Abstract
Accumulation of cholesteryl ester (CE)-enriched macrophage foam cells is central to the development of atherosclerotic lesions. Intracellular CE hydrolysis is the rate-limiting step in the removal of free cholesterol from macrophage foam cells. Enhancing this process by transgenic overexpression of CE hydrolase (CEH) resulted in a significant decrease in diet-induced atherosclerosis in LDL receptor-deficient (LDLR−/−) mice. However, for development of this step as an antiatherosclerotic target it is imperative to demonstrate that increase in CE hydrolysis after initiation of lesion formation will also attenuate further lesion progression. The objective of the present study was to directly address this issue using an animal model. LDLR−/− mice were fed a high-fat high-cholesterol diet (Western Diet) for 8 wk to initiate lesion formation and were then divided into three groups. Group 1 mice were killed to determine baseline lesion development. Mice in groups 2 and 3 were irradiated and transplanted with either LDLR−/− or LDLR−/−CEH transgenic bone marrow and maintained on Western Diet. Atherosclerotic lesion progression was assessed after 12 wk. While a more than fourfold increase in total lesions (compared to group 1) was seen in group 2 receiving LDLR−/− marrow, a significantly lower increase (<2-fold) was noted in mice reconstituted with CEH transgenic marrow (group 3). Lesions in group 3 mice were also more cellular with smaller necrotic cores. Lesion progression is associated with a switch in macrophage phenotype from anti-inflammatory M2 to proinflammatory M1 phenotype and is consistent with reduced lesion progression. Aortas from group 3 mice contained a significantly higher percentage of macrophages in M2 phenotype (Ly6Clo). These data demonstrate for the first time that enhancing macrophage CE hydrolysis even after lesion initiation can still attenuate further lesion progression and also switches the phenotype of lesion-associated macrophages to anti-inflammatory M2 phenotype establishing intracellular CE hydrolysis as an anti-atherosclerotic as well as anti-inflammatory target.
Keywords: cholesteryl ester hydrolysis, macrophage phenotype, diet-induced atherosclerotic lesion progression
accumulation of lipid-laden macrophage foam cells is central to the formation of atherosclerotic lesions. An imbalance between the uptake of modified and cholesteryl ester (CE)-rich lipoproteins via the scavenger receptors, namely CD-36 and SR-A, and efflux of unesterified or free cholesterol (FC) is recognized as the underlying mechanism. Since cholesterol is stored as CE within the foam cells and effluxed only as FC, intracellular hydrolysis of stored CE is the obligatory first and rate-limiting step in FC efflux from macrophage foam cells associated with atherosclerotic lesions in the artery wall (15). Consistently, enhancing intracellular CE hydrolysis by overexpression of CE hydrolase (CEH) leads to increased mobilization of cellular CE resulting in decreased cellular lipid burden (5). Furthermore, increased efflux of FC by all known efflux pathways is noted from human monocyte macrophage cell line THP-1 that stably overexpress CEH (21). In accordance with its role in enhancing mobilization of cellular CE and efflux of FC thus generated, macrophage-specific transgenic expression of CEH enhanced in vivo reverse cholesterol transport and consequently attenuated diet-induced atherosclerosis in LDL receptor-deficient (LDLR−/−) mice (20). These beneficial effects of enhanced intracellular CE hydrolysis underscore the importance of developing this process as a potential therapeutic target.
While we have established the antiatherogenic role of CEH by demonstrating attenuation of diet-induced atherosclerosis in LDLR−/− CEH transgenic (LDLR−/−CEHTg) mice, it needs to be emphasized that in this model, macrophages have increased intracellular CE hydrolysis from birth. Atherosclerotic lesion development in humans, starts early in life depending on various risk factors; clinical manifestations are not apparent until much later, and therapeutic interventions are only initiated thereafter (16). Therefore, to be therapeutically relevant, it is imperative to demonstrate that increased expression of CEH can prevent or reduce the progression of atherosclerosis even after the lesion formation has already begun. The objective of the present study was to directly address this issue using an animal model. We employed bone marrow transplantation (BMT) to repopulate the marrow of irradiated LDLR−/− mice with existing diet-induced lesions. Donor marrow was from either LDLR−/− or LDLR−/−CEHTg mice. We hypothesized that increasing expression of CEH in macrophages after the atherosclerotic lesion development has been initiated would attenuate the progression of diet-induced lesions in LDLR−/− mice. Data are presented to demonstrate that following irradiation when the bone marrow was reconstituted with CEHTg marrow, diet-induced lesion progression was significantly reduced.
EXPERIMENTAL DESIGN
Animals and diets.
Development and characterization of macrophage-specific CEH transgenic mice in LDLR−/− background has been described elsewhere (20). Male and Female LDLR−/− (originally obtained from The Jackson Laboratory; stock no. 002207, B6.129S7-Ldlrtm1Her/J) and CEH transgenic mice in LDLR−/− background (LDLR−/−CEHTg) were used as donors. LDLR−/− mice (of both genders) served as recipients and were fed a high-fat high-cholesterol diet(Western Diet, cat. no. TD88137; Harlan Teklad) for the indicated duration. The animals were housed in ventilated cages in aseptic animal rooms maintained on 12:12-h light-dark cycle and had free access to food (rodent chow or TD88137) and water. All procedures and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Bone marrow transplantation.
Ten-week-old LDLR−/− mice were fed Western Diet for 8 wk. Mice were then divided into three groups: mice in group 1 (baseline) were killed to determine the lesion development at the time of BMT; mice in groups 2 and 3 were irradiated (900 rads from a cesium gamma source) and reconstituted after 4 h with bone marrow (5–10 × 106 cells) isolated from either LDLR−/− or LDLR−/−CEHTg mice, respectively (Fig. 1A).All mice received antibiotics (100 mg/l neomycin and 10 mg/l polymyxin B) in drinking water for 1 wk before and 2 wk after BMT (10). The mice in groups 2 and 3 were continued on Western Diet for an additional 12 wk. At the time of death, DNA was extracted from the total bone marrow cells and analyzed by PCR to confirm reconstitution using 5′-ATGGCAAAGTGCTGGGGAAGTTC-3′ as the upstream primer and 5′-TTCACCAGAACAGAAGTGAGGGC-3′ as the downstream primer to yield a 681 bp product corresponding to the nucleotide sequence between residues 133 and 792 of human macrophage CEH cDNA (6).
Fig. 1.
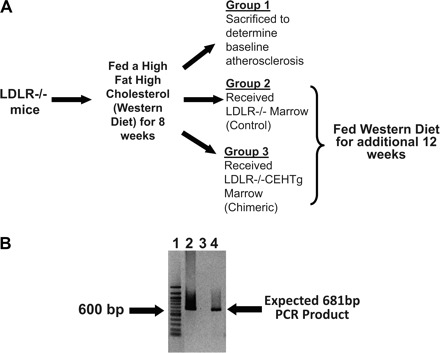
Experimental design and confirmation of bone marrow reconstitution. A: experimental design. LDLR−/−, LDL receptor-deficient. B: total DNA was isolated from the bone marrow cells, and reconstitution with cholesteryl ester hydrolase transgenic (CEHTg) marrow was confirmed by PCR as described under Bone marrow transplantation. Total PCR reaction obtained using DNA isolated from marrow cells of CEHTg mice (lane 2, positive control), group 2 mice (lane 3), and group 3 mice (lane 4) was separated on a 1% agarose gel and stained with ethidium bromide. Lane 1: 100 bp DNA ladder and band corresponding to 600 bp in this lane is marked.
Total plasma cholesterol and cholesterol distribution among plasma lipoproteins.
A modified Column Lipoprotein Profile method was used (4). Whole plasma aliquots frozen and stored at −80°C were thawed at 4°C. Total plasma cholesterol concentration was determined by a microenzymatic method. Approximately 20 μg of cholesterol was injected onto an fast protein liquid chromatography system (Superose 6 h 10/30 column; Amersham Biosciences) with online mixing of the column effluent with enzymatic reagent (Cholesterol Liquid Stable; Thermo Electron) for acquiring lipoprotein cholesterol profiles. The output generated is in millivolts. The data were acquired on a personal computer running ChromPerfect Spirit chromatography software (Justice Software). The system was optimized so that the area under the profiles was proportional to the cholesterol mass. Area percentage in each lipoprotein fraction, VLDL, LDL, and HDL, was applied to total plasma cholesterol to calculate cholesterol concentration in the lipoprotein fractions.
Quantitative atherosclerosis analyses.
The aorta was dissected from the heart to the iliac bifurcation, cleaned of any surrounding tissue, opened longitudinally, pinned on black wax, and fixed for 24 h in 10% buffered formalin. The fixed aortas were imaged on a black background using a Canon digital camera fitted with a 60-mm, f/2.8-macro lens. Total area and area occupied by the lesions in the aortic arch and total aorta were determined using Axiovision Image Analysis software (Carl Zeiss). The person quantifying the area occupied by lesions was blinded to the identity of the images. Extreme care was taken to ensure that any residual adventitial fat that appeared translucent on the images was not included in the area occupied by the lesions that were dense and opaque.
Morphological analyses of the lesions.
Hearts were fixed in buffered formalin, paraffin embedded, and sectioned. Once the aortic sinus was visible, three to four serial sections (4 μm thick) were transferred to numbered slides. Consequently numbered slides were then stained with Masson's Trichrome stain and hematoxylin and eosin. Images were acquired with Zeiss Observer A1 inverted microscope.
Flow cytometry analysis of the cells associated with the aorta.
The aortic arch was cleaned, dissected, and digested as described by Galkina et al. (3). Isolated cells were resuspended in fluorescence-activated cell sorting (FACS) buffer containing Fc receptor block and incubated with fluorescently labeled antibodies for 20 min at 4°C. After the cells were washed, specific immunofluorescent staining of individual cells was detected by flow cytometry (Canto II; BD Biosciences), and the data were analyzed using FlowJO (Tree Star) software. The following antibodies were used: anti-mouse CD45-PE (leukocytes), anti-mouse CD3a FITC (T-cells), anti-mouse CD19-PE-Cy7 (B-cells), anti-mouse CD11b-PerCP-Cy5.5 (macrophages), anti-mouse Ly6C-APC, and the respective isotype controls (all antibodies were obtained from eBiosciences). Spleens from C57BL/6 mice were collected and digested as described, and the cell suspension was used as positive control.
Real-time PCR.
Total RNA was extracted using RNeasy kit (Qiagen). Complementary DNA was synthesized using high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed on a Stratagene Mx3000P machine, using TaqMan Universal PCR Master Mix and optimized probe and primer sets from Applied Biosystems. The following probes were used: IL-1β (Mm00434228_m1), TNF-α (Mm00443258_m1), Arginase I (Mm00475988_m1), and IL-10 (Mm00439616).
Statistical analysis.
Results were analyzed by two-tailed Student's t-test with the use of GraphPad Prism Software, version 4, which tests for normality before running the t-test. While statistical significance for all comparisons was assigned at P < 0.05, the individual P values are included in the text.
RESULTS
Successful reconstitution of the bone marrow.
Total DNA extracted from the bone marrow cells at the time of death was analyzed for the presence of human CEH transgene by PCR. As shown in Fig. 1B, the expected 681 bp PCR product was obtained when DNA from group 3 mice (which received the CEHTg marrow) was used as the template confirming reconstitution with CEHTg marrow (lane 4). Consistently, this band was not seen when DNA from group 2 mice received LDLR−/− marrow (lane 3). Total DNA from the bone marrow cells of a CEHTg mouse was used as a positive control (lane 2).
Reconstitution with CEHTg marrow did not alter the plasma lipoprotein profiles.
Fasting plasma was obtained from all mice at the time of death and was analyzed for total cholesterol and distribution of cholesterol among lipoprotein fractions. As shown in Fig. 2, there was no significant difference in the total plasma cholesterol or the distribution of cholesterol among the plasma lipoproteins between the three groups, suggesting that neither an additional 12 wk of feeding with Western Diet nor the reconstitution with CEHTg marrow altered plasma or lipoprotein cholesterol concentrations. No significant difference was seen in plasma or lipoprotein cholesterol distribution between males and females. These data are consistent with our earlier data where no significant changes in plasma lipoprotein profiles were noted between LDLR−/− and LDLR−/−CEHTg mice (20).
Fig. 2.
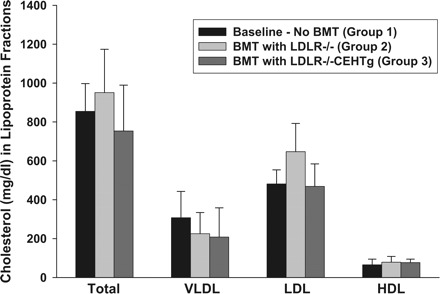
Distribution of cholesterol among plasma lipoprotein fractions. Plasma was obtained from fasted mice at the time of death. An aliquot of the plasma from each animal was applied to Superose 6 fast protein liquid chromato-graphy column (flow rate, 0.4 ml/min), and cholesterol content of individual lipoproteins was determined by an online assay as described under Total plasma cholesterol and cholesterol distribution among plasma lipoproteins. BMT, bone marrow transplantation. Data are expressed as means ± SE for n = 9 per group. No significant differences were noted between males and females.
Reconstitution with CEHTg marrow significantly reduced the progression of atherosclerotic lesion area.
The percent area of aorta covered with atherosclerotic lesions was determined by en face analyses, and Fig. 3 shows representative images of aortas obtained from group 1 (baseline), group 2 (BMT with marrow from LDLR−/− mice), and group 3 (BMT with marrow from LDLR−/−CEHTg mice). While very few lesions were noticeable in the thoracic and abdominal aorta, as expected with this mouse model, the aortic arch displayed the most profound lesions. Compared with group 1, there was a marked increase in atherosclerotic lesions in group 2, and this increase was noticeably less in group 3. The quantification of the surface area occupied by atherosclerotic lesions is shown in Fig. 4. Compared with baseline after 8 wk of feeding (group 1), an additional 12 wk of feeding led to a significant increase in total surface area occupied by atherosclerotic lesions in group 2 mice that received LDLR−/− marrow (males: 1.01 ± 1.02 vs. 4.4 ± 2.8, P = 0.022; females: 1.06 ± 0.89 vs. 4.9 ± 0.9, P = 0.0001). Although, compared with group 1, there was a noticeable increase in the total surface area in group 3 mice that were reconstituted with LDLR−/−CEHTg marrow, this increase did not reach statistical significance (males: 1.01 ± 1.02 vs. 1.7 ± 0.9, P = 0.202; females: 1.06 ± 0.89 vs. 2.1 ± 0.9, P = 0.089) (See Fig. 4A). Consistently, there was a significant increase in the area occupied by the lesions in the aortic arch in group 2 compared with group 1 (males: 2.9 ± 3.3 vs. 10.6 ± 7.3, P = 0.042; females: 2.9 ± 2.1 vs. 14.1 ± 3.8, P = 0.0004) and this difference did not reach statistical significance for group 3 (males: 2.9 ± 3.3 vs. 4.5 ± 3.8, P = 0.44; females: 2.9 ± 2.1 vs. 5.5 ± 2.6, P = 0.101) (See Fig. 4B).
Fig. 3.
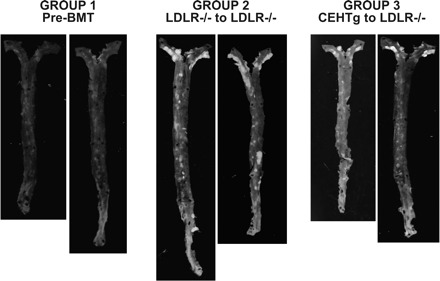
Decreased progression of lesions in mice reconstituted with marrow from LDLR−/−CEHTg mice (group 3) compared with those reconstituted with marrow from LDLR−/− mice (group 2). LDLR−/− (10 wk old) were fed Western Diet (cat. no. TD88137; Harlan Teklad) for 8 wk, and group 1 (baseline) mice were killed before BMT. Group 2 and group 3 mice were irradiated and reconstituted with marrow from LDLR−/− mice or LDLR−/−CEHTg mice, respectively. After an additional 12 wk of feeding with Western Diet, aortas (from aortic sinus to the iliac bifurcation) were isolated, opened, and pinned to expose the lesions and then imaged on a black background. Representative images of the aortas are shown.
Fig. 4.
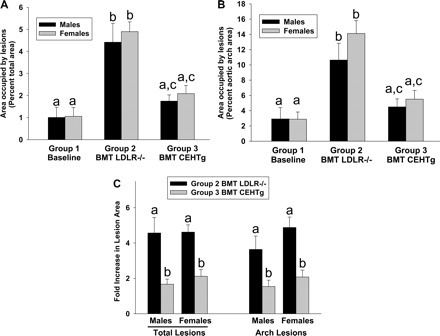
Reconstitution with CEHTg marrow attenuates diet-induced lesion progression. Images of the aortas were analyzed by AxioVision Software as described in Quantitative atherosclerosis analyses. A: quantification of the surface area occupied by the lesions in the entire aorta. B: lesion area in the aortic arch. Data (% area occupied by the lesions) are expressed as means ± SE. Number of animals in each group were males (n = 5, 11, or 11) and females (n = 5, 5, or 6), respectively, for baseline (group 1), mice reconstituted with LDLR−/− marrow (group 2), or LDLR−/−CEHTg marrow (group 3). C: lesion progression (total or arch alone) above the baseline (group 1). Dissimilar letters above the bars indicate significant (P < 0.05) differences. Individual P values are included in results.
Lesion progression was also calculated as fold increase over baseline (group 1) and is shown in Fig. 4C. There was more than fourfold increase in the area occupied by lesion in total aorta in mice reconstituted with LDLR−/− marrow compared with less than twofold increase in mice reconstituted with LDLR−/−CEHTg marrow (males: 4.6 ± 2.9 vs. 1.7 ± 0.9, P = 0.007; females: 4.6 ± 0.9 vs. 2.1 ± 0.9, P = 0.0008). Consistently, the progression of the aortic arch lesions was significantly decreased in mice reconstituted with LDLR−/−CEHTg marrow compared with mice reconstituted with LDLR−/− marrow (males: 3.6 ± 2.5 vs. 1.5 ± 1.3, P = 0.022; females: 4.9 ± 1.3 vs. 2.1 ± 0.9, P = 0.001). Collectively, these data indicate that reconstitution with LDLR−/−CEHTg marrow significantly attenuated the progression of total as well as aortic arch lesions in both males and females. This observed lack of gender differences is consistent with our earlier results showing similar (>50%) reduction in diet-induced atherosclerosis in LDLR−/−CEHTg mice (20).
Detailed analyses of lesion morphology due to macrophage-specific transgenic over expression of CEH have been reported before and were not repeated in this study. Nonetheless, Fig. 5 shows representative hematoxylin and eosin and Masson's Trichrome-stained images of the aortic root lesions, and, consistent with our earlier results with LDLR−/−CEHTg mice (20), lesions from mice reconstituted with LDLR−/−CEHTg marrow were more cellular and with reduced necrotic core.
Fig. 5.
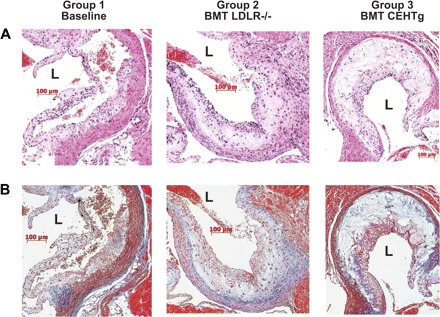
Lesions in mice reconstituted with marrow from LDLR−/−CEHTg are more cellular. Hearts from mice fed Western Diet were fixed, paraffin embedded, and sectioned as described under Morphological analyses of the lesions. Representative aortic sinus lesion on 1 of the leaflets stained with hematoxylin and eosin (A) or with Masson's Trichrome stain (B) is shown. The lumen of the aortic sinus is marked (L) for orientation. The increased number of visible nuclei illustrates the highly cellular nature of the lesion from mice reconstituted with LDLR−/−CEHTg marrow. Limited blue staining in the core of the plaque indicates reduced collagen levels in the lesion from mice receiving marrow from LDLR−/− mice.
Reconstitution with CEHTg marrow increases the macrophages with M2 phenotype in the lesions.
Lesion progression is associated with a switch in macrophage phenotype from M2 (anti-inflammatory and tissue repair phenotype) to M1 (classically activated and proinflammatory phenotype) (9). We, therefore, examined leukocyte infiltration into the aorta and changes in macrophage phenotype following BMT. Cells obtained after digestion of spleen were used as positive control, and a representative cell separation is shown in Fig. 6A. Using similar analyses, distribution of total leukocytes in aortic arches was quantified. No significant differences were noted either in the total leukocyte infiltration or in the population of T-cells (CD45+CD3a+), B-cells (CD45+CD19+), or macrophages (CD45+CD11b+) (Fig. 6B), although there was a trend toward a decrease in the total number of macrophages that did not reach statistical significance.
Fig. 6.
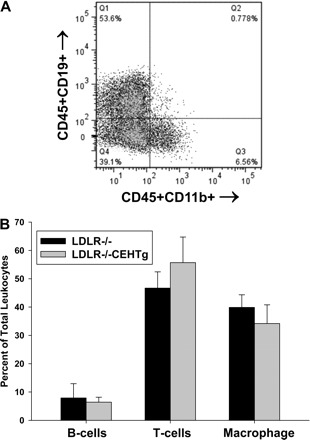
Infiltrated leukocytes into aortas. Aortic arches from mice reconstituted with marrow from LDLR−/− or LDLR−/−CEHTg mice were isolated and digested for the isolation of total leukocytes. Isolated cells were stained for leukocytes (CD45), B-cells (CD19), T-cells (CD3a), and macrophages (CD11b) and analyzed by FACS. Total cells obtained by digesting the spleen using the same enzyme cocktail were used as a positive control. A: typical spleen cell separation where CD45+CD19+ B-cells are in Q1, CD45+CD11b+ macrophages are in Q3, and CD45+CD19-CD11b-T cells are in Q4. T cells were also identified as CD45+CD3a+ cells. B: quantification of the distribution of leukocytes in aortas and data are presented as means ± SD, n = 6.
To distinguish between M1 and M2 phenotype macrophages, CD45+CD11b+Ly6C+ population was separated into Ly6Clo or M2 and Ly6CHi or M1 subpopulations. These two populations of cells were sorted, and phenotypes were confirmed by the selective expression of marker genes (IL-1β and TNF-α for M1 and IL-10 and Arginase-1 for M2; data not shown). The representative histograms shown in Fig. 7A show the separation of these two populations in aortic arch-associated macrophages. Reconstitution with LDLR−/−CEHTg marrow significantly decreased M1 macrophages and increased M2 macrophages (Fig. 7B).
Fig. 7.
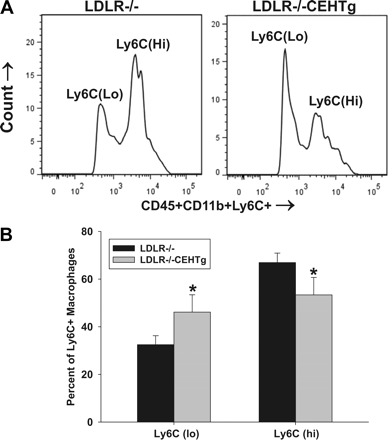
Increased % macrophages with M2 phenotype in aortic lesions of mice reconstituted with LDLR−/−CEHTg marrow. Aortic arches from mice reconstituted with marrow from LDLR−/− or LDLR−/−CEHTg mice were isolated and digested for the isolation of total leukocytes. Isolated cells were stained for leukocytes (CD45), B-cells (CD19), T-cells (CD3a), macrophages (CD11b), and Ly6C and analyzed by FACS. A: histogram depicting a representative separation of CD45+CD11b+Ly6C+ population into Ly6Clo and Ly6CHi subpopulations. B: quantification of the data where the distribution of Ly6Clo and Ly6CHi is plotted as % total Ly6C positive cells. Data are presented as means ± SD, n = 6, *P < 0.05.
DISCUSSION
Intracellular CE hydrolysis is the obligatory first step in mobilization of cellular CE since cholesterol is effluxed from the cells to extracellular acceptor as FC. This step is also recognized as the rate-limiting step in cellular cholesterol efflux from CE-loaded cells. Consistent with this view are the reports of an inverse relationship between CE hydrolytic activity and intracellular cholesterol accumulation (8). Decreased levels of CEH expression are observed in macrophages from atherosclerosis-susceptible species (7, 19), and its overexpression results in enhanced mobilization of CE from cells in vitro (5, 8). Our earlier studies have demonstrated increased FC efflux from human macrophages overexpressing CEH (21) and macrophage-specific transgenic expression of CEH led to attenuation of diet-induced atherosclerosis in LDLR−/− mice (20).
In the present study, we assessed the ability of CEH overexpression to prevent or reduce atherosclerotic lesion progression. Our results demonstrate for the first time that increasing macrophage CEH expression, even after the lesion formation has started, significantly attenuates further progression of atherosclerotic lesion development in both males and females (Figs. 3 and 4) without significantly affecting plasma lipoprotein profiles (Fig. 2). It needs to be emphasized that, while in the present experimental setup progression of any individual existing lesion could not be monitored, the effects of increasing macrophage CEH expression on total lesion progression is reported. Lesion progression occurs as a result of an imbalance between accumulation of CE in macrophage foam cells and extracellular acceptor-mediated efflux of FC. Since macrophages from CEH transgenic mice accumulate significantly less CE and increase in vivo reverse cholesterol transport (20), reconstitution with CEHTg marrow significantly reduced lesion progression. Consistent with our earlier data, the lesions in mice receiving CEHTg marrow were also more cellular with reduced necrotic area. These data clearly demonstrate that enhancing CEH-mediated CE mobilization, even after lesion development has started, will attenuate atherosclerosis as well as lesion necrosis suggesting that strategies leading to increased CE mobilization from macrophages will be antiatherogenic. It is important to note that in the present study we monitored effects on CEH-mediated enhanced CE hydrolysis on diet-induced lesion progression and not regression of existing lesions. However, it is noteworthy that a significant decrease in aortic CE is also reported in ApoE−/− mice during plaque regression (14), indicating enhanced CE hydrolysis and extracellular acceptor-mediated FC efflux. The central role of CE mobilization and efflux of resulting FC is further supported by the recent demonstration of plaque regression when atherosclerotic aortic arches were transplanted into ApoA1 transgenic mice where FC efflux is enhanced by increased expression of extracellular acceptor (2).
Examination of lesion development in humans has revealed that fatty streaks are evident as early as 15–17 yr of age and these fatty streaks progress to raised lesions after the age of 25 yr (12). Thus, even if the presence of these early lesions was adequately determined, ideal therapeutic intervention should either reverse the existing lesions or prevent further progression. The results from the present study are particularly relevant in this regard since enhancing CE mobilization by transplantation with CEHTg marrow significantly attenuates further lesion progression. While mechanisms of lesion formation are extensively studied and described, mechanisms that may reverse the disease are less understood. Possible mechanisms responsible for lesion regression include decreased retention of ApoB containing lipoproteins within the arterial wall, efflux of cholesterol from plaques, emigration of foam cells out of the arterial wall, and influx of healthy phagocytes that remove necrotic debris and other components of the plaque (18). Although plaque regression was not specifically being evaluated in the present study, it is tempting to speculate that CEHTg macrophages, with lower cellular CE content, enhanced FC efflux, and increased ability to promote in vivo reverse cholesterol transport will facilitate plaque regression. It is noteworthy that conditions promoting reduced cholesterol accumulation, such as low levels of circulating cholesterol, promote plaque regression in a surgical regression model (11), and cholesterol has a direct negative effect on the motility of macrophages (13).
Macrophage content of plaque is variable during the development of plaques (from fatty streaks to complex plaques) and is likely to be determined by processes involved in macrophage recruitment, apoptosis, and egress. In addition, functional macrophages are required for efficient clearance of apoptotic cells (or efferocytosis), a decrease in that leads to secondary necrosis. We have earlier demonstrated increased macrophage content, decreased apoptosis, and consequently reduced lesion necrosis in Western Diet-fed LDLR−/−CEHTg mice (20), and lesions in the mice reconstituted with CEHTg marrow recapitulated similar lesion morphology (Fig. 5). Khallou-Laschet et al. (9) evaluated the phenotype of macrophages associated with progression of atherosclerosis in mice and demonstrated that early lesions were infiltrated with alternatively activated and anti-inflammatory M2 macrophages that favored smooth cell proliferation and deposition of extracellular matrix (tissue repair phenotype). However, a phenotypic switch of existing macrophages within the plaques from M2 to M1 (classically activated macrophages with proinflammatory properties) occurred during plaque progression resulting in increased inflammation (9). M1 macrophages, identified by high expression of Ly6C surface antigen (Ly6CHi), increase with hypercholesterolemia, actively adhere to endothelium, become lesional macrophages, and represent a newly recognized component of the inflammatory response in atherosclerosis (17). Significantly increased percentage of M2 macrophages (Ly6Clo) in the lesions from mice reconstituted with CEHTg marrow suggests that these lesions still contain macrophages of anti-inflammatory and tissue repair phenotype and consequently may be more stable. We have earlier demonstrated decreased activation of proinflammatory transcription factors, namely NF-κB and AP-1, by CEH-mediated reduction in cellular CE content and attenuated expression of proinflammatory cytokine expression from CEH transgenic macrophages (1). Taken together, these data suggest that increased cellular CE mobilization polarizes macrophages toward a less inflammatory M2 phenotype indicative of a less progressed/inflamed plaque. This is also consistent with the reported polarization of macrophages toward M2 phenotype induced by increased extracellular acceptor-mediated FC efflux (2).
The data presented here demonstrate, for the first time, that an increase in CE hydrolysis even after the lesion development has commenced can still attenuate further progression of lesions. Furthermore, these data also illustrate the anti-inflammatory role of enhanced CE hydrolysis that facilitates the polarization of artery wall-associated macrophages to M2 phenotype (anti-inflammatory and tissue-repair phenotype).
Perspectives and Significance
Taken together with our earlier results demonstrating a significant reduction in diet-induced atherosclerosis and lesion necrosis in LDLR−/−CEHTg mice the data from this present study clearly establish the importance of intracellular CE hydrolysis in attenuating atherosclerosis. The ability to reduce the progression of atherosclerotic lesions by targeted increase in macrophage CE hydrolysis is extremely relevant from the clinical standpoint where therapeutic interventions are only considered after lesion development has initiated. Identification of mechanisms by which endogenous CEH can be activated is likely to provide novel strategies to prevent lesion formation/progression. Furthermore, CEH-mediated CE mobilization is anti-inflammatory, and future studies will determine whether CEH-mediated reduction in cellular CE content of macrophages is also associated with other beneficial characteristics, such as increased efferocytosis or egress potential, which play an important role in determining plaque necrosis and stability.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-069946 (to S. Ghosh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge Drs. Alyssa Hasty and Elena Galkina for providing the protocol and expertise for BMT and FACS analyses of the cells associated with aortic lesions, respectively. The technical assistance of David Bolick, Barbara Szomju, and Jingmei Song is also acknowledged.
REFERENCES
- 1. Bie J, Zhao B, Song J, Ghosh S. Improved insulin sensitivity in high fat- and high cholesterol-fed Ldlr−/− mice with macrophage-specific transgenic expression of cholesteryl ester hydrolase: role of macrophage inflammation and infiltration into adipose tissue. J Biol Chem 285: 13630–13607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci USA 108: 7166–7171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med 203: 1273–1282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res 41: 1020–1026, 2000 [PubMed] [Google Scholar]
- 5. Ghosh S, St Clair RW, Rudel LL. Mobilization of cytoplasmic CE droplets by over-expression of human macrophage cholesteryl ester hydrolase. J Lipid Res 44: 1833–1840, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Ghosh S. Cholesteryl ester hydrolase in human monocyte/macrophage: cloning, sequencing, and expression of full-length cDNA. Physiol Genomics 2: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Hakamata H, Miyazaki A, Sakai M, Suginohara Y, Sakamoto Y, Horiuchi S. Species difference in cholesteryl ester cycle and HDL-induced cholesterol efflux from macrophage foam cells. Arterioscler Thromb 14: 1860–1865, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Ishii I, Oka M, Katto N, Shirai K, Saito Y, Hirose S. β-VLDL-induced cholesterol ester deposition in macrophages may be regulated by neutral cholesterol esterase activity. Arterioscler Thromb 12: 1139–1145, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One 5: e8852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apoE-deficient mice by bone marrow transplantation. Science 267: 1034–1037, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA 101: 11779–11784, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGill HC, Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. Arterioscler Thromb Vasc Biol 20: 1998–2004, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Nagao T, Qin C, Grosheva I, Maxfield FR, Pierini LM. Elevated cholesterol levels in the plasma membranes of macrophages inhibit migration by disrupting RhoA regulation. Arterioscler Thromb Vasc Biol 27: 1596–1602, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 121: 2025–2036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rothblat GH, Llera-Moya MDL, Favari E, Yancey PG, Kellner-Wiebel G. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis 163: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY Study: natural history, risk factors, and pathobiology. Pathobiological determinants of atherosclerosis in youth. Ann NY Acad Sci 811: 226–235, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117: 195–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med 5: 91–102, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Yancey PG, St. Clair RW. Mechanism of the defect in cholesteryl ester clearance from macrophages of atherosclerosis-susceptible White Carneau pigeons. J Lipid Res 35: 2114–2129, 1994 [PubMed] [Google Scholar]
- 20. Zhao B, Song J, Chow WN, St Clair RW, Rudel LL, Ghosh S. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr−/− mice. J Clin Invest 117: 2983–2992, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao B, Song J, St. Clair RW, Ghosh S. Stable over-expression of human macrophage cholesteryl ester hydrolase results in enhanced free cholesterol efflux from human THP1-macrophages. Am J Physiol Cell Physiol 292: C405–C412, 2007 [DOI] [PubMed] [Google Scholar]


