Abstract
Recent findings indicate that endothelial nitric oxide (NO) plays a key role in uterine artery outward circumferential remodeling during pregnancy. Although the underlying mechanisms are not known, they likely involve matrix metalloproteinases (MMPs). The goal of this study was to examine the linkage among NO inhibition, expansive remodeling, and MMP expression within the uterine vascular wall. Adult female rats were treated with NG-nitro-l-arginine methyl ester [l-NAME (LPLN)] beginning on day 10 of pregnancy and until death at day 20 and compared with age-matched controls [late pregnant (LP)]. Mean arterial pressure of LPLN rats was significantly higher than controls. LPLN fetal and placental weights were significantly reduced compared with controls. Main uterine arteries (mUA) were collected to determine dimensional properties (lumen area and wall thickness), collagen and elastin content, and levels of endothelial nitric oxide synthase (eNOS) and MMP expression. Circumferential remodeling was attenuated, as evidenced by significantly smaller lumen diameters. eNOS RNA and protein were significantly (>90%) decreased in the LPLN mUA compared with LP. Collagen and elastin contents were significantly increased in LPLN rats by ∼10 and 25%, respectively, compared with LP (P < 0.05). Both MMP-2 and tissue inhibitors of metalloproteinase-2 as assessed by immunofluorescence were lower in the endothelium (reduction of 60%) and adventitia (reduction of 50%) of LPLN compared with LP mUA. Membrane bound MMP-1 (MT1-MMP) as assessed by immunoblot was significantly decreased in LPLN. These data suggest a novel contribution of MMPs to gestational uterine vascular remodeling and substantiate the linkage between NO signaling and gestational remodeling of the uterine circulation via altered MMP, TIMP-2, and MT1-MMP expression and activity.
Keywords: matrix metalloproteinase, extracellular matrix, hypertension, pregnancy, nitric oxide
during pregnancy, the uterine vasculature undergoes significant expansive remodeling to accommodate the dramatic increase in uteroplacental blood flow that is requisite for normal pregnancy outcome. Studies from our and other laboratories (40, 41, 44, 46, 50, 61) have established that nitric oxide (NO) is a key molecule involved in vascular remodeling during pregnancy and that expression of endothelial nitric oxide synthase (eNOS) is increased during pregnancy, leading to increased synthesis and release of NO from the endothelium.
The importance of NO and NO signaling during pregnancy is underscored by the vascular and reproductive implications evident in mouse knockouts for endothelial NO synthase and in rats treated with the NO inhibitor NG-nitro-l-arginine methyl ester (l-NAME) during pregnancy (50, 64). Treatment of animals with l-NAME has been used repeatedly as a model of preeclampsia (3, 4, 50, 60, 65). NO-inhibited and eNOS-deficient animals have increased blood pressure, proteinuria, decreased fetal and neonatal weights, and a reduced number of viable newborn pups (50, 60, 64, 65).
The decrease in reproductive performance in the l-NAME model is likely due to a number of factors, including decreased uteroplacental perfusion resulting from aberrant vascular remodeling and/or tone in the uterine circulation. Indeed, both small and large uterine artery outward remodeling are significantly reduced in this animal model, although the post-NO signaling mechanisms are not known (50, 63).
Matrix metalloproteinases (MMPs) are a family of zinc-containing enzymes that degrade the extracellular matrix (ECM; Ref. 55). MMPs are secreted as proenzymes, must be cleaved to become active, and can be regulated by tissue inhibitors of metalloproteinases (TIMPs) by forming complexes that regulate their activity (55). For example, activation of MMP-2 can be accomplished by an enzyme complex consisting of TIMP-2 and membrane bound MMP-1 (MT1-MMP or MMP-14; Refs. 62, 68). MMPs may also be regulated by NO, although the effect of NO on MMPs is variable (6, 16, 47, 53). Interestingly, shear stress, a potent activator of NO release, has been shown to both decrease and increase MMP-2 activity, further suggesting a role for NO in the regulation of MMP activity but also underscoring the complexity of the underlying signal transduction mechanisms (16, 21, 31, 53).
Many MMPs are greatly increased during late pregnancy in the rat uterine artery (32). Although studies (27) implicate MMP-2 as a key component involved in pregnancy, where vascular remodeling is essential for the survival and growth of the fetus, evaluations of the effect of NO on MMP-2 in uterine arteries are lacking.
MT1-MMP is a membrane bound MMP that has an established role in vascular remodeling, both the normal and hypertensive state (reviewed by Refs. 28, 29). These effects of MT1-MMP can be separate from its role in activating MMP-2 and may be regulated by NO (17, 28). Despite current research on MT1-MMP, the role of MT1-MMP in pregnancy-induced remodeling has not been evaluated.
Aberrant expression of MMPs has been associated with a number of vascular pathologies such as hypertension, preeclampsia, and atherosclerosis (55). There are conflicting reports evaluating circulating levels of MMPs in hypertensive patients. Some reports indicate that MMPs are elevated, while others show that they are decreased (9, 72). MMP-2 is elevated in the serum of preeclamptic women but decreased in umbilical cord arteries isolated from preeclamptic women (14, 15, 37, 45, 52). Dysregulation of MMPs may contribute to the inward eutropic remodeling evident in preeclamptic myometrial arteries (45, 49).
Considering the potential role of NO in the regulation of MMPs and in vascular pathologies, we hypothesized that NO synthase inhibition during pregnancy would affect MMP expression and thereby alter uterine artery structural remodeling. Here, we present data showing that NO synthase inhibition in the main uterine artery (mUA) decreases outward remodeling, increases arterial collagen and elastin content, and decreases MMP-2, TIMP-2, and MT1-MMP expression. Specifically, our data implicate MMPs in gestational uterine vascular remodeling and complement earlier observations that have documented the MMP-2/TIMP-2/MT1-MMP enzyme complex in both pathological vascular remodeling and compromised reproductive performance.
MATERIALS AND METHODS
Animals, treatments, and tissue collection.
Age-matched pregnant Sprague-Dawley rats (Charles River) were housed at the University of Vermont College of Medicine Animal Facility and studied between 13 and 17 wk of age. All procedures were approved by the University of Vermont's Animal Care and Use Committee. On day 10 of pregnancy, osmotic pumps (Alzet, Cupertino, CA) containing l-NAME were surgically implanted subcutaneously in the periscapular region. Then, 70 mg·kg−1·day−1 (5 μl/h) of l-NAME was infused into each animal until death on day 20 of pregnancy. Day 10 of pregnancy was chosen as this time period more closely replicates human preeclampsia, which occurs during the second half of gestation, and to extend previous work published from our laboratory (50) describing l-NAME-mediated abrogation of uterine artery remodeling during pregnancy. Likewise, the dosage of l-NAME chosen reflects both our previous work and other studies (57) that have generated 70–80% reduction in eNOS signaling while not inducing overt sickness in the animals. Residual volume in the pump was measured to ensure proper ejection of l-NAME. Control animals were surgically implanted with osmotic pumps containing saline. Blood pressures were obtained noninvasively using the oscillometric method on days 5 and 10 of treatment as previously described (50).
Animals were injected intraperitoneally with 50 mg/kg of Nembutal (Ovation Pharmaceuticals, Deerfield, IL) to attain a surgical plane of anesthesia. Animals were then euthanized using a small animal guillotine. The uterus was removed as previously described (51).
Both uterine horns were pinned out in a Petri dish filled with cold HEPES (10 mM HEPES, 141.8 mM NaCl, 4.7 mM KCl, 1.7 mM MgSO4, 0.5 mM EDTA, 2.8 mM CaCl2, 1.2 mM KH2PO4, and 5 mM glucose, pH 7.4), and the mUA was dissected. Two 0.5-mm lengths of mUA were dissected and fixed in 4% paraformaldehyde (Sigma, St. Louis, MO) for 8 h and placed in 75% ethanol before embedding in paraffin blocks for subsequent immunohistochemistry. The remainder of the mUA was frozen at −80°C for subsequent protein analysis or placed in TriZOL reagent (Invitrogen, Carlsbad, CA) and homogenized for RNA analysis. All animal protocols were reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee.
Immunohistochemistry and arterial measurements.
At least three serial sections (6 μm) from each vessel were cut and transferred to slides. Elastic Van Gieson and Masson's trichrome (which specifically highlights elastic fibers and stains collagen, respectively) staining was performed using standard techniques on the paraffin sections. Images were obtained using an Olympus BX50 light microscope at ×200 magnification coupled to a CCD camera and analyzed using the color threshold (for collagen and elastin analysis) and measurement capabilities (for lumen area and wall thickness) within MetaMorph (Molecular Devices, Downington, PA) image capture and analysis software.
RNA extraction, PCR array, and quantitative RT-PCR.
Tissue from mUA was homogenized in Trizol (Invitrogen) and Garnet Matrix A (MP Bio, Solon, OH) on a Biospec Bead Beater (Bartlesville, OK). This solution was then purified using an RNeasy Micro spin column (Qiagen, Valencia, CA) following manufacturer's instructions. Residual DNA was removed using Ambion Turbo Dnase (Ambion, Austin, TX). RNA concentrations were determined by a Nanodrop spectrometer (Nanodrop, Wilimington, DE). Before quantitative (q)PCR, RNA integrity was analyzed on an Agilent Bioanalyzer (Agilent, Santa Clara, CA).
RT-qPCR studies were performed in a two-step process. The iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used to synthesize cDNA. For each sample, the cDNA was used to amplify the target genes (eNOS, MMP-2, TIMP-2, and MT1-MMP) and two housekeeping genes (Hprt1 and Ywhaz). One microliter of cDNA was used per reaction with 150 nM of the forward and reverse primers and 12.5 μl of Power Sybr Green Master Mix (Applied Biosystems, Carlsbad, CA) in a 25-μl reaction. The reactions were performed on an ABI Prism 7000 (Applied Biosystems) using an initial denaturation of 10 min at 95°C, 40 cycles of 15 s at 95°C, and 60 s at 60°C, followed by a melt curve analysis to ensure only the correct product was amplified. Standard curves were used to determine the relative quantities of each sample. Relative target mRNA values were normalized by dividing the target quantity by the geometric mean of the quantities of the housekeeping genes. Each sample was run in triplicate and averaged.
Immunoblot.
Arteries were placed in modified RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, and 10% glycerol) supplemented with Halt protease inhibitor cocktail (Pierce, Rockford, IL) and homogenized with Garnet Matrix A (MP Bio) using three 10-s pulses on a Biospec Bead Beater and rested on ice for 10 s in between each pulse. Protein quantity was determined by Bradford assay (Bio-Rad), and 25 μg from each sample were separated using a 6% polyacrylamide gel. Proteins were transferred to nitrocellulose and analyzed by immunoblot as described previously (38), using mouse monoclonal TIMP-2 (1:100; Abcam, Cambridge, MA), rabbit monoclonal anti-MT1-MMP (MMP-14; 1:200; Epitomics, Burlingame, CA) or rabbit polyclonal anti-NOS3 (eNOS; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-β-tubulin (1:250; Cell Signaling, Danvers, MA), and rabbit polycolonal anti-GAPDH (1:1000, Cell Signaling). Immunoblots were analyzed by densitometry using ImageJ (National Institutes of Health, Bethesda, MD).
Immunofluorescence.
Vessels were formalin fixed and paraffin embedded. Three serial cross-sections (6 μm) from each vessel were cut, transferred to slides, and then stained using the following protocol. Sections were deparaffinized in xylene and rehydrated in graded alcohol solutions. Sections were placed in 1× target retrieval solution (Dako Cytomation, Carpinteria, CA) at 95°C for 20 min followed by incubation at room temperature for 30 min. Sections were blocked using 10% normal goat serum (Vector Laboratories, Burlingame, CA). Sections were incubated with primary antibody (MMP-2: 2 mg/ml and TIMP-2: 1:50; Abcam, Cambridge, MA) overnight, in a humidified box, at room temperature. The MMP-2 primary antibody recognizes both pro- and active forms of MMP-2. Sections were incubated with secondary antibody, diluted 1:500, goat anti-mouse Alexa 488 for TIMP-2, and goat anti-rabbit Alexa 633 for MMP-2 (Invitrogen, Molecular Probes) for 1 h at room temperature. Finally, sections were mounted and counterstained using Prolong Gold antifade with DAPI mounting media (Molecular Probes). Fluorescence was visualized for each slide using a Zeiss confocal microscope at ×25 and ×100. The image was then captured into an 8-bit digital image. Fluorescence integrated intensity for three arterial locations, arterial wall, endothelium, and adventitia, was assessed, representing the relative amount of MMP-2 and TIMP-2, and quantified using the threshold capabilities within the MetaMorph (Molecular Devices) software and normalized to area. Only ×25 images were used for quantification.
Statistical analysis.
Statistical differences between treatment groups were determined from individual vessel data. Statistical significance for all comparisons was assessed using Student's t-test within the Sigma Plot statistical package (Systat Software, San Jose, CA). Data are presented as means ± SE, with n values representing the number of animals. P < 0.05 was considered to be statistically significant.
RESULTS
Maternal blood pressure and fetal characteristics.
Maternal mean arterial pressure was significantly higher in the LPLN rats compared with LP controls at both time points of day 5 (LP: 101 ± 2 mmHg and LPLN: 132 ± 4 mmHg; P < 0.001; n = 8 for both groups) and day 10 of treatment (LP: 92 ± 3 mmHg and LPLN: 128 ± 4 mmHg; P < 0.001; n = 8 for both groups).
Litter size and resorption rate were not affected by l-NAME treatment (P = 0.24 and P = 0.44, respectively). However, both fetal and placental weights were significantly lower in the l-NAME-treated group (fetal weights: LP: 2.4 ± 0.06 g and LPLN: 2.2 ± 0.07 g; P = 0.01; n = 8 for both groups; placental weights: LP: 0.46 ± 0.008 g and LPLN: 0.43 ± 0.01 g; P = 0.02; n = 8 for both groups).
Maternal mUA characteristics.
Relative to LP controls, LPLN-treated mUA had significantly smaller lumen diameters, unchanged wall thickness, and an increased wall-to-lumen ratio (Table 1).
Table 1.
Main uterine artery characteristics
| LP | LPLN | P Value | |
|---|---|---|---|
| Wall thickness, μ (n = 7) | 39.1 ± 2.7 | 39.3 ± 3.7 | 0.98 |
| Lumen area, μm2 (n = 4) | 21,884 ± 4,277 | 6,963 ± 1,827 | 0.02* |
| Wall-to-lumen ratio (n = 4) | 0.21 ± 0.02 | 0.45 ± 0.07 | 0.02* |
Values are means ± SE. Comparisons are between late pregnant (LP) and LP NG-nitro-l-arginine methyl ester (LPLN)-treated rats.
Statistically significant difference between late LP and LPLN.
eNOS RNA and protein expression.
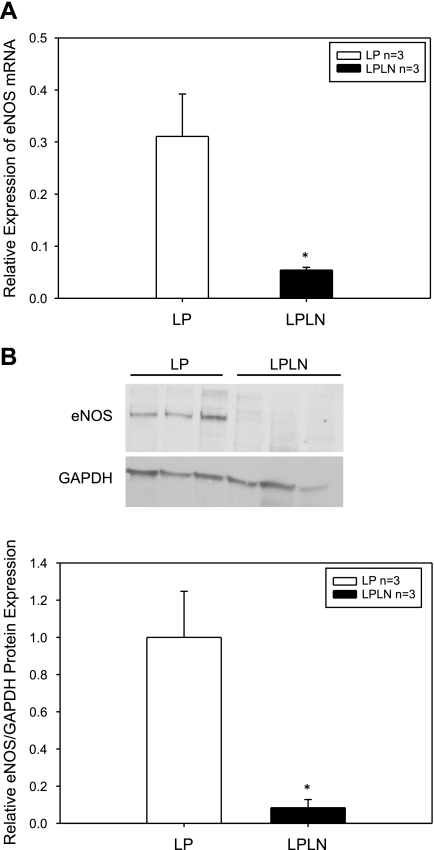
Expression of eNOS RNA in the mUA was assessed using RT-qPCR; l-NAME treatment significantly reduced eNOS RNA expression (Fig. 1A). eNOS and GADPH protein levels were assessed by immunoblot and quantified using densitometry (Fig. 1B, top and bottom, respectively). GADPH was used as a loading control and eNOS protein levels are expressed as a ratio to GADPH expression. ENOS levels were decreased by 90% in LPLN mUA compared with LP mUA (Fig. 1B, bottom).
Fig. 1.
Endothelial nitric oxide synthase (eNOS) RNA and protein expression is decreased by ∼90% in the late pregnant (LP) NG-nitro-l-arginine methyl ester (l-NAME)-treated (LPLN) main uterine artery (mUA). A: LP and LPLN mUA were dissected, placed immediately in Trizol reagent for subsequent RNA extraction, and quantitative (q)PCR analysis as described in materials and methods. Hprt1 and Ywhaz were used as control genes for the qPCR. eNOS RNA expression was significantly decreased in the LPLN mUA compared with LP. B: LP and LPLN mUA were dissected and placed in modified RIPA buffer for protein analysis as described in materials and methods. Twenty-five micrograms of protein were loaded on the gel and GAPDH was used as a loading control. Top: representative immunoblot. Bottom: densitometry was used to quantify the immunoblots probed for eNOS and normalized by GAPDH expression. eNOS is expressed relative to GADPH. eNOS is significantly decreased in LPLN-treated mUA. *P < 0.05 by Student's t-test; n = 3 animals per group.
Collagen and elastin content.
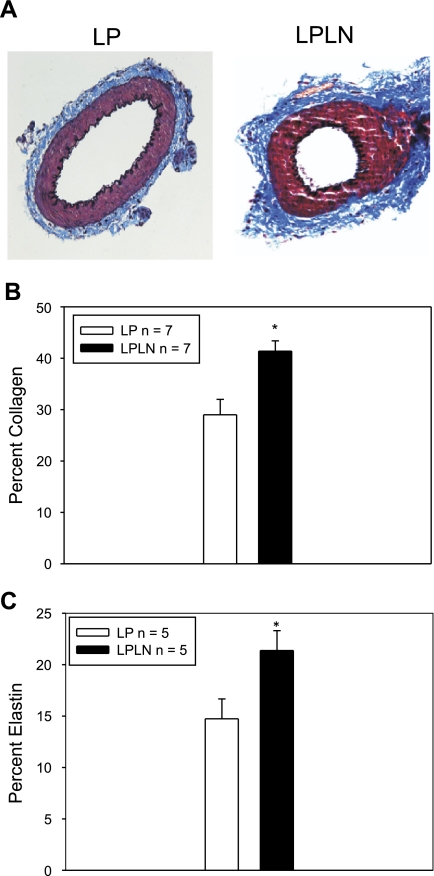
To evaluate the effect NO inhibition on ECM composition, we evaluated changes in collagen and elastin within the vessel cross-section as described above. l-NAME treatment significantly increased collagen (Fig. 2A, blue staining) content in the mUA artery cross-section compared with controls (Fig. 2, A and B). Elastin content in the mUA (Fig. 2, A and C, dark purple/black staining) was also significantly increased.
Fig. 2.
Lumen area is decreased and extracellular matrix components are increased in LPLN-treated mUA. A: LP and LPLN arteries were dissected, arterial lumens flushed of blood, fixed, and stained using elastic Van Gieson and Masson's trichrome stain as described in materials and methods. Collagen is stained blue, and elastin is stained dark purple. B: collagen content was assessed using the color threshold capability within MetaMorph set to distinguish between collagen and elastin staining. Collagen staining is significantly increased in LPLN mUA compared with LP (n = 7, both groups). C: elastin staining was determined as for collagen; however, the color threshold was set to only include dark purple stained structures within the section. Elastin staining is significantly increased in LPLN mUA (n = 5, both groups). *P < 0.05 by Student's t-test.
MMP-2, TIMP-2, and MT1-MMP RNA levels.
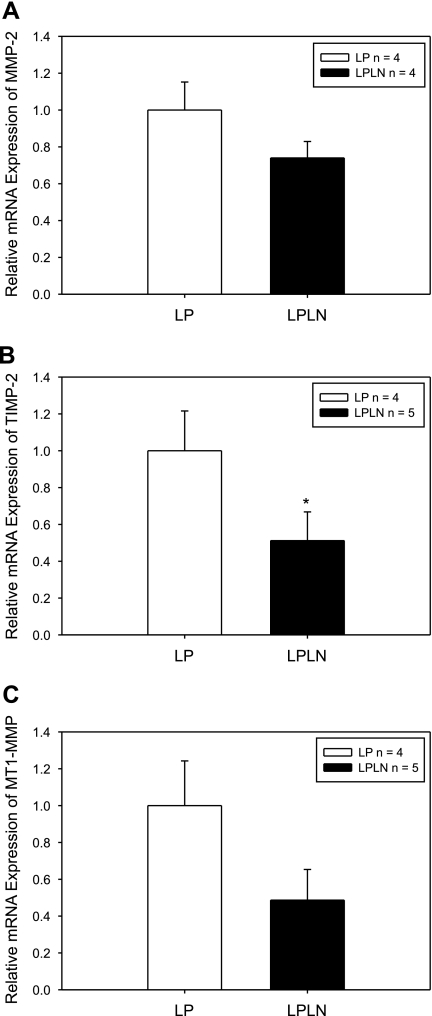
To determine the effect of NO inhibition on MMP levels, in particular the complex known to mediate MMP-2 activity, we first evaluated MMP-2, TIMP-2, and MT1-MMP RNA expression. Although mean values were reduced, l-NAME treatment did not produce a statistically significant effect on MMP-2 RNA expression (Fig. 3A; P = 0.17). However, TIMP-2 RNA expression was decreased by 50% in LPLN mUA (Fig. 3B; P = 0.05). While l-NAME treatment did not significantly decrease MT1-MMP RNA, RNA expression tended to be 50% lower in LPLN mUA (Fig. 3C; P = 0.06).
Fig. 3.
l-NAME treatment of LP rats decreases mRNA expression of tissue inhibitors of metalloproteinase-2 (TIMP-2) and membrane bound matrix metalloproteinase-1 (MT1-MMP) but not matrix metalloproteinase-2 (MMP-2) within the mUA. LP and LPLN mUA were dissected, placed immediately in Trizol reagent for subsequent RNA extraction and qPCR analysis as described in materials and methods. Hprt1 and Ywhaz were used as endogenous controls for the qPCR. A: although somewhat reduced, LPLN MMP-2 mRNA is not significantly different from LP (P = 0.17). B: l-NAME significantly decreased TIMP-2 mRNA expression in mUA, compared with LP (P = 0.05). C: MT1-MMP trended be lower in LPLN mUA, although this difference did not achieve statistical significance (P = 0.06). *P < 0.05 by Student's t-test.
MMP-2 protein expression.
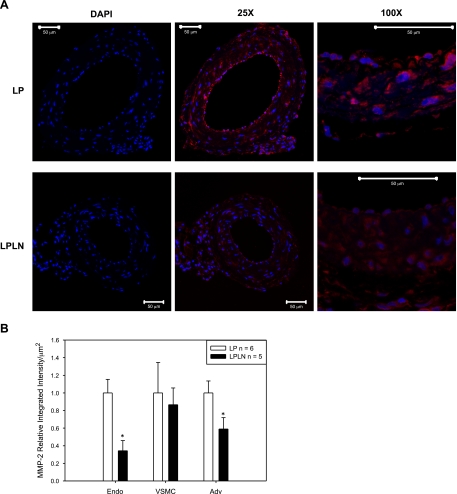
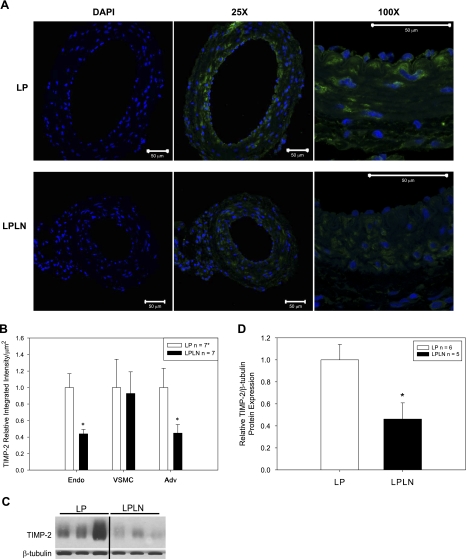
Because MMP-2 can be posttranscriptionally regulated (5, 22), we evaluated protein levels of MMP-2 using immunofluorescence (Fig. 4A). We also quantified MMP-2 levels separately in the three main areas of the mUA wall: endothelium, vascular smooth muscle cell (VSMC) and adventitia (Fig. 4B; n = 6 LP and n = 5 LPLN) and found that both endothelial and adventitial levels were significantly reduced while VSMC MMP-2 was unchanged.
Fig. 4.
Endothelial and adventitial MMP-2 protein expression is decreased in mUA in LPLN-treated rats. A: mUAs were dissected from LP and LPLN-treated animals, fixed, paraffin-embedded, cross-sectioned, and analyzed using immunofluorescence to detect both pro and active forms of MMP-2 (red). DAPI was used to stain nuclei (blue). Images were taken at ×25 and ×100 as described in materials and methods. Bar = 50 μm. DAPI images represent the secondary antibody control images where there was no incubation with primary antibody. B: MMP-2 protein immunofluorescence was quantified using the integrated intensity function on MetaMorph software and normalizing for area measured. Relative integrated intensity is expressed as a ratio to LP control per area (μm2). LPLN arteries had significantly decreased MMP-2 expression in the endothelial and adventitial layers. MMP-2 was unchanged by l-NAME in the vascular smooth muscle. *P < 0.05 by Student's t-test; n = 6 LP; n = 5 LPLN.
TIMP-2 protein expression.
Given the role of TIMPs in regulation of MMP-2 (11, 34, 66), we evaluated the expression level of TIMP-2 in mUA from LPLN vs. LP animals. Interestingly, TIMP-2 expression in LPLN arteries paralleled that of MMP-2, with reductions in endothelial and adventitial levels and no change in VSMC (Fig. 5, A and B). These findings were confirmed with immunoblotting, which showed a 60% decrease in TIMP-2 protein levels in mUA from LPLN vs. LP animals (Fig. 5, C and D).
Fig. 5.
Endothelial and adventitial TIMP-2 protein expression is decreased in late pregnant mUA isolated from lpln- rats. A: mUAs were dissected from LP and LPLN-treated animals, fixed, paraffin-embedded, cross-sectioned, and analyzed using immunofluorescence to detect TIMP-2 (green). DAPI was used to stain nuclei (blue). Images were taken at ×25 and ×100 as described in materials and methods. Bar = 50 μm. DAPI images represent the secondary antibody control images where there was no incubation with primary antibody. B: quantification of TIMP-2 protein immunofluorescence was measured using the integrated intensity function on MetaMorph software and normalizing for area measured. Relative integrated intensity is expressed as a ratio to LP control per area (μm2). TIMP-2 expression was significantly attenuated in the endothelial and adventitial layers of LPLN mUA. MUA TIMP-2 was unchanged by l-NAME in the vascular smooth muscle. *n = 7 LP; n = 7 LPLN, except n = 6 LP adventitia (Adv). C: LP and LPLN mUA were dissected and placed in modified RIPA buffer for protein analysis as described in materials and methods. Twenty-five micrograms of protein were loaded on the gel. Shown is a representative immunoblot. D: densitometry, as described in materials and methods, was used to quantify the immunoblots probed for TIMP-2 and β-tubulin. TIMP-2 is expressed as a ratio to β-tubulin. TIMP-2 is significantly decreased in late pregnant l-NAME-treated mUA. n = 6 LP; n = 5 LPLN. *P < 0.05 by Student's t-test.
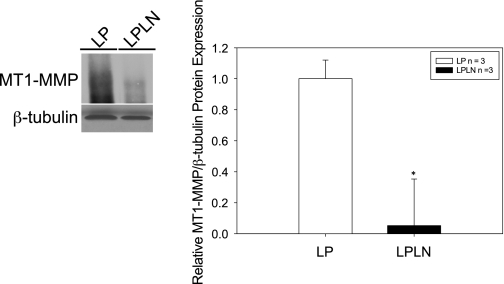
MT1-MMP protein expression.
MT1-MMP and TIMP-2 are known to form an activation complex with MMP-2 (13, 48, 62). As we found both MMP-2 and TIMP-2 protein levels to be decreased with l-NAME treatment, we also evaluated MT1-MMP levels. In l-NAME-treated animals, mUA MT1-MMP protein levels were reduced by ∼80% (Fig. 6, left, immunoblot, and right, quantification).
Fig. 6.
Treatment of rats with l-NAME decreases MT1-MMP protein expression in LP mUA. Left: LP and LPLN mUA were dissected and placed in modified RIPA buffer for protein analysis as described in materials and methods. Twenty-five micrograms of protein were loaded on a denaturing gel. Shown is a representative immunoblot. Right: densitometry, as described in materials and methods, was used to quantify the immunoblots probed for MT1-MMP and β-tubulin. MT1-MMP is significantly decreased in mUA of LPLN animals compared with LP. *P < 0.05 by Student's t-test.
DISCUSSION
To our knowledge, this study is the first to evaluate extracellular matrix components in uterine arteries from pregnant animals coupled with a potential mechanism via MMP inactivation. Likewise, the current study is the first to provide a feasible mechanism to substantiate the increased deposition of ECM components observed in myometrial arteries from hypertensive preeclamptic women (49). Further, the work presented here extends previous findings (50) showing the abrogation of uterine artery expansion during pregnancy by a reduction in NO signaling secondary to pharmacologic inhibition and provides a potential mechanism for uterine artery outward remodeling during pregnancy.
Previous research describing blood pressure responses in NO-inhibited animals show hypertension developing 2–3 days following the initiation of l-NAME administration, after which time blood pressures stabilize but then decrease in the last 3–4 days of pregnancy (3, 10, 23, 39, 50). We used a higher dose of l-NAME than in previous studies and administered l-NAME via a subcutaneous osmotic pump; mean arterial pressure (MAP) was significantly elevated on day 5 and 10 (days 15 and 20 of pregnancy, respectively) after initiation of l-NAME treatment. The consistent delivery system (5 μl/h) and increased l-NAME dosage could account for the MAP remaining high on day 10, whereas other studies have found a decrease in MAP at this time point.
Normal vascular remodeling during pregnancy is outward hypertrophic, as both lumen diameter and cross-sectional area are increased (51). Consistent with our finding of decreased mUA lumen area, l-NAME treatment has been associated with inward eutrophic remodeling characteristic of hypertension (8, 50) in both pregnant and nonpregnant animals. However, earlier studies from our laboratory (50) show that amelioration of hypertension with hydralazine cotreatment did not reverse the inward eutrophic remodeling due to l-NAME treatment. This finding points to NO inhibition, rather than hypertension, as the signaling mechanism responsible for the inward eutrophic remodeling of the mUA in l-NAME treated animals. Aberrant NO signaling has been suggested as a contributor to preeclampsia, and our data support this mechanism (18, 19, 42).
The inward eutrophic remodeling may be a symptom of decreased plasticity of the outer vessel wall due to changes in ECM composition and vessel wall content. Collagen and elastin are two ECM components that have received much attention, especially with regard to hypertensive changes in arteries. Collagen is a very stiff protein that does not contribute to the elasticity of the vessel at lower pressures but is a mediator of the passive pressure/diameter relationship of arteries at higher pressures (1). Arteries isolated from women with preeclampsia show increased collagen content in the umbilical cord artery (2). The l-NAME mUA evaluated in our study echo the literature's description of preclamptic maternal arteries as our vessels also had increased collagen and elastin content and increased wall-to-lumen ratios (2, 49).
There is inconsistency in the literature regarding the effect of l-NAME on eNOS levels. Some researchers (59) have found that eNOS levels are not changed by l-NAME treatment but that the phosphorylation of eNOS is decreased by l-NAME. Other studies (7) have shown that both eNOS protein levels and phosphorylation levels are decreased with l-NAME treatment. We found a remarkable reduction in eNOS RNA and protein (∼90%) with l-NAME treatment. In light of the previously published reports, it was surprising to find such a dramatic decrease in eNOS protein in response to l-NAME treatment. While we did not evaluate neuronal or inducible NO synthase, it is possible that inhibition of neuronal NO synthase or inducible NO synthase is contributing to the attenuation of outward remodeling induced by l-NAME.
Because lack of NO signaling rather than hypertension seems to be the primary mechanism for the lack of circumferential remodeling in our l-NAME model, and considering the increased deposition of ECM components, we hypothesized that decreased NO signaling would have a negative effect on MMP expression and thus contribute to the increased deposition of ECM. Therefore, we sought to expand on the role of MMPs in pregnancy and delineate the effect of NO inhibition on the expression of MMPs in the late pregnant mUA. MMPs are key proteases involved in remodeling and have been linked to NO signaling (17, 33, 54). Others (32, 45) have established that many MMPs and TIMPs, including MT1-MMP, MMP-2, and TIMP-2, are upregulated during rat pregnancy, especially during the day before parturition. We found a significant decrease in TIMP-2 RNA with l-NAME treatment. Differences in MMP-2 and MT1-MMP RNA secondary to l-NAME treatment were evident in terms of mean values (which differed by 30 and 50%, respectively); however, these did not reach statistical significance (P = 0.17 and 0.06, respectively). Unfortunately, our statistical power was limited due to small sample size in the RNA experiments, and we recognize this to be an unfortunate limitation of this study. Nevertheless, we found significant decreases in MMP-2, MT1-MMP, and TIMP-2 protein expression in vessels isolated from the NO synthase-inhibited animals. Based on the consistency in the pattern of change in RNA and protein levels in control vs. NO synthase-inhibited animals, we suspect that NO regulation of MMPs and TIMPs occurs primarily at the transcriptional level, with consequent changes in protein expression, although this issue cannot yet be conclusively resolved and translational or posttranslational regulation of MMPs and TIMPs by NO cannot be discounted.
Interestingly, NO inhibition only affected MMP-2 and TIMP-2 protein levels within the endothelium and adventitia. This is of particular importance since much of vascular NO signaling is initiated by the endothelium. Further, the decreased expression within the adventitia suggests a direct effect of MMPs on ECM turnover. Indeed, decreased MMP activity/expression has been associated with NO inhibition and increased collagen deposition (25, 26, 36, 59). The literature describing MMP expression in pregnancy-induced hypertension is conflicting; some studies (32, 67) suggest that MMPs are increased in preeclampsia while others found that ECM degradation is compromised in preeclampsia due to decreased MMP expression. These disparate results could be due to sampling location, since studies that evaluate serum or plasma levels of MMPs in preeclampsia generally report elevated levels of MMPs while those that evaluate MMP and TIMP expression within the umbilical cord artery or the placenta report decreased expression of MMPs and TIMPs (14, 15, 37, 45, 52).
Our findings that MMP-2, TIMP-2, and MT1-MMP are decreased in the mUA are consistent with studies evaluating MMP expression in the umbilical cord artery. Given the labile nature of enzymes, systemic regulation may not be reflective of local, vascular levels of MMPs. We suggest that MMP protein expression within the vascular wall is likely quite different than systemic levels of MMP.
Studies evaluating the effect of NO signaling on TIMP-2 are lacking. The few published reports are contradictory, showing both increased and decreased and no change in expression of TIMP-2 RNA and protein with NO inhibition (56, 70, 71, 73). The results of this study support the regulation of TIMP-2 by NO on two levels: both transcriptionally and posttranscriptionally.
Apart from its role in activating MMP-2, MT1-MMP has an established role in both physiologic and pathophysiologic vascular remodeling (12, 29). MT1-MMP null mice are infertile and have inadequate collagen turnover and decreased lifespan (24). Considering this, MT1-MMP may have an influence on pregnancy-induced vascular remodeling that is independent of MMP-2 and TIMP-2. Accordingly, we found a significant decrease in MT1-MMP with NO inhibition. In line with this finding, MT1-MMP has been implicated in outward remodeling of carotid arteries that is lost in mice that are heterozygous for MT1-MMP (12). MT1-MMP has also been shown to be regulated by NO in endothelial cells; in fact, NO increases MT1-MMP expression and, likewise, NO inhibition decreases MT1-MMP and inhibits endothelial migration and tube formation (17).
Mechanisms by which vascular NO signaling may be altered include changes in mechanical forces (e.g., strain/tensile deformation) secondary to increased intravascular pressure or by hormonal signaling (43, 69). Pregnancy-associated changes in lumen diameter secondary to uterine arterial remodeling and the biomechanical properties of the vascular wall (such as the stress-strain relationship) would each have distinct effects on wall stress and, most likely, circumferential pulsatile strain. This could directly induce MMP activation, as has been shown in isolated fibroblasts (58). Endocrine changes, particularly the elevations in relaxin and estrogen characteristic of mammalian pregnancy, may exert a synergistic effect, as both hormones have been implicated in MMP regulation and activation in the pregnant state (30, 35). Further, MMP activation is essential in blood-flow-mediated vascular enlargement, potentially through an estrogen-mediated mechanism where increased estrogen increases MT1-MMP and MMP-2 expression (20, 35, 63).
In conclusion, hypertension and/or NO inhibition leads to inward eutrophic remodeling of the uterine circulation that features decreased MMPs, in particular, MMP-2, TIMP-2, and MT1-MMP. In addition to facilitating vasodilation to normalize increased shear stress produced by increased uterine blood flow during pregnancy, our data suggest that NO is also involved in regulation of the deposition of extracellular matrix through the modulation of MMP-2, TIMP-2, and MT1-MMP protein expression. We propose that during normal pregnancy, enhanced NO signaling due to a confluence of increased estrogen levels, augmented shear stress and/or cyclic circumferential strain leads to increases in MMP-2, TIMP-2, and MT1-MMP. Conversely, in the absence of NO signaling, these MMPs and TIMP-2 decrease, contributing to increased collagen and elastin deposition and inward eutrophic remodeling that opposes the outward hypetrophic remodeling characteristic of pregnancy.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grants HL-073895 and HL-79253 (to G. Osol).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Albert van der Vliet for contributing comments and suggestions and the University of Vermont's Microscopy Imaging Facility.
REFERENCES
- 1. Arribas SM, Hinek A, Gonzalez MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111: 771–791, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bankowski E, Romanowicz L, Jaworski S. Collagen of umbilical cord arteries and its alterations in EPH-gestosis. J Perinat Med 21: 491–498, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of l-arginine, d-arginine and steroid hormones. Hum Reprod 10: 2723–2730, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Buhimschi IA, Saade GR, Chwalisz K, Garfield RE. The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Hum Reprod Update 4: 25–42, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen HH, Wang DL. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol Pharmacol 65: 1130–1140, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chen LL, Zhu TB, Yin H, Huang J, Wang LS, Cao KJ, Yang ZJ. Inhibition of MAPK signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through reduction of inflammation. Mol Biol Rep 37: 3067–3072, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Cipolla MJ, Bishop N, Vinke RS, Godfrey JA. PPARγ activation prevents hypertensive remodeling of cerebral arteries and improves vascular function in female rats. Stroke 41: 1266–1270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derosa G, D′Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Tinelli C, Cicero AF. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium 13: 227–231, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Edwards DL, Arora CP, Bui DT, Castro LC. Long-term nitric oxide blockade in the pregnant rat: effects on blood pressure and plasma levels of endothelin-1. Am J Obstet Gynecol 175: 484–488, 1996 [DOI] [PubMed] [Google Scholar]
- 11. English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem 281: 10337–10346, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med 202: 663–671, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fishman DA, Bafetti LM, Stack MS. Membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation in primary human ovarian epithelial carcinoma cells. Invasion Metastasis 16: 150–159, 1996 [PubMed] [Google Scholar]
- 14. Galewska Z, Bankowski E, Romanowicz L, Jaworski S. Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s content in the umbilical cord artery. Clinica Chimica Acta 335: 109–115, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Galewska Z, Romanowicz L, Jaworski S, Bankowski E. Matrix metalloproteinases, MMP-7 and MMP-26, in plasma and serum of control and preeclamptic umbilical cord blood. Eur J Obstet Gynecol Reprod Biol 150: 152–156, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Garanich JS, Pahakis M, Tarbell JM. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am J Physiol Heart Circ Physiol 288: H2244–H2252, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Genis L, Gonzalo P, Tutor AS, Galvez BG, Martinez-Ruiz A, Zaragoza C, Lamas S, Tryggvason K, Apte SS, Arroyo AG. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood 110: 2916–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. George E, Granger J. Mechanisms and potential therapies for preeclampsia. Current Hypertension Reports: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Grandas OH, Mountain DH, Kirkpatrick SS, Cassada DC, Stevens SL, Freeman MB, Goldman MH. Regulation of vascular smooth muscle cell expression and function of matrix metalloproteinases is mediated by estrogen and progesterone exposure. J Vasc Surg 49: 185–191, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol 293: H2429–H2437, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J 278: 28–45 [DOI] [PubMed] [Google Scholar]
- 23. Helmbrecht GD, Farhat MY, Lochbaum L, Brown HE, Yadgarova KT, Eglinton GS, Ramwell PW. l-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Am J Obstet Gynecol 175: 800–805, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 33: 569–574, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 36: 312–318, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272: 22389–22392, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Itoh Y. MT1-MMP: a key regulator of cell migration in tissue. IUBMB Life 58: 589–596, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv Exp Med Biol 612: 65–87, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol 295: H2087–H2097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly BA, Bond BC, Poston L. Gestational profile of matrix metalloproteinases in rat uterine artery. Mol Hum Reprod 9: 351–358, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Kuno Y, Iyoda M, Shibata T, Hirai Y, Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima Fatty rats. Br J Pharmacol 162: 1389–1400, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lafleur MA, Tester AM, Thompson EW. Selective involvement of TIMP-2 in the s activational cleavage of pro-MMP-2: refinement of the pro-MMP-2 activation mechanism. FEBS Lett 553: 457–463, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Lam KK, Cheng PY, Hsiao G, Chen SY, Shen HH, Yen MH, Lee YM. Estrogen deficiency-induced alterations of vascular MMP-2, MT1-MMP, and TIMP-2 in ovariectomized rats. Am J Hypertens 22: 27–34, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation 98: 535–540, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Leona CYP, Ekaterina N, Panagiotis A, Panagiotis L, Kypros HN. First-trimester maternal serum matrix metalloproteinase-9 (MMP-9) and adverse pregnancy outcome. Prenatal Diagnosis 29: 553–559, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Lounsbury KM, Beddow AL, Macara IG. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem 269: 11285–11290, 1994 [PubMed] [Google Scholar]
- 39. Lubarsky SL, Ahokas RA, Friedman SA, Sibai BM. The effect of chronic nitric oxide synthesis inhibition on blood pressure and angiotensin II responsiveness in the pregnant rat. Am J Obstet Gynecol 176: 1069–1076, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol Heart Circ Physiol 272: H1730–H1740, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NOx. Am J Physiol Heart Circ Physiol 280: H1692–H1698, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res 36: 239–247, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Meehan DT, Delimont D, Cheung L, Zallocchi M, Sansom SC, Holzclaw JD, Rao V, Cosgrove D. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int 76: 968–976, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller SL, Jenkin G, Walker DW. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol 180: 1138–1145, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno G, Franchi M, Guidi G. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal 23: 88–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased Nitric Oxide Synthase Activity and Expression in the Human Uterine Artery During Pregnancy. Circ Res 87: 406–411, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Novaro V, Colman-Lerner A, Ortega FV, Jawerbaum A, Paz D, Lo Nostro F, Pustovrh C, Gimeno MF, Gonzalez E. Regulation of metalloproteinases by nitric oxide in human trophoblast cells in culture. Reprod Fertil Dev 13: 411–420, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 272: 2446–2451, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Ong SS, Baker PN, Mayhew TM, Dunn WR. Remodeling of myometrial radial arteries in preeclampsia. Am J Obstet Gynecol 192: 572–579, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Osol G, Barron C, Gokina N, Mandala M. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J Vasc Res 46: 478–486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Palei ACT, Sandrim VC, Cavalli RC, Tanus-Santos JE. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin Biochem 41: 875–880, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Palumbo R, Gaetano C, Melillo G, Toschi E, Remuzzi A, Capogrossi MC. Shear stress downregulation of platelet-derived growth factor receptor-β and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation 102: 225–230, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Pustovrh MC, Jawerbaum A, White V, Capobianco E, Higa R, Martinez N, Lopez-Costa JJ, Gonzalez E. The role of nitric oxide on matrix metalloproteinase 2 (MMP2) and MMP9 in placenta and fetus from diabetic rats. Reproduction 134: 605–613, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Robinson EK, Seaworth CM, Suliburk JW, Adams SD, Kao LS, Mercer DW. Effect of nos inhibition on rat gastric matrix metalloproteinase production during endotoxemia. Shock 25: 507–514, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem 278: 9472–9480, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Shelton L, Rada JS. Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp Eye Res 84: 314–322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spanikova A, Simoncikova P, Ravingerova T, Pechanova O, Barancik M. The effect of chronic nitric oxide synthases inhibition on regulatory proteins in rat hearts. Mol Cell Biochem 312: 113–120, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Standley CA, Batia L, Yueh G. Magnesium sulfate effectively reduces blood pressure in an animal model of preeclampsia. J Matern Fetal Neonatal Med 19: 171–176, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Thaete LG, Kushner DM, Dewey ER, Neerhof MG. Endothelin and the regulation of uteroplacental perfusion in nitric oxide synthase inhibition-induced fetal growth restriction. Placenta 26: 242–250, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Toth M, Bernardo MM, Gervasi DC, Soloway PD, Wang Z, Bigg HF, Overall CM, DeClerck YA, Tschesche H, Cher ML, Brown S, Mobashery S, Fridman R. Tissue inhibitor of metalloproteinase (TIMP)-2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP-4 to enhance the (Membrane type 1)-MMP-dependent activation of pro-MMP-2. J Biol Chem 275: 41415–41423, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol 16: 1256–1262, 1996 [DOI] [PubMed] [Google Scholar]
- 64. van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 72: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Vanderlelie JJ, Perkins AV. Chronic nitric oxide synthase inhibition in pregnant rats does not result in placental oxidative stress. Hypertens Pregnancy 25: 103–114, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Wang H, Olszewski B, Rosebury W, Wang D, Robertson A, Keiser JA. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem Biophys Res Commun 315: 363–368, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 275: 26411–26415, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamaguchi S, Yamaguchi M, Yatsuyanagi E, Yun SS, Nakajima N, Madri JA, Sumpio BE. Cyclic strain stimulates early growth response gene product 1-mediated expression of membrane type 1 matrix metalloproteinase in endothelium. Lab Invest 82: 949–956, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Yang YL, Liu DD, Hsieh NK, Chen HI. Endothelin and gelatinases in renal changes following blockade of nitric oxide synthase in hypertensive rats. Chin J Physiol 51: 186–195, 2008 [PubMed] [Google Scholar]
- 71. Yu LB, Dong XS, Sun WZ, Zhao DL, Yang Y. Effect of a nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester on invasion of human colorectal cancer cell line SL-174T. World J Gastroenterol 11: 6385–6388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zervoudaki A, Economou E, Stefanadis C, Pitsavos C, Tsioufis K, Aggeli C, Vasiliadou K, Toutouza M, Toutouzas P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens 17: 119–124, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Zhang X, Wang HM, Lin HY, Liu GY, Li QL, Zhu C. Regulation of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) during mouse peri-implantation: role of nitric oxide. Placenta 25: 243–252, 2004 [DOI] [PubMed] [Google Scholar]