defects in skeletal muscle dramatically disrupt this relationship (21, 22, 51, 53). During exercise in mitochondrial myopathies, oxygen utilization is blocked as indicated by low peak systemic a-vO2 difference and high oxygen levels in active muscle (19). At the same time, oxygen transport is exaggerated (indicated by high cardiac output relative to workload and V̇o2) with the most severe mismatches in O2 delivery relative to utilization found in the most severe mitochondrial defects (51). High exercise cardiac output in MM patients is associated with exaggerated increases in norepinephrine and epinephrine compared with healthy subjects exercising at the same absolute or relative workload (53), indicating an exaggerated sympathetic neural response to exercise in these patients consistent with the view that an exaggerated ergoreflex, originating in working muscle is an important mechanism of the hyperkinetic circulatory response to exercise in mitochondrial myopathy. The patients in our study have a classical hyperkinetic circulatory response to exercise, so it seems plausible that a similar mechanism may contribute to exaggerated exercise ventilation in these patients.
Ventilation in exercising healthy subjects has been shown to be influenced by ergoreflexes using the method employed by Alam and Smirk to demonstrate the role of muscle metaboreflexes in the exercise pressor response (1). Postexercise restriction of limb venous return by applying suprasystolic pressure, which traps metabolites produced during exercise, results in sustained increased ventilation (11, 45). This approach isolates central command and mechanoreflexes from the metaboreflex. The metabolic effectors of this reflex are incompletely understood and may be multiple (32). Research in cats suggests that H+ accumulation in skeletal muscle arising from lactic acid is a potent metabolic stimulus for both ventilatory and cardiovascular reflexes (46). Also it has been demonstrated that acid-sensing ion channels, the receptors for lactic acid, contribute to the metaboreceptor component of the exercise pressor reflex (39). Other studies have implicated a combination of metabolites in the activation of muscle afferents, including lactate, protons, and ATP (30, 37). However, Amann et al. (2) showed that group III and IV muscle afferents augment exercise ventilation at low levels of dynamic exercise that do not increase muscle lactate or proton concentrations (2).
Our study was designed to illuminate the relative contribution of these regulatory mechanisms by assessing ventilation during exercise, when central command, ergoreflexes, and circulating metabolites may modulate ventilation and during recovery from exercise, when central command is withdrawn and ergoreflexes rapidly decline but acidosis is increasing. During early recovery, ventilation decreases precipitously in all subjects, despite continued elevation of blood lactate, and decreasing pH levels. Muscle continues to release lactate following exercise and interstitial pH falls for 1–2 min postexercise (29). Acidosis-mediated hyperventilation following exercise in control subjects is suggested by an increase in RER (Fig. 5H) and V̇e/V̇o2 (Fig. 5D) and by the fact that PaCO2 falls (Fig. 6H, Table 5). In contrast, in MM patients, RER (Fig. 5G) and V̇e/V̇o2 (Fig. 5C) rapidly falls and PaCO2 rises (Fig. 6G, Table 5). This suggests that increased ventilation that would be expected to accompany increased lactic acidosis during recovery from exercise in patients is masked by the withdrawal of exaggerated ventilatory drive that is specific to exercise.
Our results highlight the critical role of normal skeletal muscle oxidative phosphorylation in the regulation of ventilation during exercise and reveal the extent to which ventilation is exaggerated to account for dyspnea as a factor limiting exercise, when muscle mitochondrial oxidative phosphorylation is severely impaired. Muscle mitochondrial dysfunction likely promotes exaggerated ventilation in exercise, in part by lactic acidosis, mediated by increased anaerobic glycolysis; however, we conclude that enhanced lactic acidosis alone cannot fully account for hyperventilation. Our results strongly suggest that direct activation of ventilation via reflexes originating in active muscle is a key mechanism of exaggerated exercise ventilation to account for symptoms of exertional dyspnea in patients with severe mitochondrial myopathy.
Perspectives and Significance
This study demonstrates that exertional dyspnea in patients with severe mitochondrial myopathies is associated with greatly exaggerated ventilation relative to muscle metabolic rate that is not fully explained by increased lactic acidosis. Our results suggest that activation of ventilatory reflexes via metaboreceptors in working muscle contributes to increased ventilatory drive in MM and thus implicates metabolites that reflect limited muscle oxidative phosphorylation in mediating this response.
GRANTS
This work has been supported by grants from the National Institutes of Health (NIAMS Grant R01 AR050597), a Department of Veterans Affairs Merit Review, King Charitable Foundation, Cain Foundation, and Texas Health Presbyterian Hospital Dallas. TT is a Chercheur Boursier Investigator of the Fonds de Recherche en Santé du Québec.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We sincerely thank the subjects who participated in the study, and M. Newby, P. Fowler, M. Klocko, T. Semon, K. Ranasinghe who assisted with data collection and analysis. We thank Karlman Wasserman for fruitful discussions.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Thoracic Society Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 152: 1107–1136, 1995 [DOI] [PubMed] [Google Scholar]
- 3a. Andreu AL, Hanna MG, Reichmann H, Bruno C, Penn AS, Tanji K, Pallotti F, Iwata S, Bonilla E, Lach B, Morgan-Hughes J, DiMauro S. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N Engl J Med 341: 1037–1044, 1999 [DOI] [PubMed] [Google Scholar]
- 3b. Anitori R, Manning K, Quan F, Weleber RG, Buist NR, Shoubridge EA, Kennaway NG. Contrasting phenotypes in three patients with novel mutations in mitochondrial tRNA genes. Mol Genet Metab 84: 176–188, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest 124: 720–727, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Babb TG. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med Sci Sports Exerc 31: S12–S22, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Berglund E, Birath G, Bjure J, Grimby G, Kjellmer I, Sandqvist L, Soderholm B. Spirometric studies in normal subjects. I. Forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand 173: 185–192, 1963 [PubMed] [Google Scholar]
- 8. Borg G, Ljunggren G, Ceci R. The increase of perceived exertion, aches and pain in the legs, heart rate and blood lactate during exercise on a bicycle ergometer. Eur J Appl Physiol Occup Physiol 54: 343–349, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Bruschi C, Cerveri I, Zoia MC, Fanfulla F, Fiorentini M, Casali L, Grassi M, Grassi C. Reference values of maximal respiratory mouth pressures: a population-based study. Am Rev Respir Dis 146: 790–793, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am Rev Respir Dis 84: 789–806, 1961 [DOI] [PubMed] [Google Scholar]
- 11. Comroe JH, Schmidt CF. Reflexes from the limbs as a factor in hyperpnea of muscular exercise. Am J Physiol 138: 536–547, 1943 [Google Scholar]
- 12. Dandurand RJ, Matthews PM, Arnold DL, Eidelman DH. Mitochondrial disease. Pulmonary function, exercise performance, and blood lactate levels. Chest 108: 182–189, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Dempsey JA. Challenges for future research in exercise physiology as applied to the respiratory system. Exerc Sport Sci Rev 34: 92–98, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Eldridge FL. Central integration of mechanisms in exercise hyperpnea. Med Sci Sports Exerc 26: 319–327, 1994 [PubMed] [Google Scholar]
- 15. Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Flaherty KR, Wald J, Weisman IM, Zeballos RJ, Schork MA, Blaivas M, Rubenfire M, Martinez FJ. Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med 164: 425–432, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Ge RL, Stone JA, Levine BD, Babb TG. Exaggerated respiratory chemosensitivity and association with SaO2 level at 3568 m in obesity. Respir Physiol Neurobiol 146: 47–54, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Goldman HI, Becklake MR. Respiratory function tests; normal values at median altitudes and the prediction of normal results. Am Rev Tuberc 79: 457–467, 1959 [DOI] [PubMed] [Google Scholar]
- 19. Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, Vago P, Marconi C, Morandi L. Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve 35: 510–520, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hall RE, Henriksson KG, Lewis SF, Haller RG, Kennaway NG. Mitochondrial myopathy with succinate dehydrogenase and aconitase deficiency. Abnormalities of several iron-sulfur proteins. J Clin Invest 92: 2660–2666, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wibom R, Sahlin K, Areskog NH, Gunder M, Ayyad K, Blomqvist CG, Hall RE, Thuillier P, Kennaway NG, Lewis SF. Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. J Clin Invest 88: 1197–1206, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haller RG, Lewis SF, Estabrook RW, DiMauro S, Servidei S, Foster DW. Exercise intolerance, lactic acidosis, and abnormal cardiopulmonary regulation in exercise associated with adult skeletal muscle cytochrome c oxidase deficiency. J Clin Invest 84: 155–161, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haouzi P. Theories on the nature of the coupling between ventilation and gas exchange during exercise. Respir Physiol Neurobiol 151: 267–279, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hirshman CA, McCullough RE, Weil JV. Normal values for hypoxic and hypercapnic ventilatory drives in man. J Appl Physiol 38: 1095–1098, 1975 [DOI] [PubMed] [Google Scholar]
- 25. Hooper RG, Thomas AR, Kearl RA. Mitochondrial enzyme deficiency causing exercise limitation in normal-appearing adults. Chest 107: 317–322, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J Appl Physiol 53: 799–804, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Janssen AJ, Schuelke M, Smeitink JA, Trijbels FJ, Sengers RC, Lucke B, Wintjes LT, Morava E, van Engelen BG, Smits BW, Hol FA, Siers MH, Ter Laak H, van der Knaap MS, Van Spronsen FJ, Rodenburg RJ, van den Heuvel LP. Muscle 3243A→G mutation load and capacity of the mitochondrial energy-generating system. Ann Neurol 63: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Jones NL. Clinical Exercise Testing. Philadelphia: PA: WB Saunders, 1988 [Google Scholar]
- 29. Juel C. Regulation of pH in human skeletal muscle: adaptations to physical activity. Acta Physiol 193: 17–24, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Kaufman MP. Control of breathing during dynamic exercise by thin fiber muscle afferents. J Appl Physiol 109: 947–948, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447 [Google Scholar]
- 32. Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127: 725–734, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis 113: 587–600, 1976 [DOI] [PubMed] [Google Scholar]
- 35. Kory RC, Callahan R, Boren HG, Syner JC. The Veterans Administration-Army cooperative study of pulmonary function. I. Clinical spirometry in normal men. Am J Med 30: 243–258, 1961 [DOI] [PubMed] [Google Scholar]
- 36. Larsson LE, Linderholm H, Mueller R, Ringqvist T, Soernaes R. Hereditary metabolic myopathy with paroxysmal myoglobinuria due to abnormal glycolysis. J Neurol Neurosurg Psychiatry 27: 361–380, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linderholm H, Muller R, Ringqvist T, Sornas R. Hereditary abnormal muscle metabolism with hyperkinetic circulation during exercise. Acta Med Scand 185: 153–166, 1969 [DOI] [PubMed] [Google Scholar]
- 39. McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 297: H443–H449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mochel F, Knight MA, Tong WH, Hernandez D, Ayyad K, Taivassalo T, Andersen PM, Singleton A, Rouault TA, Fischbeck KH, Haller RG. Splice mutation in the iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am J Hum Genet 82: 652–660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore B, Brubaker PH, Stewart KP, Kitzman DW. V̇e/V̇co2 slope in older heart failure patients with normal versus reduced ejection fraction compared with age-matched healthy controls. J Card Fail 13: 259–262, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Olsson A, Lind L, Thornell LE, Holmberg M. Myopathy with lactic acidosis is linked to chromosome 12q23.3–2411 and caused by an intron mutation in the ISCU gene resulting in a splicing defect. Hum Mol Genet 17: 1666–1672, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Peronnet F. Lactate as an end-product and fuel. Dtsch Z Sportmed 61: 112–116, 2010 [Google Scholar]
- 44. Peronnet F, Aguilaniu B. Lactic acid buffering, nonmetabolic CO2 and exercise hyperventilation: a critical reappraisal. Respir Physiol Neurobiol 150: 4–18, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol Heart Circ Physiol 269: H1428–H1436, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989 [DOI] [PubMed] [Google Scholar]
- 47. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 166: 1443–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Taivassalo T, Abbott A, Wyrick P, Haller RG. Venous oxygen levels during aerobic forearm exercise: An index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol 51: 38–44, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 126: 413–423, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Taivassalo T, Shoubridge EA, Chen J, Kennaway NG, DiMauro S, Arnold DL, Haller RG. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol 50: 133–141, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Vissing J, Galbo H, Haller RG. Exercise fuel mobilization in mitochondrial myopathy: a metabolic dilemma. Ann Neurol 40: 655–662, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Wasserman K. Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am J Physiol Endocrinol Metab 266: E519–E539, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 56. Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol 39: 354–358, 1975 [DOI] [PubMed] [Google Scholar]
- 57. Weil JV. Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol 135: 239–246, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Weil JV, Byrne-Quinn E, Sodal IE, Filley GF, Grover RF. Acquired attenuation of chemoreceptor function in chronically hypoxic man at high altitude. J Clin Invest 50: 186–195, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. West JB, Peters RM, Jr, Aksnes G, Maret KH, Milledge JS, Schoene RB. Nocturnal periodic breathing at altitudes of 6,300 and 8,050 m. J Appl Physiol 61: 280–287, 1986 [DOI] [PubMed] [Google Scholar]
- 60. Williamson JW. The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95: 1043–1048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]