Abstract
Transduction of sound in the inner ear demands tight control over delivery of oxygen and glucose. However, the mechanisms underlying the control of regional blood flow are not yet fully understood. In this study, we report a novel local control mechanism that regulates cochlear blood flow to the stria vascularis, a high energy-consuming region of the inner ear. We found that extracellular lactate had a vasodilatory effect on the capillaries of the spiral ligament under both in vitro and in vivo conditions. The lactate, acting through monocarboxylate transporter 1 (MCT1), initiated neuronal nitric oxide (NO) synthase (nNOS) and catalyzed production of NO for the vasodilation. Blocking MCT1 with the MCT blocker, α-cyano-4-hydroxycinnamate (CHC), or a suppressing NO production with either the nonspecific inhibitor of NO synthase, NG-nitro-l-arginine methyl ester (l-NAME), or either of two selective nNOS inhibitors, 3-bromo-7-nitroindazole or (4S)-N-(4-amino-5[aminoethyl]aminopentyl)-N′-nitroguanidine (TFA), totally abolished the lactate-induced vasodilation. Pretreatment with the selective endothelial NO synthase inhibitor, l-N5-(1-iminoethyl)ornithine (l-NIO), eliminated the inhibition of lactate-induced vessel dilation. With immunohistochemical labeling, we found the expression of MCT1 and nNOS in capillary-coupled type V fibrocytes. The data suggest that type V fibrocytes are the source of the lactate-induced NO. Cochlear microvessel tone, regulated by lactate, is mediated by an NO-signaled coupling of fibrocytes and capillaries.
Keywords: cochlear capillary, monocarboxylate transporter 1, neuronal nitric oxide synthase
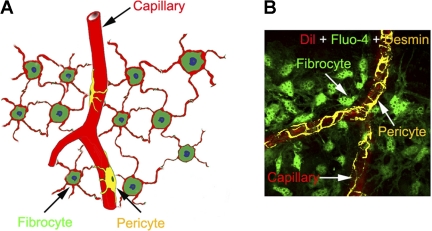
cochlear blood flow is tightly regulated to match metabolic demand and to maintain the cochlear homeostasis, including the endocochlear potential, which is essential for normal audition (21, 28). Although cochlear blood flow is thought to be primarily regulated in the spiral modiolar artery and its branching arterioles (10, 16, 27), an end-arterial system of the cochlea, finer grade control of blood distribution is exercised at the level of the capillary network (23). Capillary-mediated local control of perfusion was first reported by Sadanaga et al. (20). However, until recently, the mechanism that regulates microvessel flow in the lateral wall was not fully understood. Recent studies in our laboratory have shown that pericytes densely populate capillaries of the cochlear spiral ligament (24). These pericytes contain contractile proteins, such as α-smooth muscle actin and tropomyosin, which enable cochlear pericytes to regulate local blood flow by contraction or relaxation (1). Moreover, fibrocytes in the superstrial region of the spiral ligament closely connect with capillaries through one or more end-foot processes (1a) (Fig. 1), forming fibrovascular signaling units that “bridge” cochlear metabolic need and cochlear blood flow.
Fig. 1.
A: morphology of fibrocyte- and pericyte-populated microvessels in the spiral ligament. The fibrocytes make contact with microvessels through their end feet. B: immunofluorescence shows the relative location of fibrocytes (green), pericytes (yellow), and endothelium (red) in the spiral ligament. Dil, 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate.
Lactate, a major product of metabolism, is vasoactive and plays an important role in the regulation of blood flow in several different organ systems (4, 9, 12, 13). Both animal and human studies have shown that lactate increases retinal blood flow and induces dilation in retinal arteries (7, 12). Gordon et al. (4) point out that lactate is an important vasodilatory factor regulating blood flow in brain. Cochlear perilymph has a much higher concentration of lactate than blood and cerebrospinal fluid, and the concentration is upregulated by sound stimulation (11, 22), indicating glycolysis is important for cochlear function. Moreover, monocarboxylate transporters (MCTs), which facilitate cell uptake of lactate, are expressed in different cell types in the cochlea (18, 26).
The present study was initiated to further explore the metabolic regulation of local blood flow in the cochlea and determine the influence of lactate on cochlear blood flow and vessel tone. We found that increased local blood flow from lactate-induced dilation of cochlear microvessels is mediated by MCT1. Moreover, nitric oxide (NO) is involved in this regulation through fibrocyte vascular-coupled activity. Lactate-based regulation enables an efficient blood flow response to metabolic demand in the cochlea.
MATERIALS AND METHODS
Animals.
Albino guinea pigs (CRL, Duncan-Hartley, both sexes, 4 to 5 wk old, 300–450 g body wt), C57BL/6J mice (4 wk old, 18–20 g body wt, Jackson) and B6.129P2-Nos3tm1Unc/J mice (4–6 wk old, 18–20 g body wt, Jackson Laboratory) were used in this study. All procedures in this study were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University.
Intravital fluorescence microscopy in vivo.
The guinea pigs were anesthetized with an injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) (Abbott, Chicago, IL) and were then wrapped in a heating pad with rectal temperature maintained at ∼38°C. The head was fastened to a manipulator, which was heated to prevent conductive cooling. A ventrolateral surgical dissection was carried out to expose the bulla, which was then opened over its ventral surface. To observe the blood circulation in the vessels of the spiral ligament, we used a method previously described (17, 25). In brief, a rectangular fenestration (0.2 × 0.3 mm) into the cochlea was made by creating a window opening over the spiral ligament after scoring a rectangle of scratch marks into the bone of the fourth turn. Vessels of the cochlear lateral wall were visualized by intravital microscopy through this window. The window is referred to as the “vessel window.” A thin cover slide was used to cover the vessel window to preserve normal physiological conditions and also to have the best optical view for recording vessel images. Vessels of the spiral ligament were visualized by adjusting the optical focus. The vessels within the window were monitored with intravital video microscopy using a long-working distance objective lens [×20, 0.4 numerical aperture (NA)]. A physiological solution containing lactate (10 mM) was superfused into the cochlea through the vessel window. Capillaries of the spiral ligament were visualized using an Olympus BXFM fluorescence microscope equipped with a long distant objective (×20, 0.4 NA). The resolution of the in vivo imaging system, limited by pixel size of the imaging CCD camera, was 0.48 μm, sufficient for image analysis. To document changes in capillary diameter, time-lapse images were recorded at 1-s intervals.
Isolation of whole mounted lateral wall tissue.
The cochleae were dissected from the auditory bullae immediately after the anesthetized guinea pigs were euthanized by exsanguination. The lateral walls were carefully removed from the cochleae. The spiral ligament was isolated by detaching the stria vascularis from the cochlear lateral wall. The lateral walls were rapidly removed and transferred to a petri dish filled with a physiological solution composed of 125 mM NaCl, 3.5 mM KCl, 5 mM glucose, 10 mM HEPES, 1.3 mM CaCl2, 1.5 mM MgCl2, and 0.51 mM NaH2PO4, bubbled with 95% O2-5% CO2. The osmolarity of the solution was adjusted to 310 mosmol/l with NaCl, and the pH was adjusted to 7.4 with NaOH. All experiments were performed at 37°C using a temperature control chamber (Warner Instruments). The tissues were maintained in the physiological solution until needed.
Time-lapse microscopy in vitro.
The detached spiral ligament was positioned in an eight-channel perfusion chamber (Bioscience Tool, San Diego, CA). Capillaries of the spiral ligament were visualized by differential interference contrast optics at ×400 magnification on a Nikon Eclipse E600 with a water-immersion objective (×40, 0.8 NA). Time-lapse images were recorded at 1-s intervals using a Photometrics Sensys digital camera to record the changes in capillary diameter with perfusion of a physiological solution containing 10 mM lactate. In experiments using α-cyano-4-hydroxycinnamate (CHC), NG-nitro-l-arginine methyl ester (l-NAME, Cat No. 0665, Tocris Bioscience, Ellisville, MI), 3-bromo-7-nitroindazole (3-Br-7-NI, Cat No. 0735, Tocris Bioscience), (4S)-N-(4-amino-5[aminoethyl]aminopentyl)-N′-nitroguanidine (TFA, Cat No. 490070, EMD Chemicals, Gibbstown, NJ), or l-N5-(1-iminoethyl)ornithine (l-NIO) (Cat No. 0546, Tocris Bioscience), we exposed freshly isolated tissues to these chemicals for 30 min before performing time-lapse microscopy. ImageJ software was used to measure the lumen diameter at the beginning of the perfusion and at the time when the changes in diameter were maximal (1).
Capillary visualization in vivo and in vitro.
The cochlear capillaries were prelabeled with the fluorescent dye, 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate (Dil) (19). Before intravenous injection, the stock solution was diluted with saline to a concentration of 3 mg/ml, which was slowly administrated to the guinea pig over the course of 10 min.
NO assessment.
NO production was assessed using an NO-sensitive dye, 4,5-diaminofluorescein diacetate (DAF-2DA; Cat No. 251505, EMD Chemicals), under experimental conditions as previously described (25). The detached lateral wall tissues were incubated in a petri dish with a physiological solution containing 10 μM DAF-2DA for 30 min at room temperature and washed to remove excess dye. DAF-2DA was excited at a wavelength of 480 nm on an Olympus Fluoview FV1000 confocal laser microscope, and images of emission were acquired before and after treatment with 10 mM lactate. Change in NO was assessed as the relative increase of fluorescence from the baseline intensity (ΔF/F). ΔF/F = [(F1 − FB1) − (F0 − FB0)]/(F0 − FB0), where F1 is the fluorescence intensity in the cells after lactate treatment, F0 is the fluorescence intensity at the beginning of the experiment, and FB is the background fluorescence.
Reverse transcription polymerase chain reaction.
Total RNA from the cochlear lateral wall of three mice was separately extracted with an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's suggestions. Slc16a (MCT) family mRNA was also analyzed. One microgram of total RNA was reverse transcribed using a RETROscript kit (Ambion, Austin, TX). Conserved regions spanning introns were selected for the primers of MCTs and GAPDH. The primers used were as follows: Slc16a1 (MCT1) forward, GAGGTCCTATCAGCAGTATCTT, and reverse, CCAGTGGTCGCTTCTTGT, 220-bp product; Slc16a7 (MCT2) forward, CGCTTCCACAACCACTTC, and reverse, CCTCCCACTATCACCACA, 269-bp; Slc16a8 (MCT3) forward, GGCACTCGGGCTCTTTGT, and reverse, GCAGGCAGCAGTAGGTGGTAA, 518-bp; Slc16a3 (MCT4) forward, ATGCTCTATGGCACAGGACC, and reverse, GGCGACGCTTGTTGAAGTA, 229-bp product; and GAPDH forward, 5′-ATGTGTCCGTCGTGGATCTGAC-3′, and reverse, 5′-AGACAACCTGGTCCTCAGTGTAG-3′, 132-bp product. The RT-PCR was cycled at 95°C for 2 min, up to 35 cycles at 95°C for 30 s, 60°C for 45 s, 72°C for 30 s, and a final 5-min extension at 72°C. The products of the reverse transcription polymerase chain reaction were visualized by agarose gel electrophoresis.
Fluorescent in situ hybridization.
The animals were anesthetized with an injection of ketamine and xylazine and transcardially perfused with a solution of 4% paraformaldehyde in PBS. The cochleae were isolated from the skull and soaked in 4% paraformaldehyde for 24 h. The spiral ligaments were carefully dissected in RNase-free PBS and in situ hybridized with LNA probes (Exiqon, Woburn, MA). The tissues were dehydrated with ethanol, graded from 25 to 100%, and treated with proteinase K (10 mg/ml) for 15 min. After incubation in the prehybridization mix for 1 h (54°C, rotating), the tissues were hybridized overnight with the LNA probes. After a wash in 2× SSC for 10 min, the tissues were mounted with mounting medium with 4,6-diamidino-2-phenylindole (DAPI, Cat No. H-1500, Vector, Burlingame, CA) and observed on an Olympus IX81 inverted microscope fitted with a Fluoview FV1000 confocal laser system.
NO synthase activity assay.
Cochleae were obtained from anesthetized endothelial NO synthase (eNOS) knockout mice. The spiral ligament tissues were removed and incubated in artificial labyrinth buffer for 30 min with different concentrations of the neuronal NO synthase (nNOS) inhibitor TFA. The tissues were homogenized in extraction buffer and centrifuged for 15 min at 4°C. The supernatants were collected, and 20 μl of the homogenate were incubated in 40 μl of 1 mM NADPH, l mM l-arginine, 1 mM CaCl2, 10 μM FAD, 0.5 mM DTT and 0.1 mM (6R)-BH4 for 30 min at 37°C. The reaction was heat terminated (100°C for 1 min), and the products were centrifuged for 15 min at 4°C. The supernatants were collected, and the NO synthase (NOS) activity was measured using a Nitric Oxide Synthase Assay Kit per the manufacturer's recommendations (Cat No. 482702, EMD Chemicals).
Immunohistochemistry.
The primary antibodies used in the experiments included anti-desmin (rabbit monoclonal to desmin, Cat No. ab32362, Abcam, Cambridge, MA), anti-MCT1 (Cat No. AB3538P, Millipore, Billerica, MA), anti-S100 (Cat No. ab8330, Abcam), anti-nNOS (Cat No. 610310, BD Transduction, Lexington KY), and anti-eNOS (Cat No. ab66127, Abcam).
Secondary antibodies (Invitrogen, Carlsbad, CA) included Alexa fluor 568 conjugate goat anti-rabbit (Cat No. A11011), Alexa fluor 647 conjugate goat anti-rabbit (1:100, Cat No. A21245, Invitrogen), Alexa fluor 488 conjugate goat anti-mouse IgG (H + L) (1:100, Cat No. A11001), and Alexa fluor 647 conjugate goat anti-guinea pig (Cat No. A21450).
After the animals were euthanized, segments of the spiral ligament were removed and incubated for 30 min in a petri dish with a physiological solution containing 10 μM DAF-2DA. The tissues were fixed in 4% formaldehyde for 2 h, washed in 0.02 phosphate-buffered saline (PBS, pH 7.4) for 30 min, permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO) for 1 h, and immunoblocked in a solution of 10% goat serum with 1% bovine albumin in 0.02 M PBS for 30 min. The specimens were incubated overnight with the primary antibody diluted in 1% BSA-PBS. The specimens were washed in 0.02 M PBS for 30 min and incubated with a secondary antibody (diluted 1:100 in 1% BSA-PBS) for 1 h. After being washed in 0.02 M PBS for 30 min, the tissues were mounted and observed on an Olympus IX81 inverted microscope fitted with a Fluoview FV1000 confocal laser system. Negative controls included tissue incubated with 1% BSA-PBS replacing the primary antibody or incubated with a blocking peptide.
Statistics
Data, presented as means ± SD, were evaluated using the Student's t-test for comparison of two groups or ANOVA for comparisons of three or more groups. A 95% confidence level was considered statistically significant.
RESULTS
Lactate increases cochlear blood flow.
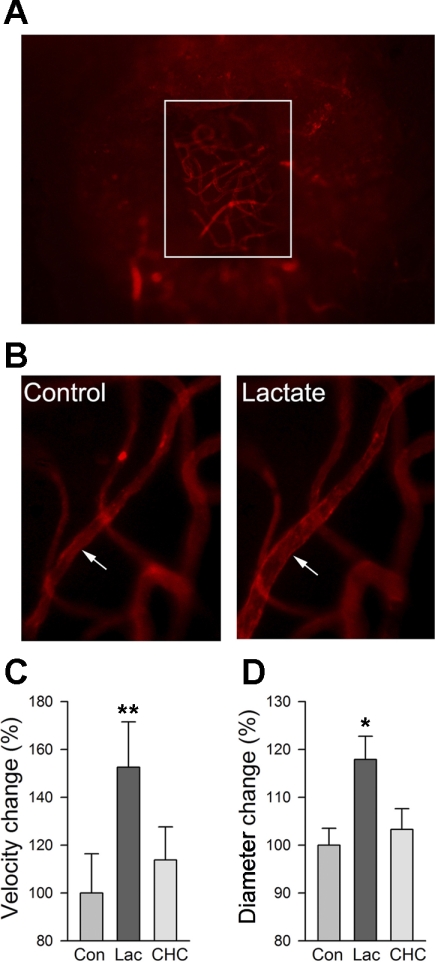
To measure blood flow and capillary diameter in guinea pig cochlea in vivo, a vessel window at the third turn of the cochlea was made in a living guinea pig (as described in materials and methods). An intravenous injection of Dil enabled visualization of the capillaries (Fig. 2A). A local perfusion system allowed the physiological conditions to be maintained and topical application of experimental agents and artificial perilymph. Superfusion of 10 mM lactate for 15 min significantly increased cochlear blood flow in the guinea pig (baseline blood flow was 128.3 ± 5.8 μm/s, and flow was increased ∼150% with the superfusion) (Fig. 2C, n = 10). However, pretreatment with the monocarboxylate transport blocker CHC (1 mM) fully prevented this response (Fig. 2C, n = 10). As shown in Fig. 2B, the increase in cochlear blood flow induced by lactate was accompanied by an increase in capillary diameter (Δdiameter = 17.8 ± 4.87%, and initial diameter = 9.32 ± 1.16 μm, n = 10) (Fig. 2B). Consistent with the effect of CHC on cochlear blood flow in the presence of lactate, CHC pretreatment diminished the dilatory effect of lactate (Fig. 2D, n = 10).
Fig. 2.
Extracellular lactate induces vasodilation and increased blood flow in the cochlear lateral wall. A: cochlear vessels in a cochlear wall (vessel window) at the third turn were visualized by a fluorescence intravital microscope with a long working distance lens. White box outlines the edges of the vessel window. B: intravital fluorescence microscopy shows that vasodilation of the cochlear capillaries to 10 mM lactate is blocked by pretreatment with the monocarboxylate transporter (MCT) blocker α-cyano-4-hydroxycinnamate (CHC). Superfusion with lactate (10 mM) to the vessel window increased both blood flow (C; n = 10) and capillary diameter (D; n = 10). Pretreatment with CHC significantly attenuated both effects. Con, control; Lac, lactate. *P < 0.01; **P < 0.001.
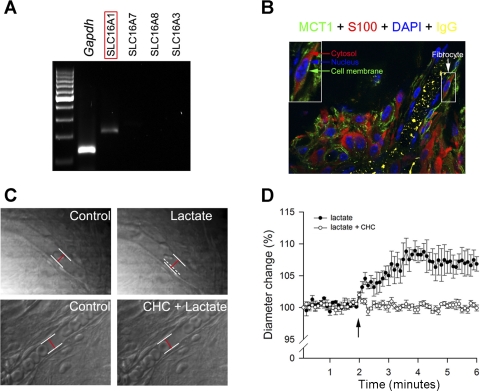
Lactate-induced vasodilation requires MCT1.
Only one subtype of MCT1 (slc16a1) was detected by agarose gel electrophoresis after 35 amplification cycles by RT-PCR (Fig. 3A). The expression of MCT1 in the spiral ligament was further confirmed by immunohistochemical labeling. MCT1 protein was expressed in the plasma membrane of type V fibrocytes (Fig. 3B), but the MCT1 immunoactivity could not be detected in the microvessels, indicating that fibrocytes are the primary cell type in the lateral wall taking up lactate.
Fig. 3.
Extracellular lactate induces vasodilation of capillaries in the spiral ligament. A: mRNA for one isoform of the SLC16A (MCT) family, SLC16A1 (MCT1), is expressed in the cochlear lateral wall. B: immunofluorescence shows MCT1 protein selectively expressed in the cell membrane (green arrow) of type V fibrocytes (white arrow) but not in vascular cells. The fibrocytes were identified by the positive cytosolic staining of S100 (red arrow), and nuclei were labeled by 4,6-diamidino-2-phenylindole (DAPI; blue arrow). C: time-lapse images show lactate-induced change in vessel diameter with or without the MCT blocker CHC. White lines outline position of the vessel wall, whereas dashed white line indicates previous position of vessel wall and red line indicates diameter of vessel. D: time-dependent changes in cochlear capillary diameter in response to extracellular lactate with and without pretreatment with MCT blocker CHC.
Tests were conducted to assess the role of MCT in lactate-induced vasodilation in vitro. Spiral ligament tissues were dissected quickly and carefully in these tests. The effect of applied lactate on capillary diameter was recorded with time-lapse microscopy. ImageJ was used to assess the change in capillary diameter from the recorded images. Consistent with the results from the in vivo study, 10 mM lactate evoked dilation of capillaries near pericytes (Δdiameter = 8.9 ± 1.9%, and initial diameter = 9.35 ± 1.41 μm, n = 24, P < 0.01). When the sample was pretreated with 1 mM CHC, a panspecific MCT blocker, lactate-initiated vasodilation was significantly diminished (Δdiameter = 1.8 ± 2.1%, and initial diameter = 9.18 ± 0.93 μm, n = 15, P > 0.05). Blockage of the MCT mechanism with CHC attenuated lactate-induced vasodilation, consistent with the results obtained in our in vivo study.
Lactate induces NO production.
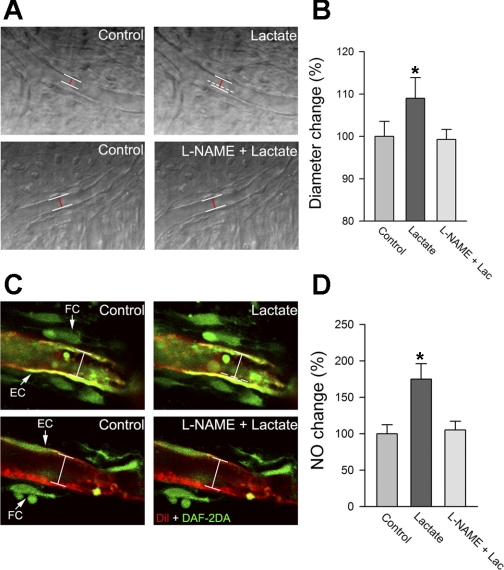
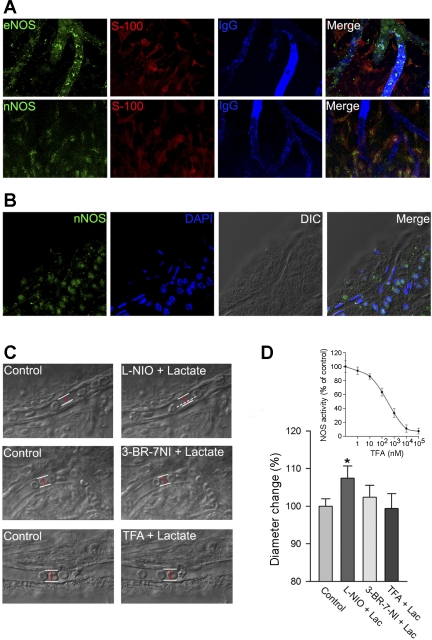
In this study, we tested whether NOS activation is required for lactate-induced vasodilation of capillaries. Tissues were pretreated with the pan-NOS inhibitor l-NAME (10 μM) for 10 min. The results showed that pretreatment with l-NAME almost completely eliminated the vasodilative response to lactate (Fig. 4, A and B, n = 15), indicating NOS contributes substantially to lactate-induced vasodilation.
Fig. 4.
A: representative time-lapse images show lactate-induced change in vessel diameter with and without the nitric oxide (NO) synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME). White lines outline the position of the vessel wall, whereas dashed white line indicates the previous position of the vessel wall and the red line indicates the diameter of the vessel. B: mean capillary diameter is significantly increased after lactate perfusion (n = 24, *P < 0.01). In contrast, mean capillary diameter shows no change in tissues pretreated with the MCT blocker (n = 10, P > 0.05). C: confocal images show 10 mM lactate increases intracellular NO [labeled by 4,5-diaminofluorescein diacetate (DAF-2DA), green; indicated by white arrows], an effect diminished by l-NAME. D: mean values of intensity of the fluorescence, indicating NO production among different treatment groups. Mean value of NO fluorescent signal intensity is significantly increased after treatment with lactate (n = 15, *P < 0.01). In contrast, there is no change in tissues treated with the NOS blocker (n = 15, P > 0.05). FC, fibrocytes; EC, endothelial cells.
To check whether lactate-induced vasodilation depends on NO production, the tissue was perfused with the NO-sensitive dye DAF-2DA (10 μM). The DAF-2DA was used as a sensor for intracellular NO in the lateral wall tissue (25). The tissues positioned in a perfusion chamber were observed on an inverted Olympus Fluoview FV1000 confocal laser microscope system. The application of lactate increased the fluorescent signal for NO in the capillary wall and in type V fibrocytes (Fig. 4C, n = 10). NO production by lactate was attenuated in the l-NAME-pretreated tissue (Fig. 4, C and D).
Lactate-induced increase in NO production via activation of nNOS.
Neuronal NOS (nNOS) and endothelial NOS (eNOS) catalyze synthesis of NO from l-arginine in neurons and endothelial cells. Immunostaining showed that both isoforms of eNOS and nNOS were present in the spiral ligament (Fig. 5A). The results revealed that eNOS was selectively expressed in vascular endothelium (Fig. 5A), whereas nNOS was selectively expressed in fibrocytes (Fig. 5A). Selective expression of nNOS in fibrocytes was further confirmed by a whole mount fluorescent in situ hybridization experiment (Fig. 5B).
Fig. 5.
A, top: expression of endothelial NOS (eNOS; green) in capillaries of the spiral ligament (red). Abutting capillaries are labeled for anti-serum protein IgG (blue). A, bottom: expression of neuronal NOS (nNOS; green) in type V fibrocytes (red) with immunohistochemistry method. B: specific expression of nNOS mRNA in type V fibrocytes showed by fluorescent in situ hybridization method. DIC, differential interference contrast. C: time-lapse images show the effect of lactate perfusion on vessel diameter after incubation with l-N5-(1-iminoethyl)ornithine (l-NIO; an eNOS inhibitor), 3-bromo-7-nitroindazole (3-Br-7-NI; an nNOS inhibitor), and (4S)-N-(4-amino-5[aminoethyl]aminopentyl)-N′-nitroguanidine (TFA; a specific nNOS inhibitor). White lines outline position of the vessel wall, whereas dashed white line indicates previous position of vessel wall and red lines indicate diameter of the vessel. D: 3-Br-7-NI and TFA abolish lactate-induced vasodilation, whereas l-NIO has no effect (*P < 0.05, n = 10). D, inset: dose-effect curve of TFA to nNOS activity of the spiral ligament tissue.
The results, shown in Fig. 4, implicate NOS in vessel dilation by lactate but do not address the type of NOS involved. In this study, TFA (a potent nNOS inhibitor), 3-Br-7-NI (a relatively selective nNOS inhibitor), and l-NIO (a relatively selective eNOS inhibitor) were used to intervene with lactate-induced changes. Lactate-induced vasodilation was inhibited by pretreatment with 10 μM 3-Br-7-NI (Fig. 5C, Δdiameter = 2.3 ± 3.1%, and initial diameter = 9.41 ± 1.33 μm, n = 15, P > 0.05) and 10 μM TFA (Fig. 5C, Δdiameter = 0.7 ± 3.9%, and initial diameter = 9.08 ± 1.06 μm, n = 15, P > 0.05) but was unaffected by pretreatment with 10 μM l-NIO (Fig. 5C, Δdiameter = 7.4 ± 3.2%, and initial diameter = 9.26 ± 1.17 μm, n = 15, P < 0.01). The findings suggest that extracellular lactate likely causes vasodilation through a nNOS signal pathway.
DISCUSSION
The cochlea has rapidly changing metabolic requirements, and fast-acting mechanisms are needed to adjust vessel tone. Although little is known about how microvascular blood flow in the cochlea is regulated, there is increasing evidence that vasoactive agents are involved in the fine control over capillaries in the lateral wall. Lactate acts as a dynamic vasoactive agent in retinal and brain microvasculature (12, 30), indicating that lactate is the bridging agent which links metabolic need to local blood flow. In the present investigation of cochlear capillaries, lactate modulation of vessel diameter was demonstrated both in vitro and in vivo. This is the first demonstration that lactate causes a significant increase of local blood flow in the cochlea by affecting dilation of microvessels. The lactate-induced vasodilation requires the activation of nNOS in fibrocytes.
MCTs facilitate transport and cellular uptake of lactate (14). Our results show that MCTs play a major role in lactate-induced vasodilation, as inhibition of CHC significantly diminishes vasodilation. Of the fourteen MCTs identified (5), only MCT1-MCT4 are shown to catalyze proton-coupled transport of lactate (2, 6, 29). Our PCR and immunofluorescence results indicate that MCT1 is the predominant subtype expressed in cochlear lateral wall tissue. More importantly, the expression of MCT1 is localized in the plasma membranes of fibrocytes, whereas the expression of MCT1 is virtually undetectable in microvessels. This suggests that the locus of lactate vasoactivity is the MCT1 expressed in fibrocytes.
Since NO has been shown to be essential for lactate-induced vasodilation in various other organs (12), it is reasonable to assume that NO also plays a role in lactate-induced cochlear vasodilation. In agreement with studies in other organs, lactate-induced vasodilation in the cochlea was almost completely blocked by l-NAME, indicating NO is the major mediator of lactate-induced vasodilation in cochlear microvessels. Two isoforms of calcium-dependent constitutive NOS are detected in the spiral ligament: eNOS is mainly expressed by vascular endothelium, whereas nNOS expression is localized in fibrocytes. Specific blocking of nNOS and eNOS enabled identifying nNOS as the source of the NO in lactate-induced cochlear vasodilation. Pretreatment with a selective nNOS inhibitor significantly reduced NO production and inhibited vasodilation in response to extracellular lactate. On the other hand, vasodilation caused by lactate was marginally changed with the suppression of eNOS from pretreatment of tissues with the selective eNOS inhibitor l-NIO. Since MCT1 is also expressed in fibrocytes and the fibrocytes are in close contact with endothelial cells and pericytes, we speculate that extracellular lactate activates nNOS in fibrocytes through MCT1. The direct measurement of lactate-initiated NO production in fibrocytes confirmed the hypothesis. A significant increase in DAF-2DA fluorescence (an NO indicator) in fibrocytes is detected after stimulation with lactate.
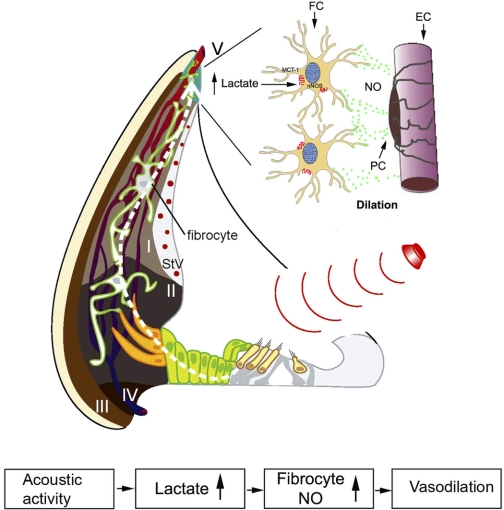
Blood supply for the cochlea is supplied by two microvessel networks located in the stria vascularis and spiral ligament. Capillaries of the spiral ligament, arteriovenular anastomosing vessels, are considered to be the primary network regulating cochlear blood flow, whereas the capillaries of the stria vascularis are considered secondary. Our previous studies showed that type V fibrocytes in contact with microvessels nearby in the superstrial region form fibrovascular-coupling units. We speculate that this fibrovascular-coupling unit, similar to neurovascular coupling units in the brain and retina, functionally regulates local blood flow to meet the local metabolic demand (3, 8, 15, 31). As we propose in the working model of Fig. 6, increased cochlear metabolism (e.g., under sound stimulation) also increases glycolysis and lactate production. Increased lactate acts as a signal that increases local blood flow by regulating the fibrovascular-coupling unit.
Fig. 6.
The working model illustrates how cochlear blood flow is locally regulated to meet metabolic demand. Increased cochlear activity (such as sound stimulation) leads to increased glycolysis and lactate production. The local extracellular lactate activates the lactate transporter MCT1 expressed in type V fibrocytes, resulting in nNOS activation. The NO produced by nNOS diffuses into the perivascular space to elicit vasodilatation and increased blood flow. PC, pericyte; StV, stria vascularis; I–IV, types I–IV fibrocytes, respectively.
In summary, the present study provides the first direct evidence that lactate causes dilation of cochlear capillaries. The uptake of lactate by fibrocytes through an MCT1 transport pathway leads to the activation of nNOS and the production of NO, which induces vasodilation.
GRANTS
This work was supported by the National Institute of Deafness and Other Communications Disorders Grants R03-DC-008888, DC-008888-02-S1, and R01-DC-010844 (to X. Shi) and P30-DC-005983 and R01-DC-00105.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Dai M, Nuttall A, Yang Y, Shi X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear Res 254: 100–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. Dai M, Shi X. Fibro-vascular coupling in the control of cochlear blood flow. PLos One 6: e20652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278: 40128–40135, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: 619–628, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343: 281–299, 1999 [PMC free article] [PubMed] [Google Scholar]
- 7. Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci 47: 693–699, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Ido Y, Chang K, Williamson JR. NADH augments blood flow in physiologically activated retina and visual cortex. Proc Natl Acad Sci USA 101: 653–658, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang ZG, Shi X, Zhao H, Si JQ, Nuttall AL. Basal nitric oxide production contributes to membrane potential and vasotone regulation of guinea pig in vitro spiral modiolar artery. Hear Res 189: 92–100, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Lotz P, Posse D, Haberland EJ, Kuhl KD, Ernst A. The metabolic reaction of the cochlea to unphysiological noise exposure. Acta Otolaryngol 102: 20–26, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Mendrinos E, Petropoulos IK, Mangioris G, Papadopoulou DN, Stangos AN, Pournaras CJ. Lactate-induced retinal arteriolar vasodilation implicates neuronal nitric oxide synthesis in minipigs. Invest Ophthalmol Vis Sci 49: 5060–5066, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Mintun MA, Vlassenko AG, Rundle MM, Raichle ME. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci USA 101: 659–664, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J 10: 311–321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res Brain Res Rev 43: 17–28, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Nuttall AL. Techniques for the observation and measurement of red blood cell velocity in vessels of the guinea pig cochlea. Hear Res 27: 111–119, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Okamura H, Spicer SS, Schulte BA. Developmental expression of monocarboxylate transporter in the gerbil inner ear. Neuroscience 107: 499–505, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Ravnic DJ, Jiang X, Wolloscheck T, Pratt JP, Huss H, Mentzer SJ, Konerding MA. Vessel painting of the microcirculation using fluorescent lipophilic tracers. Microvasc Res 70: 90–96, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Sadanaga M, Liu J, Wangemann P. Endothelin-A receptors mediate vasoconstriction of capillaries in the spiral ligament. Hear Res 112: 106–114, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97: 984–991, 1987 [PubMed] [Google Scholar]
- 22. Scheibe F, Haupt H, Hache U. Postmortem changes in the perilymphatic lactate and pyruvate concentrations of guinea pigs. [In German.] Arch Otorhinolaryngol 232: 293–297, 1981 [DOI] [PubMed] [Google Scholar]
- 23. Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P, Voigtlaender-Bolz J, Lidington D, Bolz SS. Tumor necrosis factor-alpha enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke 41: 2618–2624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation 15: 515–529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi X, Nuttall AL. The demonstration of nitric oxide in cochlear blood vessels in vivo and in vitro: the role of endothelial nitric oxide in venular permeability. Hear Res 172: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Shimozono M, Scofield MA, Wangemann P. Functional evidence for a monocarboxylate transporter (MCT) in strial marginal cells and molecular evidence for MCT1 and MCT2 in stria vascularis. Hear Res 114: 213–222, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Wangemann P. Cochlear blood flow regulation. Adv Otorhinolaryngol 59: 51–57, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Wangemann P. K+ cycling and the endocochlear potential. Hear Res 165: 1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem 273: 15920–15926, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 290: H925–H934, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003 [DOI] [PubMed] [Google Scholar]