Abstract
Indoleamine 2,3-dioxygenase (IDO) metabolizes l-tryptophan to l-kynurenine, promotes immunosuppression, and has been described as a consumer of superoxide. We discovered IDO expression in periaortic fat and tested the hypothesis that periarterial IDO functionally reduces agonist-induced contraction. Our model was the thoracic aorta, abdominal aorta, and superior mesenteric artery of the male Sprague-Dawley rat. Periaortic fat from the thoracic aorta stained intensely for IDO, the brown fat marker uncoupling protein-1, and oil red O as a general lipid marker. White fat around the mesenteric artery and abdominal aorta stained less for IDO; brown fat was less abundant. IDO activity (kynurenine-to-tryptophan ratio via HPLC) was detected in visceral and mesenteric artery fat (ratio: ∼4) but was highest in perithoracic aortic fat (ratio: 10 ± 1.1). In isometric contractile experiments, periadventitial fat reduced ANG II-induced thoracic aortic (with fat: 34% of without fat) and mesenteric artery (with fat: 63% of without fat) maximal contraction. In contrast, periadventitial fat did not reduce agonist-induced contraction in the abdominal aorta. The IDO inhibitor 1-l-methyltryptophan (1-MT) reversed the fat-induced reduction of ANG II-induced contraction in the thoracic aorta but not in the mesenteric artery. The IDO metabolite kynurenine relaxed the thoracic aorta only at high (9 mM) concentrations, whereas the downstream metabolite quinolinic acid (1 mM) relaxed the contracted thoracic aorta (∼80%). 1-MT did not correct the reduction in basal superoxide levels observed in the presence of perithoracic aortic fat. We conclude that IDO is an enzyme active primarily in brown fat surrounding the thoracic aorta and depresses aortic contractility.
Keywords: tryptophan, brown fat, angiotensin II, immunosupression, kynurenine
blood vessels are classically defined as having three functional layers: the tunica intima (endothelium and intima), tunica media (smooth muscle), and tunica adventitia (collagen and fibroblasts). Most vessels are also surrounded by adipose tissue. Our knowledge of the functional blood vessel has expanded dramatically in the last several decades with the recognition that the endothelial cell layer is vasoactive and the adventitia is a source of ROS (12). We have begun to understand that the periadventitial adipose layer serves as a functional fourth layer of a blood vessel, in that the fat cells of this layer secrete vasoactive substances. This was initially recognized 20 yr ago by Soltis and Cassis (60). Many of these substances are also recognized as players in inflammation, and adipose tissue has been established as a site of T cell migration (30). Thus, the adipose tissue around a vessel is important when considering the overall function of a blood vessel.
A long-term interest of our laboratory is the role of serotonin [5-hydroxytryptamine (5-HT)] in vascular regulation. 5-HT is synthesized from the essential amino acid l-tryptophan, but only a minority of tryptophan (5–10%) ultimately becomes 5-HT. The majority is used by the enzymes indoleamine 2,3-dioxygenase (IDO) or tryptophan dioxygenase. IDO metabolizes tryptophan to N-formylkynurenine, followed by enzymatic or spontaneous conversion to a measurable metabolite, kynurenine; at least one form of IDO is found in rodents and humans (1, 2, 63). We were interested in the expression of this enzyme because its activity would necessarily draw tryptophan away from 5-HT by alternative metabolism, and this theory has been put forward as relevant to depression (44). Another reason that IDO is interesting is because it has been described as a consumer of superoxide (32, 34, 63) and thus is one of the few enzymes, outside of SODs, to reduce superoxide levels. In initial immunohistochemical experiments using sections of the rat thoracic aorta, we discovered that IDO was not robustly expressed in the vessel proper but in the fat around the blood vessel. In an elegant series of experiments, Wang et al. (70) described kynurenine, a primary product of IDO, as an endothelium-derived relaxing factor produced in inflammation. Kynurenine, in millimolar concentrations, caused direct relaxation of the contracted mouse aorta and pig coronary artery. This observation allowed us to formulate the hypothesis that IDO present in the periarterial fat released kynurenine to reduce agonist-induced contraction. Kynurenine would thus be added to the following growing group of substances derived from adipose tissue that are vasoactive (5, 21, 29, 33, 73): adiponectin (25), adipokines (62), angiotensinogen (47), an unknown K+ channel activator (41, 69), hydrogen sulfide (24), nitric oxide (19, 28), superoxide (27, 35, 36), and potentially ANG(1–7) (42). Evidence suggests that periadventitial fat may also play a role in vascular remodeling (64, 65), making it important for us to understand the biological composition and function of periadventitial fat.
The type of fat expressed around or near all blood vessels is not the same. As we will demonstrate, the periadventitial fat around the thoracic aorta is primarily brown fat, whereas that of the abdominal aorta is white fat. This raises the important question of whether the type of fat itself, instead of and in addition to its placement in the body, determines the substances made that can influence contractility. As such, we investigated three different arteries: the thoracic aorta, abdominal aorta, and superior mesenteric artery. Using a host of techniques, we discovered that IDO is preferentially expressed in the fat around the thoracic aorta and that a product from IDO, likely not kynurenine, depresses contraction relatively selective to ANG II.
MATERIALS AND METHODS
All procedures that involved animals were performed in accordance with institutional guidelines and with approval from the Animal Use Committee of Michigan State University.
Animal use.
Normal male Sprague-Dawley rats (8–10 wk of age, 250–300 g, Charles River Laboratories, Portage, MI) were used. Before all dissection, rats were anesthetized with Fatal Plus (60 mg/kg ip).
Materials.
Unless stated otherwise, all chemicals were from Sigma Chemical (St. Louis, MO).
Histochemistry.
Thoracic aortae, abdominal aortae, and superior mesenteric arteries were removed from anesthetized animals. Tissues were left with the fat layer and either formaldehyde fixed or fresh frozen. Paraffin-embedded sections (8 μm) were cut, dewaxed, and taken through a standard protocol using a Vector kit. Arterial sections were incubated 24 h with IDO (20 μg/ml, clone 10.1, Millipore, Temecula, CA) or uncoupling protein (UCP)-1 primary antibody (10 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) or no primary antibody. Sections were developed according to the manufacturer's instructions using a 3,3-diaminobenzidine developing solution (Vector Laboratories, Burlingame, CA). Binding was observed as a dark brown-black precipitate, and specific binding of the primary antibody was determined as that which was absent in parallel sections incubated without primary antibody. All slides were counterstained with Vector hematoxylin (30–240 s), with nuclei stained blue. Sections were dried, coverslipped, and photographed on a Nikon TE2000 inverted microscope using MMI Cellcut Software. Fresh-frozen sections were stained using a standard protocol for oil red O (Michigan State University Investigative Pathology Services).
Western blot analysis.
Thoracic aortic fat and the thoracic aorta, superior mesenteric fat and the superior mesenteric artery, perinephric fat (next to the left kidney), subcutaneous fat (white fat under the back skin), epididymal fat (above the right testes), and the brown fat pad (from between scapulae) were dissected out and immediately placed on dry ice. Fats were dissected away from the adventitia of the blood vessel. Standard protein isolation and Western blot procedures were performed, with equivalent amounts of total protein loaded per lane (49). Immobilon polyvinylidene difluoride membranes were blocked for 3 h in 4% chick egg ovalbulmin [4°C, Tris-buffered saline (TBS) with 0.1% Tween and 0.025% NaN3 or blocking buffer (Li-COR Bioscience, Lincoln, NE)]. Primary antibody [IDO (clone 10.1, 1:200, Millipore), UCP-1 (1:1,000, LS-B705, Lifespan Biosciences, Seattle, WA), or peroxisome proliferator-activated receptor (PPAR)-γ (B-5, 1:1,000, Santa Cruz Biotechnologies)] was incubated with blots overnight at 4°C. Blots were then rinsed three times in TBS and 0.1% Tween with a final rinse in TBS and incubated with IRDye 800 goat anti-mouse IgG (1:1,000) for testing IDO and horseradish peroxidase-coupled anti-mouse secondary antibody for testing UCP-1 and PPAR-γ (1:5,000, GE Healthcare) for 1 h at 4°C with rocking. IDO blots were visualized using an Odyssey Infrared Imaging system (LI-COR Biosciences). ECL reagents (Amersham Life Sciences, Arlington Heights, IL) were used to visualize PPAR-γ and UCP-1. Blots were reprobed for β-actin (clone AC74, 1:1,000, Sigma Chemical) or α-actin (A14, EMD Chemicals, Gibbstown, NJ) as a loading control.
IDO activity assay: HPLC measures of the kynurenine-to-tryptophan ratio.
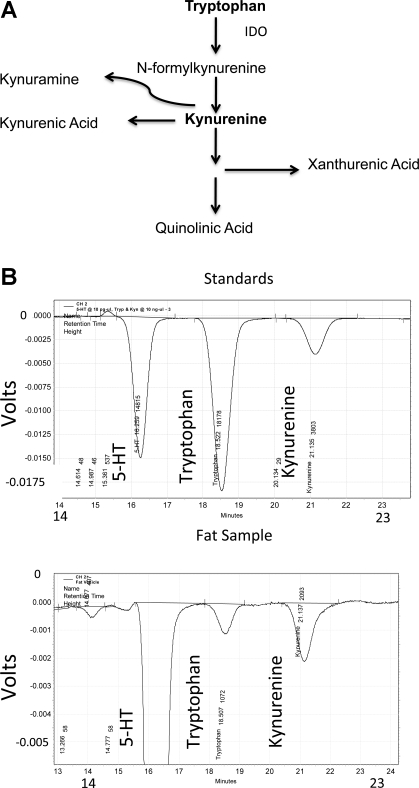
Fat samples were removed from the animal and directly frozen on dry ice or taken through a protocol in which segments of fat from the thoracic aortae were incubated with vehicle or tryptophan (1 μM) with or without the IDO inhibitor 1-methyltryptophan (1-MT; 1 mM) for 1 h at room temperature in physiological salt solution (PSS) [composed of (in mM) 130.00 NaCl, 4.70 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 1.60 CaCl2·2H2O, 14.90 NaHCO3, 5.50 dextrose, and 0.03 CaNa2EDTA; pH 7.2]. Tissues were homogenized in 0.1M perchloric acid and sonicated briefly three times. Concentrations of kynurenine and tryptophan in tissue supernatants were determined by isocratic HPLC (HPLC/ESA) coupled with an electrochemical detector. A Dionex/ESA Cat-A-Phase II Mobile phase [100 mM sodium phosphate (pH ∼3), 16% methanol, 8% acetonitrile, and a proprietary amount of sodium octyl sulfate] was used on an ESA HR-80 column. The detection electrode was set at −350 mV. Concentrations of kynurenine and tryptophan were determined by standard curves. HPLC standards (including kynurenine, tryptophan, and 5-HT) were run every day to verify the retention time of each compound. An example of this tracing is shown in Fig. 6B.
Fig. 6.
A: diagram of tryptophan metabolism through IDO and the resultant alternative metabolites. B: representative tracing of standards (top) and fat samples (bottom) from HPLC detection of tryptophan and kynurenine.
Isometric contraction.
Aortic or superior mesenteric rings (endothelium intact) either cleaned of fat [without (−)fat] or with endogenous fat intact [with (+)fat] were mounted in tissue baths for isometric tension recordings using Grass transducers and PowerLab data-acquisition software (AD Instruments, Colorado Springs, CO). Baths contained standard PSS. Rings were placed under optimum resting tension [4 g for the aorta (thoracic and abdominal) and 1.2 g for the superior mesenteric artery] and equilibrated for 1 h, with washing, before exposure to compounds. Tissue baths contained warmed (37°C), aerated (95% O2-5% CO2) PSS. Administration of an initial concentration of 10 μM phenylephrine (PE) was used to test arterial viability, and the presence of the endothelium was verified by ACh (1 μM)-induced relaxation of a half-maximal PE-induced contraction. Tissues were then washed out and incubated with either vehicle (0.1% of 10% of 4 N HCl + 90% DMSO), the IDO inhibitor 1-MT (1 mM), or the IDO product l-kynurenine (6 mM) for 1 h. Abdominal aortae were not incubated with inhibitor or vehicle. Cumulative response curves to the agonist ANG II (10−10–10−6 M) or PE (10−9–3 × 10−5 M) were generated. In some experiments, rings were contracted first with a half-maximal concentration (EC50) of PE or thromboxane mimetic U-46619, and either a vehicle (neutral, basic, or acid) or an IDO-related metabolite (l-kynurenine, kynuramine, kynurenic acid, xanthurenic acid, or quinolinic acid) was added cumulatively to 0.1 and 1 mM concentrations to test metabolite-induced relaxation.
Lucigenin-based measures of superoxide.
Aortae were placed in chilled Krebs-HEPES buffer (pH 7.4) containing (in mM) 20 HEPES, 11.9 NaCl, 0.46 KCl, 0.10 MgSO4·7H2O, 0.015 Na2HPO4, 0.04 KH2PO4, 0.5 NaHCO3, 1.2 CaCl2, and 5.5 dextrose. For some rings, all fat was removed, and aortae were cut into rings (>10 mg wet wt); in other rings, fat was left intact, and tissues were cleaned only of blood. Aortae were incubated in Krebs-HEPES buffer with 1-MT (1 mM) or vehicle (0.1% of 10% of 4 N HCl + 90% DMSO) and equilibrated for 30 min at 37°C. Diethyldithiocarbamic acid (DDC; 10 mM), an inhibitor of SOD, and either vehicle or ANG II (100 nM) were added, and aortae were incubated for 1.5 h. Aortae were transferred to fresh Krebs-HEPES buffer with lucigenin (5 μM) for 10 min, and chemiluminescence was measured using a luminometer (TD 20/20 Luminometer, Turner Designs, Sunnyvale, CA). Ten chemiluminescent measurements, each integrated over 30 s, were taken, and the superoxide scavenger 4,5-dihydroxy-1,3-dibenzenedisulfonic acid disodium salt (tiron; 10 mM) was added to aortae. After a 10-min incubation, 10 chemiluminescent measurements were made. Aortae were then blotted dry and weighed.
Data analysis and statistical procedures.
Immunohistochemical results are shown in sections incubated with and without primary antibody and are representative of a minimum of four separate animals. Photographic images were processed only for color balance, contrast, and brightness, and all of the whole images underwent the same process. Actual blots for Western blot analyses are shown, and results for IDO were quantified using ImageJ (National Institutes of Health) and normalized for actin expression. The only manipulation of these computer-generated figures was to process for contrast and brightness. All quantitative measures are reported as means ± SE for the number of animals reported. IDO activity is reported as the kynurenine-to-tryptophan ratio (product/substrate). Contractility results are reported as means ± SE. Contraction from baseline tone is reported as a percentage of the initial contraction to PE. When relaxation was tested, data are reported as a percentage of the initial contraction to half-maximal PE or U-46619. For lucigenin experiments, the superoxide signal was taken to be the difference of the average luminescence before and after tiron, and superoxide levels (in nmol·min−1·mg−1) were calculated using a cytochrome c oxidase standard curve. These data were normalized to tissue wet weight. For two group comparisons, the appropriate Student's t-test was used. For three or more groups, ANOVA followed by a Tukey's post hoc test was used. P values of ≤0.05 were considered statistically significant.
RESULTS
Immunohistochemical detection of IDO in the thoracic aorta and mesenteric artery.
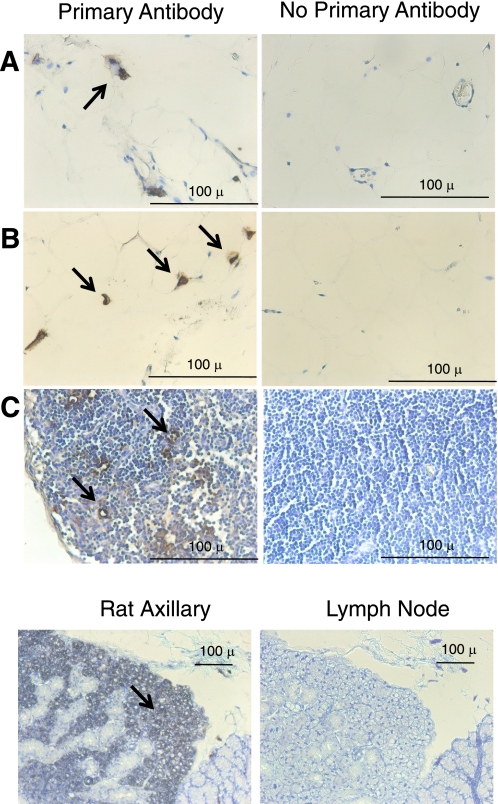
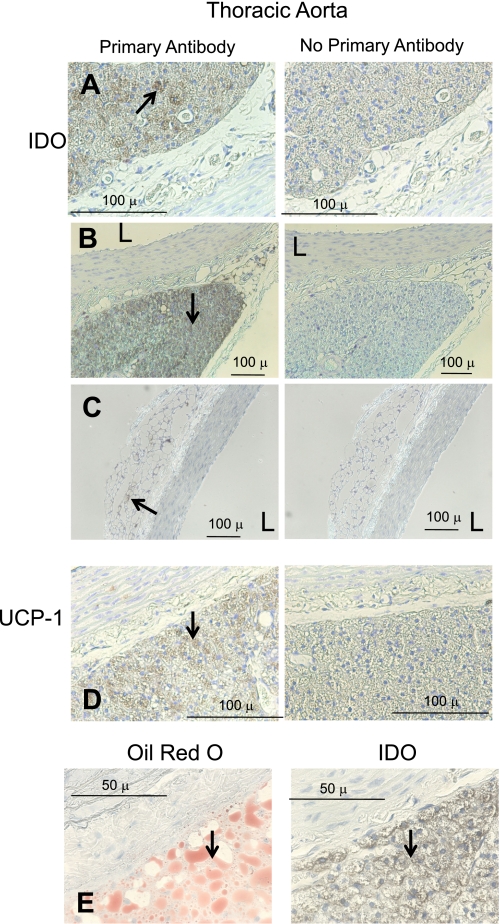
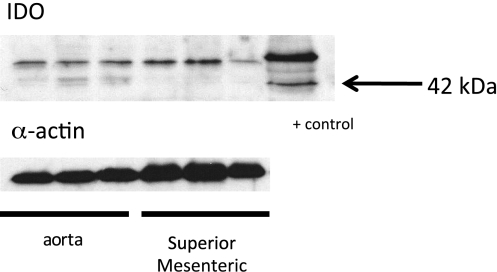
We first determined the site of expression of IDO in visceral fat (abdominal; Fig. 1) and compared this with sections of the thoracic aorta and superior mesenteric artery with intact adipose tissue (Figs. 2 and 3). Sections were incubated with (left) or without (right) primary antibody against IDO. The lacy network of white fat cells of visceral fat is apparent in Fig. 1, A and B, where the cytoplasm did not stain for IDO but resident cells (macrophages or dendritic cells) did (arrows, Fig. 1, A and B). In contrast, the lymph nodes present in the visceral fat presented with significant staining of the endothelial cells of the vasculature that was significant in the nodes (Fig. 1C). Thus, IDO is present in normal white fat. As a positive control, rat axillary lymph nodes were incubated with the same IDO antibody, and significant staining throughout the node was observed. Figures 2 and 3 show results from the isolated thoracic aorta and superior mesenteric artery, respectively. In the thoracic aorta, the vessel media and endothelial cell layer did not stain for IDO, but the fat around it stained intensely (Fig. 2, A and B, representing two separate aortae). Lack of significant IDO expression (∼42 kDa) in homogenates of the aorta (and superior mesenteric artery) without fat was validated through Western blot analyses (Fig. 4). While a protein was recognized by this antibody at ∼55–60 kDa, this was not consistent with the expected molecular weight of IDO. The periaortic fat that stained significantly appeared multilocular and dense, and there were small pockets of white, unilocular cells that stained positively for IDO (Fig. 2C). Identification of the dense fat as brown adipose tissue was verified by the significant immunolocalization of UCP-1 to the same fat (Fig. 2D). Figure 2E shows that the cells that stained positive for IDO are adipose cells, as evidenced by the clear oil red O staining of lipid droplets, both large and small.
Fig. 1.
Immunohistochemical detection of indoleamine 2,3-diooxygenase (IDO) in visceral (abdominal) fat from a normal Sprague-Dawley rat. Left: with IDO primary antibody; right: without IDO primary antibody. A and B: representative staining of IDO near white adipose cells (lacy network with condensed cytoplasm). C: lymph node within the fat. Bottom: positive control in the rat lymph node. Arrows mark regions of interest. Images are representative of four different animals.
Fig. 2.
Immunohistochemical detection of IDO (A–C) and uncoupling protein-1 (UCP-1; D) in the thoracic aorta of a normal Sprague-Dawley rat. Left: with antibody; right: without antibody. A and B: representative staining of IDO in dense multilocular fat. C: IDO staining of the aorta with unilocular fat. D: UCP-1 staining. E: oil red O compared with IDO staining. Arrows point to regions of interest. Images are representative of four different animals. L, lumen of the vessel. Bars = 100 μm in A–D and 50 μm in E.
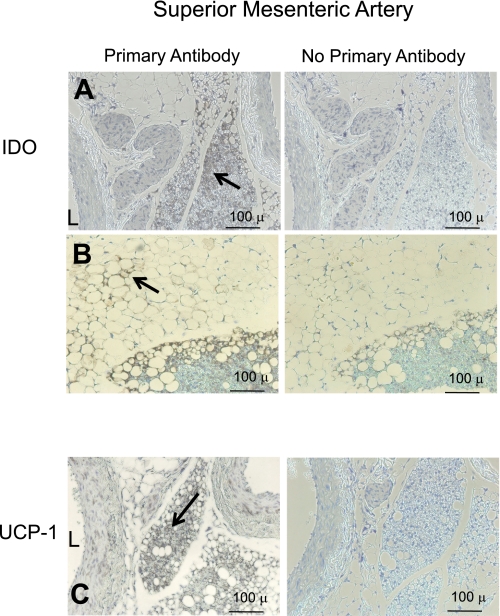
Fig. 3.
Immunohistochemical detection of IDO (A and B) and UCP-1 (C) in the superior mesenteric artery of a normal Sprague-Dawley rat. Left: with antibody; right: without antibody. A: representative staining of IDO in dense multilocular fat. B: IDO staining of the artery with mixed unilocular and multilocular fat. C: UCP-1 staining. Arrows point to regions of interest. Images are representative of four different animals. Bars = 100 μm.
Fig. 4.
Western blot analyses of thoracic aorta and superior mesenteric artery homogenates (no fat) from a normal male Sprague Dawley rat for the protein IDO and the housekeeping protein α-actin. The + control lane represents an interferon-γ-incubated HeLa cell homogenate. Blots are representative of six to eight animals, with fats from within the same animal used in each experiment.
The association of brown fat that stained for IDO with the blood vessel was different in the superior mesenteric artery. The fat immediately adjacent to the vessel was a mix of white and brown fat, unlike the dense predominant brown fat around the aorta. Both fats expressed IDO (Fig. 3, A and B). UCP-1 positive staining of tissue was observed near the superior mesenteric artery (Fig. 3C); modest UCP-1 staining was observed in the media of one of four sections.
Western blot detection and activity of IDO in fats.
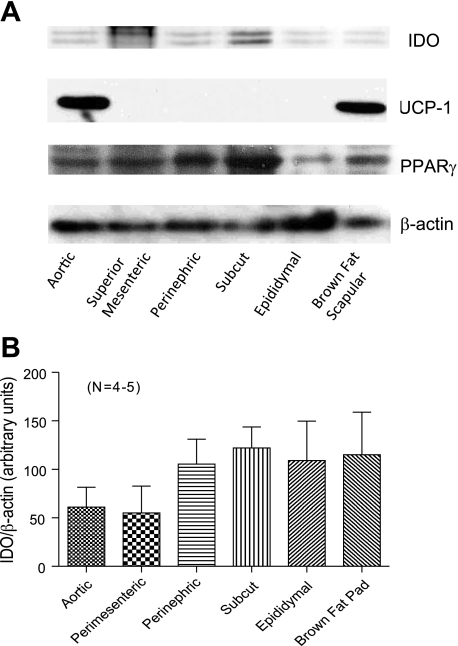
We next used Western blot analyses to quantify IDO expression in the two periadventitial fats examined above (the aorta and superior mesenteric artery) and compared expression of IDO with other body fats. An important comparison was made to the scapular brown fat pad to determine if it was a property of brown versus white fat to express IDO. Figure 5A shows representative blots for IDO (top), UCP-1, the general fat marker PPAR-γ, and normalized β-actin protein. IDO was detectable but not robustly expressed in any tissues examined and was routinely recognized as a doublet in fats. In contrast, UCP-1 was detected only in aortic fat and the brown fat pad. PPAR-γ, as expected, was expressed in all tissues, as was β-actin. IDO expression was quantified using densitometry and is shown in Fig. 5B. Statistically, these amounts were not different from one another (P > 0.05 by ANOVA).
Fig. 5.
A: Western blot analyses of fats from a normal male Sprague Dawley rat for the proteins IDO, UCP-1, and peroxisome proliferator-activated receptor (PPAR)-γ and the housekeeping protein β-actin. B: normalization of IDO expression to β-actin. Results are representative of four to five animals, with fats from within the same animal used in each experiment. Values are means ± SE.
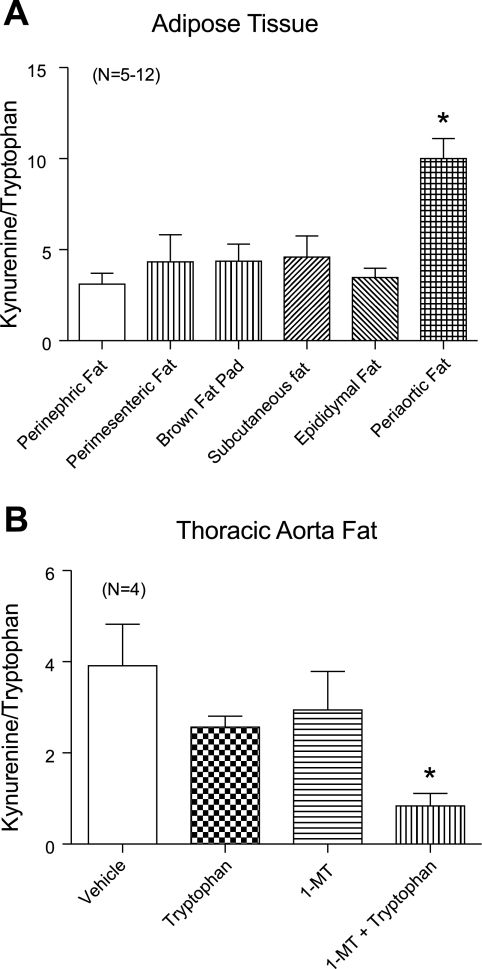
A measure of IDO activity was made by quantifying the kynurenine-to-tryptophan ratio of each fat, with kynurenine being the most immediate stable metabolite of IDO (Fig. 6A). Figure 6B shows a representative tracing of the standards (top) used to identify and quantify tryptophan and kynurenine in fat samples using HPLC (bottom). As expected based on the above Western blot analyses, kynurenine and tryptophan were detected in all samples. However, the kynurenine-to-tryptophan ratio was highest in the periaortic fat (P < 0.05 by ANOVA; Fig. 7A). Using the same HPLC measures, we verified that 1-MT, a classic IDO inhibitor, reduced IDO activity. Thoracic aortic fat was exposed to vehicle [0.1% (DMSO + 10% of 4 N HCl)] or 1-MT (1 mM) and tryptophan (1 μM) for 1 h. IDO inhibition was observed as a significant decrease in the kynurenine-to-tryptophan ratio in samples treated with 1-MT and tryptophan versus tryptophan or 1-MT alone (Fig. 7B).
Fig. 7.
A: IDO activity measured as the ratio of kynurenine to tryptophan as detected through HPLC. B: effect of the IDO inhibitor 1-l-methyltryptophan (1-MT; 1 mM) on kynurenine-to-tryptophan conversion within fat of the thoracic aorta. Values are means ± SE for the numbers of animals in parentheses, where fats were taken from the same animals where possible for comparison. *Significant difference from other experimental groups (P < 0.05 by ANOVA).
Effect of fat and IDO on contractility in the thoracic aorta and superior mesenteric artery.
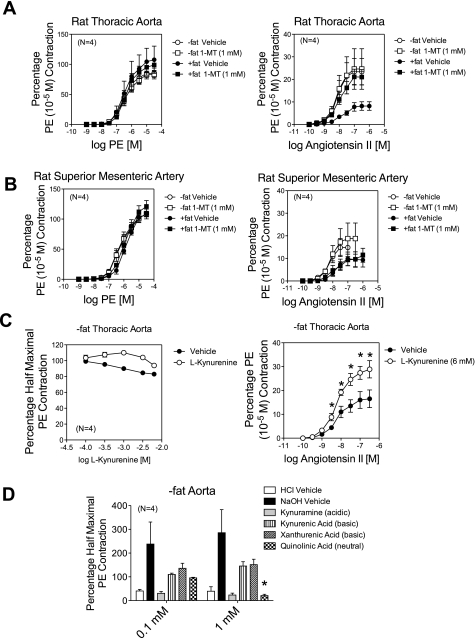
We next investigated the role of periarterial fat on agonist-induced contraction, focusing on PE and ANG II. The initial contraction of the arteries (all endothelium intact) to PE was reduced in the +fat versus −fat arteries. For the thoracic aorta, this contraction was 2,068 ± 112 mg −fat and 1,708 ± 122 mg +fat (P < 0.05). For the superior mesenteric artery, contraction was 972 ± 34 mg −fat and 688 ± 152 mg +fat (P = 0.09). These were the values used to normalize the responses in the later parts of the experiment. PE-induced contraction was not affected by leaving the fat in place on the thoracic aorta or mesenteric artery (Fig. 8, A and B, left). By normalizing with the initial PE contraction, which was reduced in the +fat arteries, we could be skewing these data. However, the actual maximum thoracic aortic contraction to PE (3 × 10−5 M) was not statistically different in any one group (+fat plus vehicle: 1,596 ± 151 mg, +fat plus vehicle: 1,876 ± 315 mg, −fat plus 1-MT: 1,841 ± 72 mg, and +fat plus 1-MT: 1,598 ± 220 mg, P > 0.05 by ANOVA). In contrast, ANG II-induced maximum contraction in both the thoracic aorta (Fig. 8A, right) and superior mesenteric artery (Fig. 8B, right) was significantly reduced by the presence of periarterial fat compared with the contraction of tissues without fat. In the thoracic aorta, the IDO inhibitor 1-MT reversed the inhibition of ANG II-induced contraction observed in the presence of fat. This reversal was not observed in the superior mesenteric artery, in which ANG II-induced contraction of the +fat vessel remained reduced in the presence of 1-MT compared with the responses of the +fat plus vehicle-treated vessel.
Fig. 8.
A and B: effect of the IDO inhibitor 1-MT on phenylephrine (PE)-induced (left) and ANG II-induced (right) contraction in without (−)fat and with (+)fat endothelium-intact thoracic aortae (A) and superior mesenteric arteries (B). C, left: direct effect of the IDO metabolite kynurenine on PE-induced (left) contraction in the −fat thoracic aorta. Right, effect of a 1-h incubation of kynurenine (6 mM) on ANG II-induced contraction in the −fat thoracic aorta. D: change in PE-induced contraction in the −fat thoracic aorta caused by basic and acidic vehicles and the downstream IDO metabolites kynuramine, kynurenic acid, xanthurenic acid, and quinolinic acid. Values are means ± SE for the numbers of animals in parentheses. *Significant differences from the appropriate vehicle control (P < 0.05).
We next investigated whether kynurenine, the most immediate stable product of IDO, could relax the isolated thoracic aorta. In the cleaned aorta, kynurenine did not cause a relaxation compared with vehicle. In this instance, vessels were contracted with a half-maximal concentration of PE (Fig. 8C, left). When kynurenine (6 mM) was incubated with vessels for 1 h, the ANG II-induced maximum contraction was enhanced, not depressed (Fig. 8C, right). When a half-maximal concentration of the thromboxane mimetic U-46619 (20 nM) was used as the contractant instead of PE, kynurenine-induced relaxation above and beyond that caused by vehicle was only statistically significant at a concentration of 9 mM (percent U-46619 contraction remaining: vehicle 59.7 ± 16.1% and kynurenine 21.6 ± 4.1%, P < 0.05). These data suggest that kynurenine may not be the IDO metabolite that reduces the ANG II-induced contraction. As such, we next investigated four additional metabolites downstream from kynurenine itself (metabolism shown in Fig. 6A; data shown in Fig. 8A). With the exception of kynuramine, these compounds required significant adjustment of pH to solubilize them at a high enough concentration so as to be able to achieve near millimolar concentrations in the tissue bath (stock solutions were 0.1 M). Kynuramine was dissolved straight in water but resulted in an acidic pH (pH 2). Kynurenic acid and xanthurenic acid had a pH of ∼11, whereas the quinolinic acid solution was neutral. None of these metabolites, up to a concentration of 3 mM, caused direct aortic constriction (not shown). Half-maximal contraction to PE was established (statistically similar contraction achieved in each group) and followed by one of the following additions. The acid vehicle (pH 2 for kynuramine) caused direct relaxation, whereas the basic vehicle (pH ∼11 for xanthurenic and kynurenic acid) caused contraction. Neutral vehicle had no effect on PE-induced contraction. Although kynuramine caused relaxation at both 0.1 and 1 mM concentrations, the relaxation was not significantly greater than that caused by vehicle. Neither kynurenic acid nor xanthurenic acid relaxed PE-induced contraction from baseline or compared with the basic vehicle. In contrast, quinolinic acid (1 mM, neutral pH) relaxed PE contraction. In separate experiments, quinolinic acid (1 mM) did not reduce the maximal contraction to ANG II in the cleaned thoracic aorta [vehicle: 50.4 ± 12.7% PE (10−5 M) contraction, quinolinic acid: 47.6 ± 12.8%, n = 4, P > 0.05].
IDO as a superoxide quencher in the thoracic aorta.
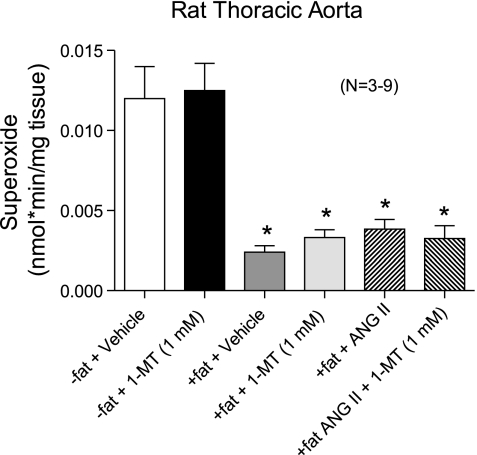
Figure 9 shows the results from experiments in which superoxide was measured with lucigenin-enhanced chemiluminescence. ANG II has the well-known actions of increasing superoxide production in the vasculature, and IDO has been described as an enzyme that metabolizes superoxide. If IDO metabolizes superoxide to reduce contractility and 1-MT reverses this, then changes in superoxide that parallel these data should be observable. In −fat thoracic aortic rings, 1-MT did not alter basal superoxide levels compared with vehicle. The presence of fat significantly reduced the basal amount of superoxide, but this was not reversed with incubation with the IDO inhibitor 1-MT. ANG II (10−7 M, 90 min) did not cause a significant elevation of superoxide in +fat rings, and this was not altered by the presence of the IDO inhibitor 1-MT. We verified the ability of ANG II (10−7 M) to increase superoxide in −fat thoracic aortic rings (superoxide: vehicle 0.036 ± 0.0047 nmol·min−1·mg tissue−1 and ANG II 0.14 ± 0.06 nmol·min−1·mg tissue−1, n = 4, P < 0.05).
Fig. 9.
Effect of intact periaortic fat on basal superoxide production or ANG II (100 nM)-stimulated superoxide in the thoracic aorta and effect of vehicle or IDO inhibitor 1-MT (1 mM). Values are means ± SE for the numbers of animals in parentheses. *Significant difference from −fat values (P < 0.05 by ANOVA).
Comparison with the abdominal aorta.
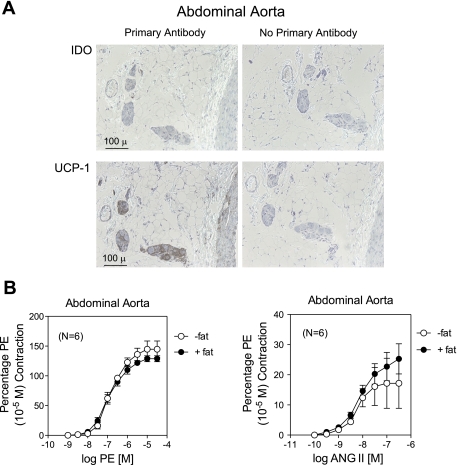
We performed some of the protocols described above on the abdominal aorta, a tissue contiguous to the thoracic aorta but in a different environment. Immunohistochemistry demonstrated that the majority of the fat surrounding the abdominal aorta did not stain for IDO and appeared to be white fat, given the minimal staining of the tissue with an antibody against UCP-1 and the cellularity of the fat (Fig. 10A). In isolated tissue baths, the presence of periadventitial fat in abdominal aorta did not affect the contraction (potency or maximum) stimulated by PE (Fig. 10B, left) or ANG II (Fig. 10B, right).
Fig. 10.
A: immunohistochemical detection of IDO and UCP-1 in the abdominal aorta of a normal Sprague-Dawley rat. Left: with antibody; right: without antibody. B: effect of periadventitial fat on contraction induced by PE (left) or ANG II (right) in the abdominal aorta. Values are means ± SE for the numbers of animals in parentheses.
DISCUSSION
The ability of adipose cells to produce substances that have the potential to modify vascular reactivity was recognized nearly 2 decades ago, with the discovery that fat around the aorta made angiotensinogen (47). However, it has only been in the last 8 yr that the direct influence of periadventitial fat factors on vascular function has been studied. This group is rich in chemical diversity (a gas, peptides, and small soluble molecules), the mechanisms by which they modify contractility (direct relaxation and blockade of channels), and the enzymes that produce these substances [adiponectin (25), adipokines (62), angiotensinogen (47), an unknown K+ channel activator (41, 69), hydrogen sulfide (24), nitric oxide (19, 28), and superoxide (27, 35)]. We add to this IDO and downstream IDO metabolites. This work is important because we introduce several new findings and ideas: 1) it is the first time IDO has been recognized in any type of fat, 2) the selective enrichment of IDO around the thoracic aorta is novel, 3) and the potential role for brown fat in regulating vascular tone is new.
IDO.
IDO is an ancient enzyme, closely related to liver-expressed tryptophan dioxygenase (72). The majority of work on IDO has been in the immune system and pregnancy, where two theories predominate on how IDO functions. First, because IDO metabolizes tryptophan, it depletes tryptophan for T cells, and T cells have a seemingly strong dependence on tryptophan (an essential amino acid) to function (14, 23, 61). Without it, T cells enter anergy, and IDO therefore acts as an immunosuppressive and allows for tolerance of the maternal/fetal interface. Second, it has been hypothesized that some of the downstream metabolites investigated here, such as quinolinic acid, serve as T cell toxins that reduce immune function. IDO has been identified in immune dendritic cells, and, although their exact role remains unclear, the presence of IDO in dendritic cells suggests yet another important immune function of IDO (6, 46, 52).
We have identified IDO as being present in arteries and expressed significantly in the fat surrounding the artery and less so in the artery itself. An important fact that makes the investigation of IDO relevant is that it is a regulated enzyme, most notably inhibited by nitric oxide (67), and thus may play a dynamic role in vascular tone. The presence of functional IDO is supported by immunohistochemical, Western blot, and HPLC activity assays. The expression of IDO was not as robust as UCP-1 was in aortic fat and the brown fat pad. We did not expect robust IDO expression in normal tissues (media and intima, not fat), as IDO expression is normally low in an unchallenged state. Immunohistochemical experiments were negative, and Western blot analysis demonstrated a protein recognized by the IDO antibody that was not of the expected molecular weight. Together, these findings suggest either minimal to no expression of IDO in the media and intima or expression of a modified IDO protein not previously described in other tissues. Interferon (IFN)-γ is the best known activator of IDO expression, and others (13) have demonstrated the ability of IFN-γ to upregulate IDO expression in vascular smooth muscle cells. We did not observe IDO expression in endothelial cells, as been observed with infection, where infection is considered a challenge that stimulates IDO expression (31, 70). Moreover, IDO expression was not limited to the fat around blood vessels but was detected in resident cells and lymph nodes in abdominal fat as well as homogenized fats from multiple regions of the body. The function of IDO in these sites is unknown, but the IDO mechanism can be examined using an inhibitor of IDO. IDO is inhibited competitively by the compound 1-MT. This isomer was our choice as the d-isomer has been described as a less-effective inhibitor (7, 38, 39). Use of 1-MT in isolated blood vessels was used to test whether the fat, and the IDO present in it, had effects on vascular smooth muscle function. 1-MT, at the concentration used in contractile experiments (1 mM), significantly reduced the kynurenine-to-tryptophan ratio in the fat around the thoracic aorta when the tissue was incubated with tryptophan, validating its use as an IDO inhibitor.
Vessel selectivity: the aorta versus the superior mesenteric artery.
Our results first suggest that periadventitial fat can reduce arterial contraction. This finding confirms that of several other laboratories. Moreover, we demonstrated that the fat-induced reduction of contractility was relatively selective to ANG II in both the thoracic aorta and superior mesenteric artery and was not observed in the abdominal aorta. This suggests significant differences in the function of regionally distinct periadventitial fat. The inability of periadventitial adipose tissue to reduced contraction to PE (in all tissues) differs from the findings of others, although the degree of inhibition caused by fat in these studies varied significantly. Specifically, fat reduced the maximum contraction to PE, ANG II, and 5-HT in the male Sprague-Dawley rat aorta (41); reduced the maximum contraction to 5-HT and PE in the male Sprague-Dawley rat aorta (21); reduced the maximum contraction to PE, modestly to 5-HT, and not to U-46619 in male Sprague Dawley superior mesenteric arteries (69); and had modest inhibitory effects on PE-induced contraction in thoracic aorta from spontaneously hypertensive rats (42). In our hands, the initial contraction to PE (first agonist addition to the tissue) was reduced in the +fat thoracic aorta compared with the −fat thoracic aorta, and there was a trend for a similar fat-induced reduction in the superior mesenteric artery. However, the full concentration-response curves to PE were not different between groups. We understand that normalization of the PE curves to this initial, reduced contraction may cancel out any difference, and thus we also reported the maximum contraction to PE for each of the full concentration-response curves. The differences in contraction to PE were lost over the time of the experiment, suggesting that PE, unlike ANG II, stimulates the production of a factor that is lost or not able to be synthesized during the whole course of the experiment. These are the best reasons we can offer for our results involving PE being different from other groups.
The central finding was that 1-MT reversed the depression caused by fat for ANG II-induced contraction in the thoracic aorta but not the superior mesenteric artery. Because IDO activity was higher in the thoracic aortic fat versus mesenteric fat, this suggests that the expression of IDO in the thoracic aorta was sufficient to produce/metabolize substrates that could reduce contractility. There must be a different enzyme in the fat around the superior mesenteric artery, or at least one that is 1-MT insensitive, as 1-MT was not able to reverse the fat-induced reduction of ANG II-induced contraction. In contrast, fat provided for no reduction in agonist-induced contraction in the abdominal aorta. We took several approaches to determine what function of IDO was responsible for the dimunition of the ANG II-induced contraction. Kynurenine, the most immediate stable metabolite of IDO, has recently been shown to function as a vasodilator, such that inhibition of production of kynurenine through IDO would promote contractility (70). We used l-kynurenine as it is l-tryptophan that is metabolized through IDO. We were unable to observe a kynurenine-induced relaxation of the cleaned aorta (−fat) contracted with PE (Fig. 8) and observed significant relaxation in the U-46619-contracted aorta only at high (9 mM) kynurenine concentrations. Moreover, incubation with kynurenine (6 mM) did not reduce ANG II-induced contraction but enhanced maximal contraction. The results differ significantly from those found by Wang et al. (70). Wang et al. demonstrated the ability of kynurenine (from 1 to 6 mM) to concentration dependently relax the porcine coronary artery contracted with the thromboxane mimentic U-46619 and the mouse aorta contracted with PE. In their work (70), kynurenine was solubilized in DMSO and was used as high as 50 mM in in vitro experiments. We can point to two differences in our studies. First, we were unable to solubilize kynurenine (or any other metabolites) in DMSO without a significant adjustment of pH. Our vehicles exactly match the percentage of DMSO and pH of the stock solution introduced in the bath. Second, we used the rat vasculature, whereas Wang et al. used the mouse and pig. Thus, the effects of kynurenine and/or vehicle may be different in these three species. Regardless, multiple approaches suggested that kynurenine was likely not the substance responsible for the fat-induced reduction of ANG II-induced aortic contraction.
We investigated four other metabolites that are downstream of kynurenine. Of these metabolites, only quinolinic acid caused a reduction in the PE-induced contraction above that caused by its appropriate vehicle. However, quinolinic acid did not reduce the maximum contraction to ANG II in the −fat thoracic aorta, suggested that IDO-dependent production of quinolinic acid is not the cause for the fat-induced reduction of the maximum ANG II-induced contraction. Because metabolites had to be solubilized in either acidic (pH 2) or basic (pH 11) conditions, it is difficult to completely rule out the ability of these substances to modify vascular tone. We tested these substances at lower concentrations (0.1 and 1 mM) than kynurenines/tryptophan (up to near 10 mM) because kynurenines have been reported to be present in ∼1,000× greater concentrations than these downstream metabolites (53). We also cannot exclude the possibility that these compounds together, rather than individually, may affect vascular tone. We do not have sufficient information about relative metabolite concentrations to mimick this condition in vitro.
The other mechanism we investigated was the ability of IDO to inhibit ANG II-induced contraction by metabolizing superoxide. Although controversial, IDO has been described as one of the few enzymes outside of SOD that uses superoxide in its reaction. The controversy comes from the different standpoints as to the use of superoxide by IDO, with some suggesting that superoxide is directly used, whereas others suggesting that cytochrome b5 is the important cofactor (32, 34, 43, 63). Nonetheless, we examined superoxide production first in the basal state in the absence and presence of IDO inhibition. Consistent with this general hypothesis was the finding that periadventitial fat significantly reduced the basal amount of superoxide in the thoracic aorta. ANG II was unable to elevate superoxide in the +fat aorta, and this was not modified by 1-MT. ANG II, in −fat vessels, was able to elevate superoxide levels, although only modestly. One speculation is that DDC, used in all samples to inhibit SODs and improve the superoxide signal, could inhibit IDO or chelate metals important to IDO function. Structurally, 1-MT and DDC are not overtly similar but do contain central nitrogen atoms. No information is available to determine if DDC has an affinity for IDO, so this remains a speculation.
Adipose tissue: white versus brown.
One of the most interesting findings was the mosaic of expression of brown fat throughout vascular tissues. The fat around the thoracic aorta of the rat is clearly not white–it is a caramel brown color. This observation, as well as the fact that hematoxylin staining demonstrated a very dense multilocular fat, suggested that this was something different than white fat (for a review, see Ref. 11). UCP-1 was expressed in the thoracic aortic fat, as detected by both immunohistochemical and Western blot analyses. Western blot analyses of the six fats taken from the rats (aortic, superior mesenteric, subnephric, subcutaneous, epididymal, and brown fat pad) convincingly demonstrated that the thoracic aorta and brown fat pad express UCP-1, but the other tissues possess far less UCP-1 (virtually undetectable). The abdominal aorta and superior mesenteric artery express less UCP-1, and the fat around these tissues histologically is consistent with white cells. This difference between the thoracic and abdominal aorta has been described in the mouse (56). However, IDO expression was not specific to brown fat. IDO activity was equivalent in the brown fat and the other four white fats sampled; the thoracic aortic fat was unique in its high activity of IDO. The ability of UCP-1 to dampen superoxide production from the mitochondria makes brown fat of great interest (20, 51). We recognize that other adipocytes not in the class of brown fat can express a UCP-1-like protein (55), so, strictly speaking, we can only call those cells around the aorta as UCP-1-expressing fat cells.
The presence and potential function of this special reservoir of brown fat deserves attention because of the recent discovery of the expression and function of brown fat in the adult human (14, 22, 48). Brown fat had been thought of as expressed solely in neonates to aid in nonshivering thermogenesis, and, to be sure, neonates express brown fat. However, adults do as well. These groups have demonstrated regions of brown fat in the human that were activated (e.g., took up glucose mimetics) under cold conditions. In the adult, brown fat is primarily supraclavicular (48) but is also found near the thoracic aorta (8). Brown fat is currently the subject of intense discussion as a potential means to burn energy and reduce weight (15).
Clinical ramifications.
Our findings of the expression of brown fat around the thoracic aorta raise the question of the role of this fat on important parameters of aortic function, such as the Windkessel effect of blood movement and arterial stiffness. Excess body weight is associated with aortic stiffness (17, 71). One can speculate that the type of fat added to the aorta upon adiposity may be less beneficial/more harmful than the existing brown fat with IDO function; this remains to be tested. In terms of cardiovascular function and disease, IDO could play multiple roles. IDO has been located to dendritic cells (6, 46, 52), and we showed strong expression of IDO in the vessels within the lymph node of the fat. Periaortic fat in the human is associated with increased adiposity, metabolic risk, and coronary and abdominal calcification, so the specific function of the fat around the aorta is important (37). IDO has been implicated in general cardiovascular disease (45, 50), general inflammation (4, 31, 57), inflammation of obesity (3), and stroke (18). Our findings point to a potentially important intersection with the role of periadventitial fat in atherosclerosis (40, 54, 59, 66, 68), vascular remodeling (64, 65), and hypertension (26).
Limitations.
We recognize several limitations in this study. All the work was done with the arterial endothelium intact, so as to best mimic the normal situation, and nitric oxide could serve to inhibit IDO activity. Removal of the endothelium might reveal whether the fat-induced reduction of contraction was mediated by the endothelium, but we have not done these experiments as our interest has focused on the role of IDO and IDO was not observed in endothelial cells. It is possible that IDO produces a substance that enhances endothelial or nitric oxide synthase function, and these are avenues of future research. We have not investigated the role of IDO in vascular function in an inflammatory state, a condition that strongly stimulates IDO expression. As such, we speculate the role of IDO in pathologies of inflammation/elevation of IFN-γ would be significant. IDO knockout mice have been created (70), and we have not used them in this study because of the data suggesting that there are species differences in the ability of IDO metabolites to cause relaxation. All contractility experiments were performed under normal glucose conditions, as this is consistent with a majority of contractile experiments published. Different fat depots may have differing glucose requirements (9, 10), and we do not know the optimal glucose levels for thoracic aorta versus superior mesenteric versus abdominal aorta periadventitial fat. Arterial tissues were not normalized to weight. We observed an average ring (∼4–5 mm long) of the thoracic aorta: −fat was ∼21 mg and +fat was ∼62 mg, or three times the mass. Throughout our experiments, these weights were consistent, and this is one reason that the thoracic aorta may be valuable to the field, because of the high level of consistency of the fat strips expressed along the thoracic aorta. The superior mesenteric artery was more difficult given the fatty area of the body in which it was positioned, but we took the same length of segment for each tissue. We used one IDO inhibitor (1-MT). Multiple other IDO inhibitors are being developed (58), but we chose to use that inhibitor best accepted in the field presently and validated the use of this compound as an inhibitor of IDO in our system. We must also address the idea that while we can observe the effects of periadventitial fat in vitro, the substances released from the fat may not diffuse to the smooth muscle or endothelium. This statement holds true for all in vitro tissue experiments done.
Summary.
Here, we demonstrate, for the first time, the expression and function of IDO in periadventitial fat, most specifically that around the thoracic aorta. This enzyme imparts an inhibition of ANG II-induced contraction in the thoracic aorta, whereas periarterial fat caused a reduction in ANG-induced II contraction in the superior mesenteric artery. Contraction to the α-adrenergic agonist PE was less affected. Although kynurenine was produced through IDO activity in the fat around the thoracic aorta, kynurenine does not appear to be the substance that promotes the inhibition of agonist-induced contraction. We introduce the idea of brown fat in the regulation of vascular tone.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-081115 (to S. W. Watts) and P01-HL-70687 (to S. W. Watts).
REFERENCES
- 1. Ball HJ, Sanchez-Perez A, Weiser S, Austin CJB, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396: 203–213, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2: new enzymes in the kynurenine pathway. Int J Biochem Cell Biol 41: 467–471, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Barbarroja N, Lopez-Pedrera R, Mayas MD, Garcia-Fuentes D, Garrido-Sanchez L, Macias-Gonzalez M, El beka R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J 430: 141–149, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Barth MC, Ahluwalia N, Anderson TJ, Hardy GJ, Sinha S, Alvarez-Cardona JA, Pruitt IE, Rhee EP, Colvin RA, Gerszten RE. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem 284: 19189–19195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandes RP. The fatter the better: perivascular adiopose tissue attenuates vascular contraction through different mechanisms. Br J Pharmacol 151: 303–304, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun D, Longman RS, Albert ML. A two step induced of indoleamine 2,3 dioxygenase (IDO) activity during dendritic cell maturation. Blood 106: 2375–2381, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cady SG, Sono M. 1-Methyl-dl-tryptophan, β-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan) and β-[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Archiv Biochem Biophys 291: 326–333, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Chiba S, Katsuragi I, Simada T, Adachi I, Satoh Y, Noguchi H, Gotoh K, Tsubone T, Fujiwara K, Masaki T, Kakuma T, Kang M, Tanaka K, Hamaguchi K, Wada C, Yoshimatus H. Evaluation of human brown adipose tissue using positron emission tomography, computerized tomography and histochemical studies in association with body mass index, visceral fat accumulation and insulin resistance (Abstract). Obes Rev 7, Suppl 2: 87, 2006 [Google Scholar]
- 9. Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, Libby P. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. J Am Coll Cardiol Img 3: 843–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cigolini M, Bonora E, Querena M, Moghetti P, Cacciatori V, Zancanaro C, Benati D, Muggeo M. Differences in glucose metabolic enzyme activities in human adipose tissue from abdominal and gluteal regions. Metabolism 37: 820–823, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Cinti S. The adipose organ. Prostgl Leukotr Ess Fatty Acids 73: 9–15, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Csayni G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, Lakkis FG, Fuchs D, Pober JS, Tellides G. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-γ contributes to medial immunoprivilege. J Immunol 179: 5246–5254, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Curti A, Travanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygeanase in the induction of immune tolerance: focus on hematology. Blood 113: 2394–2401, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Cur Opin Endocrinol Diabetes Obes 17: 143–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo F, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danias PG, Tritos NA, Stuber M, Botnar RM, Kissinger KV, Manning WJ. Comparison of aortic elasticity determined by cardiovascular magnetic resonance imaging in obese vs. lean adults. Am J Cardiol 91: 195–199, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Darlington LG, Mackay GM, Forrest CM, Stoy N, Georg C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci 26: 2211–2221, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vasc Res 44: 175–181, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Dlaskova A, Clarke KJ, Porter RK. The role of UCP1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophysica Acta 1797: 1470–1476, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Enerback S. Human brown adipose tissue. Cell Met 11: 248–252, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Diff 9: 1069–1077, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C, Liu X, Geng B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens 27: 2174–2185, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzales MC, Ruiz-Gayo M, Gollasch M, Fernandez-Alfonso MS. A reduction in the amount and anticontractile effect of periaventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res 31: 1415–1423, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 71: 363–373, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, Ruiz-Gayo M, Fernandez-Alfonso MS, Somoza B. Adaptive nitric oxide overproduction in perivascular adipose tissue during early diet induced obesity. Endocrinology 151: 3299–3306, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci 25: 647–653, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exper Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, Hunt NH. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localized to the vascular endothelium. Internat J Parasitol 34: 1309–1319, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Hirata F, Hayaishi O. Studies on indoleamine 2,3-dioxygenase; superoxide anion as substrate. J Biol Chem 250: 5960–5966, 1975 [PubMed] [Google Scholar]
- 33. Ichiki T. Perivascular adipose tissue, a janus-faced regulator of vascular function. Circ J 7: 1300–1301, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Keskin DB, Marshall B, Munn D, Mellor A, Gerhart DA. Decreased protein nitration in macrophages that overexpress indoleamine 2,3-dioxygenase. Cell Mol Biol Lett 12: 82–102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 74: 1479–1487, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens 27: 782–790, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart study. Atherosclerosis 210: 656–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine 2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 9: 445–452, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111: 2152–2154, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Lohmann C, Schafer N, von Lukowicz T, Stein MAS, Boren J, Rutti S, Wahli W, Donath MY, Luscher TF, Matter CM. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver and pancreatic islets. Atherosclerosis 207: 360–367, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Lohn M, Dubrovska G, Lauterback B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol 656: 68–73, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Maghzal GJ, Thomas SR, Hunt NR, Stocker R. Cytochrome b5, but not superoxide anion radical, is a major reductant of indoleamine 2,3-dioxygenase in human cells. J Biol Chem 288: 12014–12025, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the knurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 11: 198–209, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Montani JP, Carroll FJ, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogeneis of cardiovascular diseases. Int J Obesity 28: S58–S65, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Munn DH, Sharma MD, Lee JR, Javer KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297: 1867–1870, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Naftilan AJ, Zuo WM, Inglefinger J, Ryan TJ, Pratt RE, Dzau VJ. Localization and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest 87: 1300–1311, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Ni W, Thompson JM, Northcott CA, Lookingland K, Watts SW. The serotonin transporter is present and functional in peripheral arterial smooth muscle. J Cardiovasc Pharmacol 43: 770–781, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Niinisalo P, Raitala A, Pertovaara M, Oja SS, Lehtimaki T, Kahonen M, Reunanen A, Jula A, Moilanen L, Kesaniemi YA, Nieminem MS, Hurme M. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest 68: 767–770, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Oelkrug R, Kutschke M, Meyer CW, Heldmaier G, Jastroch M. Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. J Biol Chem 285: 21961–21968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogasawara N, Oguro T, Sakabe T, Matsushima M, Takikawa O, Isobe KI, Nagase F. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-κB and the generation of reactive oxygen species. J Cell Biochem 108: 716–725, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynureninemetabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol 54: 175–189, 2003 [PubMed] [Google Scholar]
- 54. Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-β-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H460–H465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPAR-γ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP-1 containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood 106: 2383–2390, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rohrig UF, Awad L, Grosdidier A, Larrieu P, Stroobant V, Colau D, Cerundolo V, Simpson AJ, Vogel P, Van den Eynde BJ, Zoete V, Michielin O. Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem 53: 1172–1189, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 153: 907–917, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Sorensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN, Svane IM, Becker JC, Straten P, Andersen MH. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLos ONE 4: e6910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 17: 115–130, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev 1: 609–620, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res 105: 906–911, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Atheroscler Thromb Vasc Biol 30: 1576–1582, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Tellides G. Periadventitial fat: regional source of inflammation in atherosclerosis. Arch Pathol Lab Med 131: 346–347, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Thomas SR, Terentis AC, Cai H, Takikawa O, Levina A, Lay PA, Freewan M, Stocker R. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem 282: 23778–23787, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Vela D, Buja M, Madjid M, Burke A, Naghavi M, Willerson JT, Casscells SW, Litovsky S. The role of periadventitial fat in atherosclerosis. Arch Pathol Lab Med 131: 481–487, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Hunt NH, Stocker R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nature Med 16: 279–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 42: 468–473, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Yuasa HG, Ball HJ, Ho YF, Austin CJD, Whittington CM, Below K, Maghzal GJ, Jermiin LS, Hunt NH. Characterization and evolution of vertebrate indoleamine 2,3-dioxygenases IDOs from monotremes and marsupials. Comparative Biochem Physiol Part B 153: 137–144, 2009 [PubMed] [Google Scholar]
- 73. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signaling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820, 2005 [DOI] [PubMed] [Google Scholar]