Abstract
Agonist-induced Ca2+ entry into the pulmonary endothelium depends on activation of both store-operated Ca2+ (SOC) entry and receptor-operated Ca2+ (ROC) entry. We previously reported that pulmonary endothelial cell SOC entry and ROC entry are reduced in chronic hypoxia (CH)-induced pulmonary hypertension. We hypothesized that diminished endothelial Ca2+ entry following CH is due to derangement of caveolin-1 (cav-1) containing cholesterol-enriched membrane domains important in agonist-induced Ca2+ entry. To test this hypothesis, we measured Ca2+ influx by fura-2 fluorescence following application of ATP (20 μM) in freshly isolated endothelial cells pretreated with the caveolar-disrupting agent methyl-β-cyclodextrin (mβCD; 10 mM). Cholesterol depletion with mβCD attenuated agonist-induced Ca2+ entry in control endothelial cells to the level of that from CH rats. Interestingly, endothelial membrane cholesterol was lower in cells isolated from CH rats compared with controls although the density of caveolae did not differ between groups. Cholesterol repletion with a cholesterol:mβCD mixture or the introduction of the cav-1 scaffolding peptide (AP-cav; 10 μM) rescued ATP-induced Ca2+ entry in endothelia from CH arteries. Agonist-induced Ca2+ entry assessed by Mn2+ quenching of fura-2 fluorescence was also significantly elevated by luminal AP-cav in pressurized intrapulmonary arteries from CH rats to levels of controls. Similarly, patch-clamp experiments revealed diminished inward current in response to ATP in cells from CH rats compared with controls that was restored by AP-cav. These data suggest that CH-induced pulmonary hypertension leads to reduced membrane cholesterol that limits the activity of ion channels necessary for agonist-activated Ca2+ entry.
Keywords: adenosine triphosphate, store-operated calcium entry, receptor-operated calcium entry
caveolins are integral constituents of plasmalemmal invaginations termed caveolae. These distinct lipid microdomains are thought to compartmentalize a variety of signaling molecules and facilitate signal transduction (12). Caveolin-1 (cav-1) is abundantly expressed in the vascular endothelium as a 22-kDa protein and is sufficient and necessary to promote caveolae formation (11). Cav-1 also binds cholesterol, and its overexpression has been shown to elevate cholesterol content in lipid microdomains (reviewed in Ref. 25). A critical functional motif of cav-1 is its scaffolding domain (CSD). This region is believed to act as a membrane-delimited anchor of various signaling and regulatory proteins like albumin-binding protein gp60, Src family tyrosine kinases, small GTPases, and endothelial nitric oxide synthase (eNOS; Refs. 1, 9, 26). In addition to serving as a scaffolding element, cav-1 can function either by inhibiting or augmenting ancillary protein activity in health and disease. For example, enhanced coupling of cav-1 with eNOS is associated with diminished production of nitric oxide (NO) in a chronic hypoxia (CH) model of pulmonary hypertension (17).
The role of cav-1 has also been investigated using genetic approaches that demonstrate abnormal morphological characteristics of precapillary pulmonary endothelium (13) and development of pulmonary hypertension in cav-1 null mice (27). Interestingly, endothelial cell-specific cav-1 reexpression rescues endothelium-dependent relaxation, prevents pulmonary artery remodeling, reduces collagen deposition in hearts, and normalizes right ventricular systolic pressures of cav-1 knockout mice (15). Recent data also show that cav-1-deficient mice exhibit a reduction in surface localization of canonical transient receptor potential channels (TRPC4) and agonist-induced Ca2+ entry in pulmonary endothelial cells (16). Similar to the restoration of the normotensive phenotype by transgenic reintroduction of cav-1, this approach also rescues membrane expression of TRPC4 and normalizes agonist-induced Ca2+ entry. These findings illustrate the role of cav-1 to regulate Ca2+ entry by trafficking TRP channels to the plasma membrane and are consistent with numerous reports of store-operated Ca2+ (SOC) entry dependence on cav-1 and/or membrane cholesterol (23, 28).
In addition to cav-1 knockouts exhibiting pulmonary defects, monocrotaline (MCT)-treated rats also demonstrate cav-1-related endothelial dysfunction (7). Specifically, MCT-induced pulmonary hypertension is associated with progressive loss of endothelial cav-1 membrane surface expression that contributes to hyperactivation of promitogenic signaling pathways in the vascular wall leading to pulmonary arterial hypertension. These deleterious responses to MCT are prevented by systemic administration of a cell-permeable CSD peptide (8), suggesting a critical role for impaired cav-1 function in this model of pulmonary hypertension.
Similar to cav-1 knockouts and administration of MCT, CH-induced pulmonary hypertension is also associated with endothelial dysfunction. Aberrations in pulmonary artery endothelial morphology are evident in neonatal rats exposed to hypobaric hypoxia (10) and cultured pulmonary arteries exposed to hypoxia (18). In addition to ultrastructural abnormalities following CH, Golgi trapping of eNOS (14) and enhanced association of eNOS with cav-1 have been reported (17). Organellar sequestration of cav-1 and tight coupling to eNOS following CH may not only limit NO synthesis but are thought to restrict delivery of the enzyme to caveolar microdomains.
Although cav-1 function may be deranged in the CH model of pulmonary hypertension, the effects of CH on cav-1 regulation of Ca2+ -conducting ion channels have not been investigated. Interestingly, endothelial Ca2+ entry appears to be reduced following CH. For example, the endothelium in CH-hypertensive pulmonary arteries exhibits a blunted sustained rise in endothelial cell intracellular calcium concentration ([Ca2+]i) in response to carbachol (17). In addition, reduced whole cell store-operated Ca2+ current (ISOC) and cyclopiazonic acid (CPA)-induced Ca2+ influx are seen in endothelial cells freshly isolated from pulmonary arteries of CH exposed rats (21). Given the recently described role for cav-1 in this process, we hypothesized that diminished agonist-induced endothelial cell Ca2+ entry in pulmonary arteries following CH is due to impaired cav-1-mediated regulation of Ca2+ channels. We tested this hypothesis by examining the effect of manipulation of membrane cholesterol and introduction of the CSD on agonist-induced Ca2+ influx in freshly isolated cells and pressurized pulmonary arteries from control and CH rats.
METHODS
All protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Exposure of rats to CH.
Male Sprague-Dawley rats (Harlan Industries; 200–250 g) were used for all studies. Rats exposed to CH were placed in a hypobaric chamber with barometric pressure maintained at ∼380 Torr for 4 wk. Age-matched control rats were housed in similar cages under ambient barometric pressure (∼630 Torr). The hypobaric chamber was opened three times per week to provide fresh rat chow, water, and clean bedding. All animals were maintained on a 12:12-h light-dark cycle.
Isolation and preparation of pulmonary artery endothelial cells.
Following CH or normoxic exposure, rats were euthanized with pentobarbital sodium (200 mg/kg ip) and the heart and lungs were exposed by midline thoracotomy. The left lung was rapidly excised and placed in HBSS. The HBSS contained the following (in mM): 150 NaCl, 6 KCl, 1 MgCl2, 1.8 CaCl2, 10 HEPES, and 10 glucose, titrated to pH 7.4 with NaOH. Intrapulmonary arteries (3rd and 4th order, 200–400 μm inner diameter) were dissected from the cranial most region of the left lung and carefully cleaned of surrounding lung parenchyma. Endothelial sheets were enzymatically dissociated and identified as previously described (21) and then released by gentle trituration with a small bore fire-polished Pasteur pipette and stored at 4°C. One to two drops of solution containing freshly isolated rat pulmonary artery endothelial cells were placed on poly-l-lysine-coated glass bottom coverslips (BD Biosciences) and incubated 30 min at 37°C in the presence or absence of the cholesterol binding compound methyl-β-cyclodextrin (mβCD; 10 mM) to deplete membrane cholesterol. In separate experiments, cholesterol repletion was performed in previously untreated endothelial sheets taken from animals from both groups by incubation with a 1:5 ratio of cholesterol:mβCD for 30 min at 37°C. In addition to protocols designed to deplete or replete cholesterol, endothelial sheets were incubated for 30 min at 37°C with 10 μM of the cell permeant cav-1 scaffolding domain peptide (AP-cav; 21st Century Biochemicals) or 10 μM of inactive scrambled peptide (AP-scrambled peptide).
Cannulation of intrapulmonary arteries and introduction of the cav-1 scaffolding domain.
Intrapulmonary arteries (3rd and 4th order, 200–400 μm inner diameter) were dissected from the left lung in HBSS as described above before transferring to an arteriograph (CH-1; Living Systems). Next, the proximal end of the artery was cannulated and secured with a single strand of silk suture. The vessel lumen was gently flushed to remove any blood with physiological saline solution (PSS) containing the following (in mM): 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose. The distal end of the vessel was then cannulated, and the artery was pressurized with a servo-controlled peristaltic pump (Living Systems) to 12 mmHg. This preparation was subsequently superfused with PSS, transferred to an inverted microscope (Nikon, TS-100F), and equilibrated with a normoxic gas mixture (10% O2, 6% CO2, balance N2) at 37°C. AP-cav or AP-scrambled peptide (10 μM) was introduced to the endothelium by luminal perfusion, allowed to incubate for 30 min at 37°C, and then washed before fura-2 loading.
Endothelial fura-2 loading.
Agonist-induced Ca2+ influx was determined in freshly isolated endothelial cells and in endothelium of intact pressurized pulmonary arteries with the ratiometric Ca2+-sensitive fluorescent indicator fura-2 AM (Invitrogen). Following cholesterol depletion, repletion, or introduction of either AP-cav or AP-scrambled peptides, freshly isolated pulmonary artery endothelial cells were loaded with fura-2 AM (3 μM and 0.05% pluronic acid) in HBSS for 5 min at ∼23°C and washed for 15 min at 37°C. Ratiometric changes in endothelial cell [Ca2+]i were determined by alternating a xenon arc lamp light source between 340- and 380-nm bandpass filters at 1 Hz (Ionoptix Hyperswitch), and the interleaved fura-2 fluorescent emissions at 510 nm were detected with a photomultiplier tube. In separate experiments, the endothelium was selectively loaded with fura-2 in pressurized pulmonary arteries. We previously reported this technique (21), which involves luminal perfusion of a 3 μM fura-2 in PSS via a pressure servo-controlled peristaltic pump (Living Systems). The Ca2+-sensitive dye was loaded at ∼23°C for 1 min at a pressure of 12 mmHg and subsequently perfused to remove the indicator before being washed for 15 min at 37°C. This allowed effective cytosolic partitioning of fura-2 that yielded more accurate Mn2+ quenching of fura-2 fluorescence described below. Following some experiments, the endothelium was disrupted with a moose mane fiber to assess the degree of fluorescence loss indicating selective endothelial loading. This maneuver typically resulted in 90–95% loss in dye fluorescence relative to vessel autofluorescence.
Agonist-induced Ca2+ influx in freshly isolated endothelial cells.
ATP-induced Ca2+ entry has been shown to enhance activity of several vasodilator pathways in the arterial segment of the pulmonary circulation (24). Therefore, we utilized this purinergic ligand to mobilize Ca2+ stores and activate SOC and receptor-operated Ca2+ (ROC) entry in the continued presence of ATP. After cholesterol or scaffolding domain peptide treatments, fura-2-loaded endothelial sheets were superfused with Ca2+-free HBSS containing 0.1 mM EGTA to chelate residual Ca2+. Next, endothelial cells were stimulated with 20 μM ATP to deplete intracellular Ca2+ stores. Calcium influx was then determined upon repletion of extracellular Ca2+ (1.8 mM) in the continued presence of ATP. The magnitude of F340/F380 was determined by quantifying the area under curve (AUC) beginning at the initial Ca2+ response upon Ca2+ repletion for a total of 4 min. ATP-induced Ca2+ mobilization transients were also quantified by AUC determination. This mode of analysis was chosen to quantify the entire Ca2+ entry response rather than a single instantaneous value for fura-2 fluorescence.
Ca2+ entry assessed by Mn2+ quenching of fura-2 fluorescence in pressurized arteries.
Endothelial cell Ca2+ influx was also determined by Mn2+ quenching of fura-2 fluorescence in intact pressurized arteries as recently described by our laboratory (21). Following introduction of AP-cav or AP-scrambled peptide and fura-2 loading, the vessel lumen was perfused with 20 μM ATP under Ca2+-free conditions. Vessels were pressurized at the estimated in vivo pulmonary artery pressures of 12 and 35 mmHg for control and CH rats, respectively. Parallel experiments were performed in the absence of ATP to assess basal Ca2+ influx. Each preparation was excited at the isosbestic wavelength (360 nm), and emission wavelength was recorded at 510 nm. Basal- and ATP-induced Ca2+ entry was quantified as the linear quench rate (percent/min) and percent change (ΔF/Fo) at the 360-nm wavelength following the addition of 500 μM MnCl2 in Ca2+-free PSS (without EGTA).
Whole cell Ca2+ currents in freshly isolated endothelial cells.
Freshly dispersed endothelial cells obtained from intrapulmonary arteries were superfused (2 ml/min) at room temperature (∼23°C) in an extracellular solution containing the following (in mM): 145 NaCl, 10 HEPES, 10 glucose, 1.5 CaCl2, and 0.5 MgCl2, titrated to pH 7.4 with NaOH. Agonist-induced transmembrane currents were measured in conventional whole cell patch-clamp configuration with an intracellular solution composed of the following (in mM): 140 CsCl, 10 HEPES, 5 EGTA, 2.3 CaCl2, 1 and MgCl2, titrated to pH 7.3 with CsOH. The osmolarity of all solutions was adjusted to 290–300 mosmol/l with sucrose. To assess the role of the scaffolding domain of cav-1 on agonist-induced Ca2+ entry, transmembrane cation currents were measured in the presence of AP-cav (10 μM) or AP-scrambled peptide (10 μM) in the pipette solution. Whole cell configuration was achieved following tight seal formation (>1 GΩ) on electrically isolated endothelial cells. Series resistances <25 MΩ and stable whole cell current values of less than −12 pA at a holding potential of −60 mV were deemed acceptable biophysical criteria for continuation of the experimental protocol. The contents of the pipette solution were allowed to dialyze for a minimum of 5 min before initiation of a voltage step protocol in which transmembrane currents were recorded in response to steps from −100 to +100 mV in increments of 20 mV with a pulse duration of 500 ms. Endothelial cells were subsequently stimulated with ATP (20 μM) for 5 min followed by another series of the voltage steps. The Ca2+ channel blocker La3+ (100 μM) was applied to the bath solution in the presence of ATP for 5 min before voltage steps were reapplied. ATP-induced currents were quantified by difference subtraction (off-line) and current-voltage (I/V) relationships were expressed as current density (pA/pF) for each experimental group.
Membrane cholesterol content.
Endothelial cell sheets were prepared as described above from two to three animals per group. In separate experiments, cholesterol depletion and repletion were performed by incubating small pulmonary arteries with mβCD (10 mM) or mβCD:cholesterol, respectively, for 1 h at 37°C before enzymatic dissociation in fresh HBSS. Endothelial cell sheets were plated on to poly-l-lysine coated glass slides and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. Following three washes with PBS, endothelial cell sheets were stained with filipin (50 μg/ml) for 90 min at room temperature under light-protected conditions. After two washes, coverslips were mounted on the slides using mounting media (Prolong Antifade; Invitrogen) and slides were stored at −20°C until analysis. The samples were viewed and photographed by fluorescence confocal microscopy (Zeiss LSM 510 AxioObserver; Göttingen, Germany) using 405-nm laser (excitation), a 420-nm long pass filter (emission), and a Plan-Neofluar ×40/1.3 oil objective. Filipin staining was quantified using NIH Image J. A total of 3–12 sheets per group were analyzed. Fluorescence intensity was quantified using between 60 and 200 regions of interest from up to 10 cells per sheet. Fluorescence from each region of interest was averaged to determine the mean fluorescence for that sheet.
Immunoflourescence.
Freshly isolated pulmonary arterial endothelial sheets were fixed for 10 min at room temperature with 4% paraformaldehyde and permeabilized with 0.01% Triton X-100 in PBS solution for 10 min. Cells were blocked with 3% donkey serum in PBS solution for 1 h. Following blocking and fixation, the cells were incubated overnight at 4°C with rabbit polyclonal cav-1 antibody (1:100; Santa Cruz Biotechnology). Dylight 649 donkey anti-rabbit secondary antibody (Jackson) was used to detect the primary antibodies. Nuclei were labeled with Sytox (1:10,000). Samples were visualized with confocal laser scanning microscope. (LSM 510; AxioObserver) with a ×63 oil immersion lens. Nonspecific binding of the secondary antibody was determined by omitting primary antibody.
Electron microscopy.
The heart and lungs were exposed by midline thoracotomy in pentobarbital sodium (200 mg/kg ip) anesthetized rats. Heparin (100 U) was injected into the right ventricle, and the pulmonary artery was cannulated with a 13-gauge needle stub. The lungs were perfused at a pressure of 15 cmH2O with 100 ml of PSS containing the following (in mM): 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose, with 4% (wt/vol) albumin, 10−4 M papaverine, and 1,000 U of heparin. The lungs were then perfused at the same pressure with 100 ml of fixative (PBS containing 4% paraformaldehyde, 0.1% glutaraldehyde, and 10−4 M papaverine). Samples of lung tissue were then fixed for transmission electron microscopy with 3% formaldehyde, 2% glutaraldehyde in 0.1 M sodium cacodylate buffer, post-fixed in reduced osmium tetroxide (1% OsO4 and 0.5% potassium ferrocyanide), en bloc, stained with 1% uranyl acetate, dehydrated, and embedded in epoxy resin.
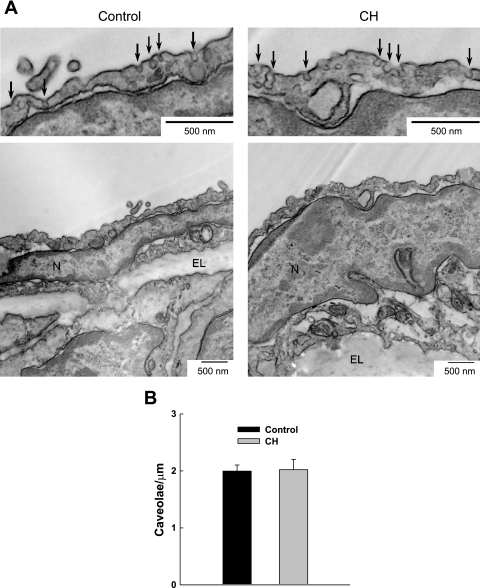
Caveolae between 60 and 100 nm in diameter were counted at the membrane of endothelial cells in arterial sections and divided by the membrane length using NIH Image J software. The diameter criterion was selected to limit analysis to caveolae; however, due to variation in the plane of cross-section, quantitative analysis of differences in caveolar dimension between groups was impossible. Vessels from three rats per group were analyzed. A total of 70 images encompassing 21 cells and 667 μm of membrane were analyzed from arteries of control rats, whereas 84 images encompassing 25 cells and 758 μm of membrane were analyzed in the CH group.
Calculations and statistics.
All data are expressed as means ± SE. Values of n refer to the number of endothelial sheets (40–100 cells/sheet) for experiments utilizing freshly dispersed cells, the number of animals in each group for intact vessel experiments, and the number of single cells examined for electrophysiological recordings. Percentage data were converted to normal distributions by arcsine transforms before parametric analysis. A one-way or two-way ANOVA was used where appropriate for all comparisons between control and CH groups. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. A probability of ≤0.05 was accepted as statistically significant for all comparisons.
RESULTS
Characterization of ATP-induced Ca2+ influx.
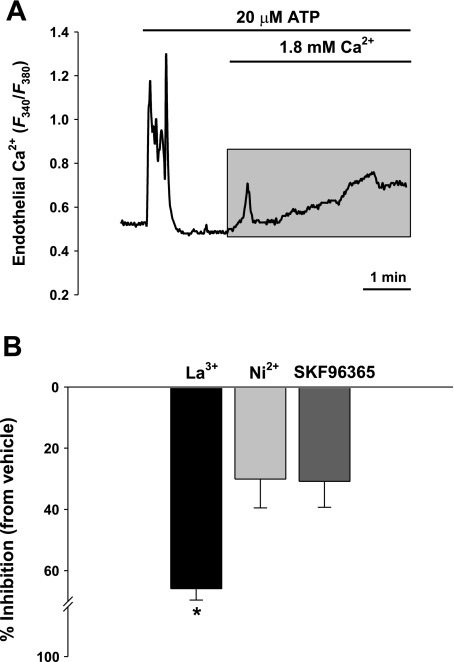
Ca2+ entry was assessed as the AUC (shaded area in Fig. 1A) following reintroduction of extracellular Ca2+ in the continued presence of ATP in endothelial cells from normoxic control rats. This response was inhibited by known antagonists of SOC and ROC channels (Fig. 1B). Lanthanum (100 μM) was a more potent blocker of agonist-induced Ca2+ entry (∼65%), whereas Ni2+ (100 μM) or SKF96365 (20 μM) only inhibited ∼30% of the response (Fig. 1B).
Fig. 1.
Effects of pharmacological antagonists of Ca2+ influx in freshly isolated pulmonary endothelial cells. A: depletion of intracellular Ca2+ stores was performed with 20 μM ATP in a nominally Ca2+-free HBSS from control rats. Shaded area depicts ATP-induced Ca2+ entry by integrating area under the curve (AUC) for statistical analysis. B: inhibitory effects of store- and receptor-operated Ca2+ channel antagonists on ATP-induced Ca2+ influx. Summary data showing percent inhibition from vehicle control in the presence of 100 μM La3+ (n = 4), 100 μM Ni2+ (n = 4), and 20 μM SKF96365 (n = 3). Values are means ± SE. *P ≤ 0.05 vs. vehicle and control.
ATP-induced Ca2+ influx is diminished following CH.
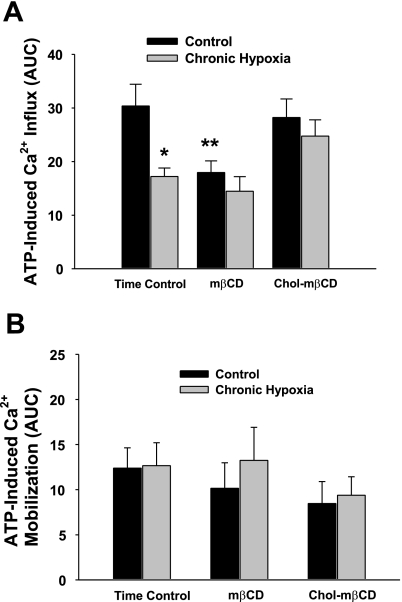
ATP-induced Ca2+ entry was diminished in isolated endothelial cells from CH rats compared with controls (Fig. 2A) consistent with our previous work (21) using CPA to passively deplete intracellular stores in similar Ca2+ add-back protocols. We also assessed the magnitude of the initial store release following ATP administration to eliminate the possibility that subsequent attenuated Ca2+ influx in the CH group was due to altered Ca2+ release or Ca2+ loading of the endoplasmic reticulum. We found that these initial Ca2+ transients (defined by AUC) due to store release did not differ between control groups (Fig. 2B).
Fig. 2.
A: ATP-induced Ca2+ entry was less in cells from chronically hypoxic (CH) rats compared with controls (time control columns). Cholesterol depletion attenuates agonist-induced Ca2+ entry in freshly isolated endothelial cells from control rats but not from CH rats. Summary data for ATP-induced Ca2+ influx in endothelial cells under cholesterol deplete (mβCD) and cholesterol replete (Chol-mβCD) conditions. Values are means ± SE; n = 4–5/group. *P ≤ 0.05 vs. vehicle and control; **P ≤ 0.05 vs. time control. B: ATP-induced Ca2+ release from stores upon stimulation. There were no differences between groups.
Cholesterol depletion attenuates ATP-induced Ca2+ influx in control cells.
Cholesterol is an important constituent of the plasma membrane that regulates bilayer fluidity and binds to the integral membrane protein cav-1. Cav-1 is thought to function as a trafficking chaperone for Ca2+ channels (2). We found that cholesterol depletion of control endothelial cells with mβCD inhibited ATP-induced Ca2+ entry compared with untreated controls and that this response was reversed by cholesterol repletion (Chol-mβCD; Fig. 2). We did not detect a further reduction in Ca2+ influx in cholesterol-depleted endothelial cells from CH rats; however, we did observe that cholesterol repletion in previously untreated sheets normalized the ATP-induced Ca2+ response to the level of controls (Fig. 2). These data suggest that endothelial membrane cholesterol is necessary for agonist-induced Ca2+ entry and that impaired cav-1 function may limit endothelial Ca2+ entry following CH-induced pulmonary hypertension.
Cav-1 scaffolding peptide rescues ATP-induced Ca2+ influx following CH.
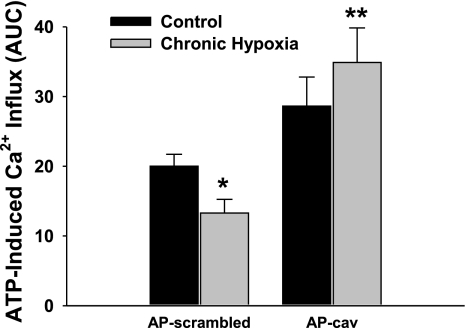
To further examine the relationship between cav-1 function and Ca2+ influx, we assessed Ca2+ entry via two different approaches, ATP-induced Ca2+ entry in isolated endothelial cells and Mn2+-quenching of fura-2 in pressurized arteries from control and CH rats. In each of these preparations, the endothelium was preincubated with the cav-1 scaffolding domain peptide (AP-cav) or control peptide (AP-scrambled peptide). Similar to our prior findings, ATP-induced Ca2+ entry was lower in endothelial cells from CH arteries treated with the scrambled peptide compared with identically treated controls (Fig. 3). Endothelial cells isolated from control rats incubated with AP-cav had a slight, but nonsignificant, tendency for augmented Ca2+ entry compared with control cells pretreated with control peptide (Fig. 3). The control peptide was without effect compared with vehicle alone in control cells (95.4 ± 5.0% of vehicle value; n = 5 in each group) demonstrating that AP-linked peptides do not affect Ca2+ entry through a nonspecific effect. Preincubation with AP-cav significantly enhanced agonist-induced Ca2+ entry in endothelial cells isolated from CH-hypertensive arteries compared with the respective scrambled peptide group and normalized the response to that of control cells treated with AP-cav (Fig. 3).
Fig. 3.
Caveolin-1 (cav-1) scaffolding domain peptide rescues agonist-induced Ca2+ entry following CH. Summary data illustrate the effect of AP-scrambled peptide (10 μM) or AP-cav (10 μM) on ATP-induced Ca2+ influx in freshly isolated endothelial cells from control and CH rats. Values are means ± SE; n = 4/group. *P ≤ 0.05 vs. control scrambled; **P ≤ 0.05 vs. CH scrambled.
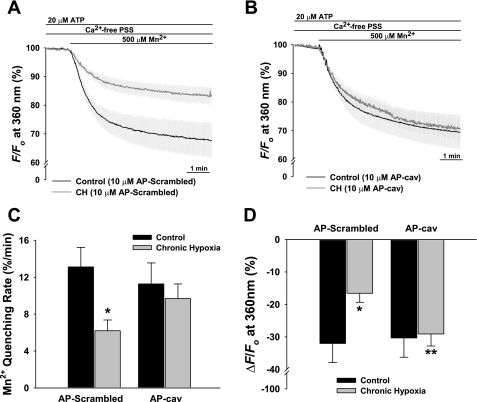
We also assessed the effects of AP-scrambled peptide and AP-cav on basal and ATP-induced Ca2+ entry in pressurized intrapulmonary arteries by Mn2+-quenching of fura-2 fluorescence. These vessels do not demonstrate basal tone. Luminal application of ATP appeared to selectively stimulate the endothelium as evidenced by an endothelial Ca2+ transient measured at the 340/380 wavelengths accompanied by no change in vessel diameter (data not shown). Figure 4A illustrates reduced Mn2+-quenching of fura-2 fluorescence in CH vessels pretreated with the scrambled peptide compared with controls. Traces are depicted as means of F360 before and after addition of Mn2+ in the continued presence of ATP. Introduction of Mn2+ resulted in a steady decay of fura-2 fluorescence indicative of cation entry. Fura-2 quench rate in the presence of ATP was significantly less in CH vessels treated with the scrambled peptide compared with similarly treated control vessels (Fig. 4C). Similar to the results in freshly isolated cells, AP-cav restored agonist-induced endothelial entry of the Ca2+ surrogate and resultant quenching of fura-2 fluorescence in pressurized arteries from CH rats to the level of controls (Fig. 4B). When the degree of Mn2+-quenching of fura-2 fluorescence was examined in CH vessels preincubated with AP-cav, this response was normalized to the level of controls (Fig. 4D). We also examined basal (unstimulated) endothelial Mn2+ influx and failed to detect a significant difference in the initial rate of quenching between groups, although there was a strong tendency for less quenching in the CH group (P = 0.078).
Fig. 4.
Introduction of AP-cav restores Mn2+-quenching of fura-2 fluorescence in the continued presence of luminal ATP (20 μM) in isolated small pulmonary arteries from CH rats. Percent change in fura-2 fluorescence at 360-nm excitation where trace data are expressed as means in the presence of AP-scrambled peptide (A) and AP-cav (B). C: fura-2 quench rate (initial decline) expressed as percent change during the first 60 s after addition of 500 μM Mn2+. D: quench values (during the final min) represented as percent change relative to baseline at 360 nm. Experiments were performed at the estimated in vivo pressures for control (12 mmHg) and CH (35 mmHg) arteries. Values are means ± SE; n =4–6 rats/group. *P ≤ 0.05 vs. control and **P ≤ 0.05 vs. CH AP-scrambled.
Cav-1 scaffolding peptide enhances ATP-induced cationic currents following CH.
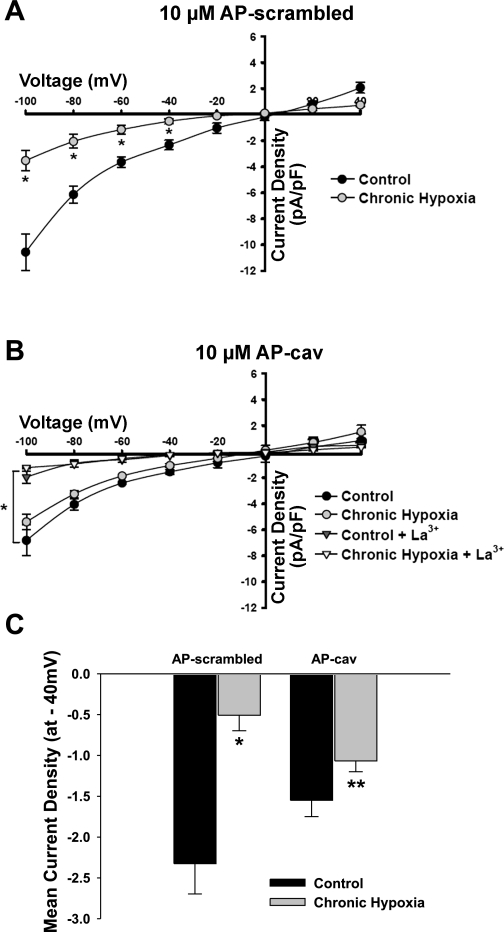
Electrophysiological recordings of ATP-induced cation influx were performed in which the control (scrambled peptide) or the scaffolding domain of cav-1 (AP-cav) was present in the pipette solution. Figure 5A illustrates reduction of an inwardly rectifying current with a reversal potential near 0 mV in endothelial cells from CH rats compared with controls. The introduction of the cav-1 scaffolding peptide enhanced this current in the CH group to the level of controls (Fig. 5, B and C) and was sensitive to trivalent blockade with 100 μM La3+. Figure 5C summarizes mean inward currents evoked at −40 mV, which corresponds to endothelial membrane potentials in intact pulmonary arteries (21). Although there was a tendency for AP-cav to blunt inward current in controls, no significant difference was detected compared with control endothelial cells dialyzed with the AP-scrambled peptide.
Fig. 5.
Whole cell ATP-induced inward currents are reduced following CH and are improved in the presence of the cav-1 scaffolding element. ATP-induced (20 μM) currents were difference subtracted from an initial voltage pulse during which extracellular ATP was absent. Current-voltage (I/V) relationships were generated from voltage pulses applied to freshly isolated endothelial cells from control and CH arteries where either the AP-scrambled peptide or AP-cav was introduced via the patch pipette (A). I/V relationships depict the effect of AP-cav on ATP-stimulated endothelial cells from control and CH arteries in the presence or absence of 100 μM La3+ (B). Summary data indicating inward current density at −40 mV for control and CH endothelial cells in the presence of control peptide or cav-1 scaffolding element (C). Values are means ± SE; n = 6 cells/group. *P ≤ 0.05 vs. control AP-scrambled and **P ≤ 0.05 vs. CH AP-scrambled.
Membrane cholesterol content is decreased following mβCD treatment and CH.
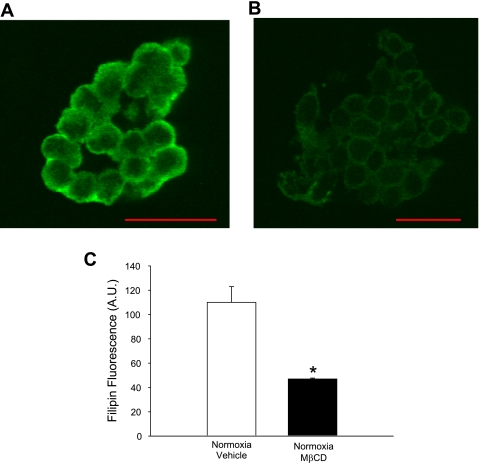
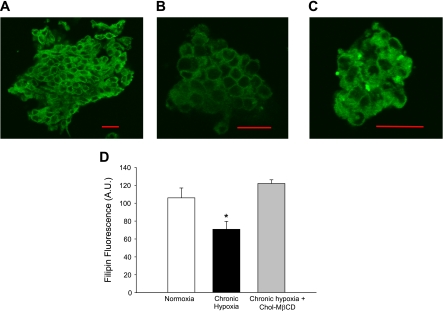
Membrane cholesterol content was assessed by quantifying filipin fluorescence in endothelial cells. As expected, treating endothelial cell sheets with mβCD significantly depleted membrane cholesterol content compared with control cells (Fig. 6C). We also assessed endothelial cell membrane cholesterol content following CH. Membrane cholesterol content was significantly lower in endothelial cells from CH animals compared with controls, whereas treating cells from CH animals with Chol-mβCD normalized membrane cholesterol content (Fig. 7D).
Fig. 6.
Effect of mβCD on membrane cholesterol content. Filipin fluorescence as a marker of membrane cholesterol content in pulmonary artery endothelial cells pretreated with vehicle (A) or 10 mM mβCD (B). Summary data showing the effect of mβCD on mean fluorescence intensity (C). Values are means ± SE. A.U., arbitrary units. *P ≤ 0.05 vs. control. Scale bar = 20 μm.
Fig. 7.
Effect of CH on membrane cholesterol content. Filipin fluorescence as a marker of membrane cholesterol content in pulmonary artery endothelial cells from control animals (A), CH animals (B), and cholesterol (Chol)-mβCD-treated cells from CH animals (C). Summary data illustrating the effect of CH and Chol-mβCD treatment on membrane cholesterol content (D). Values are means ± SE. *P ≤ 0.05 vs. normoxia and Chol-mβCD. Scale bar = 20 μm.
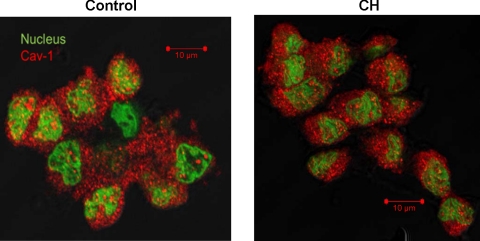
Cav-1 immunofluorescence and number of caveolae.
Cells freshly isolated from control and CH rats demonstrated similar immunofluorescent staining for cav-1 (Fig. 8). No staining was observed in sections treated only with secondary antibody. In addition, quantification of caveolar density from transmission electron micrographs revealed no difference between groups (Fig. 9).
Fig. 8.
Immunofluorescence images of cav-1 (red) in freshly dispersed pulmonary arterial endothelial sheets from control (left) and CH (right) rats. Nuclei are labeled green.
Fig. 9.
Transmission electron microscopy was used to determine the number of caveolae in the endothelium of pulmonary arteries from control and CH rats. A: representative electron micrographs at low and high magnification of caveolae (denoted by arrows) in arteries from control (left) and CH (right) rats. N, nucleus; EL, elastic lamina. B: caveolar density is expressed as the number of caveolae per micrometer length of membrane (n = 3 rats/group). There were no significant differences.
DISCUSSION
The present study examined the contribution of the exogenous cav-1 scaffolding element AP-cav on Ca2+ entry following CH-induced pulmonary hypertension. The major findings of this study were 1) ATP-induced Ca2+ entry was reduced following CH; 2) cholesterol depletion blunted agonist-induced Ca2+ influx in controls but was without effect in cells from CH rats; 3) cholesterol repletion did not augment the response to ATP in controls; however, Ca2+ entry was nearly restored in endothelial cells from CH rats to the level of controls; 4) introduction of AP-cav rescued ATP-induced Ca2+ entry in isolated endothelial cells and in pressurized arteries from CH rats compared with controls; 5) similarly, inward cation currents were less in cells from CH rats than controls and enhanced by AP-cav; and 6) membrane cholesterol content was lower in cells from CH rats compared with controls, although cav-1 staining and number of caveolae did not differ between groups. These results suggest that reduced agonist-dependent Ca2+ entry following CH is due to impairment of membrane microdomains related to altered cav-1 function and/or cholesterol homeostasis.
The effect of hypoxia on pulmonary endothelial Ca2+ influx remains controversial. Earlier reports documenting increased [Ca2+]i (4) utilized chronic (72 h) hypoxic exposure in cultured cell models. In contrast, our recent studies (21) demonstrating reduced endothelial SOC entry were conducted in freshly dissociated cells and intact pressurized arteries from pulmonary hypertensive rats following CH. Although our earlier study utilized a less relevant stimulus (CPA) directed specifically at SOC entry, we observed similar reductions in Ca2+ influx following CH in the present design with the physiological agonist ATP. We (22) have recently observed similarly reduced ROC entry in endothelial cells from CH rats that appears to be linked to altered PKC activity. Since both targeted-store depletion and agonist administration likely involve SOC entry, it is possible that CH affects the degree of filling of Ca2+ stores that could influence our results. However, we observed no difference in the magnitude of the initial Ca2+ release event following ATP administration between groups (Fig. 2B).
The present study focused on the role of membrane cholesterol as a regulating influence on endothelial agonist-induced Ca2+ entry. Inhibition of receptor-mediated Ca2+ influx by cholesterol depletion with mβCD has been reported in cell types other than endothelial cells (3, 5), and these findings are consistent with our data. Although we did not observe an effect of cholesterol depletion on ATP-induced Ca2+ entry following CH, there was a strong tendency for enhancement of this response after cholesterol repletion (Fig. 2). Administration of mβCD and Chol-mβCD to alter membrane cholesterol and caveolar structure is a commonly used approach; however, these manipulations may affect a wide range of signaling pathways dependent on lipid microdomains. Thus, although these experiments yielded the predicted effects on membrane cholesterol (Figs. 6 and 7), many pathways could be affected. However, one intriguing possibility is that CH is associated with decreased endothelial cholesterol synthesis. This hypothesis is supported by our observation of reduced membrane cholesterol in cells from CH rats compared with controls (Fig. 6). Interestingly, Nyugen et al. (19) recently showed that hypoxia diminishes cholesterol synthesis through a hypoxia-inducible factor-α-dependent mechanism. Hypoxic downregulation of de novo cholesterol synthesis may be critical since induction of cav-1 expression is cholesterol dependent and cholesterol is thought to be rate limiting for cav-1 trafficking to the plasma membrane necessary for caveolar formation (6).
In addition to caveolar biogenesis, cav-1 plays an important role in trafficking endothelial Ca2+ entry pathways to the plasma membrane. Murata et al. (16) proposed a model of endothelial Ca2+ entry in which cav-1 integrates caveolar TRPC4 and endoplasmic reticulum-associated IP3 receptors as a functional signaling unit. Furthermore, pulmonary vascular defects and hypertension are abrogated by cav-1 reexpression in cav-1-deficient mice or by administration of the cell permeant AP-cav to MCT-treated rats (8, 15). Whereas these reports focused primarily on the effect of cav-1 on proliferative and morphometric indexes of pulmonary hypertension, endothelial Ca2+ was not examined. Our findings that introduction of the cell permeant CSD rescues agonist-induced Ca2+ entry in endothelial cells from CH-hypertensive rats suggest a potential mechanism for the reduced Ca2+ influx in this setting that we have previously reported (22). In addition to the Ca2+ fluorescence data, our electrophysiological recordings also support the idea that the cav-1 scaffolding element acts to promote endothelial cation influx. It is not clear whether CH downregulates cav-1-related trafficking phenomena, although the restorative effects of AP-cav on Ca2+ entry suggest that ion channel expression may not be affected.
Caveolae are complex microdomains containing a myriad of signaling components. Interestingly, cav-1 immunofluorescence (Fig. 8) and the number of endothelial caveolae (Fig. 9) were not affected by CH exposure, although membrane cholesterol content was reduced. Thus deranged Ca2+ influx following CH may be due to diminished cholesterol within caveolae that interferes with direct cav-1 regulation of ion channels or through other aspects of signaling. Our previous work has suggested that activities of various protein kinase C (PKC) isoforms may be differentially regulated in the pulmonary arterial endothelium following CH that affect Ca2+ entry. Specifically, we found that a component of agonist-induced Ca2+ entry dependent on PKCε was absent after CH exposure. Interestingly, PKCε has been shown to associate with the CSD, but unlike some other PKC isoforms, it is not inhibited by this interaction (20). Thus the restoration of ATP-induced Ca2+ entry by introduction of the cell-permeable CSD in our experiments could be related to reintroduction of PKCε to this signaling platform. Further experiments are necessary to delineate this possible interaction and its effect on ion channel function.
In conclusion, we examined the effects of cholesterol depletion/repletion along with the role of the cav-1 scaffolding element to modulate ATP-induced Ca2+ entry in pulmonary endothelium. Our findings reveal an effect of CH to impair Ca2+ entry that is associated with reduced membrane cholesterol content and that is resolved by cholesterol replenishment or introduction of the CSD. The results of our study suggest that CH-induced pulmonary hypertension has detrimental effects on membrane microdomains and/or cholesterol homeostasis that decrease endothelial Ca2+ entry following CH.
GRANTS
Fluorescent images in this study were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Facility, which received support from National Center for Research Resources Grants 1-S10-RR-14668, P20-RR-11830, S10-RR-19287, S10-RR-016918; National Science Foundation Grant MCB9982161; National Cancer Institute Grant P30-CA-118100; the University of New Mexico Health Sciences Center; and the University of New Mexico Cancer Center. This work was supported by National Heart, Lung, and Blood Institute Grants HL-58124, HL-95640, and HL-07736 (to B. R. Walker) and HL-88192 (to T. C. Resta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Tamara Howard for generation of electron micrographs and Minerva Murphy for technical assistance.
REFERENCES
- 1. Chatterjee S, Cao S, Peterson TE, Simari RD, Shah V. Inhibition of GTP-dependent vesicle trafficking impairs internalization of plasmalemmal eNOS and cellular nitric oxide production. J Cell Sci 116: 3645–3655, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Everson WV, Smart EJ. Influence of caveolin, cholesterol, and lipoproteins on nitric oxide synthase: implications for vascular disease. Trends Cardiovasc Med 11: 246–250, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Fagan KA, Smith KE, Cooper DM. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem 275: 26530–26537, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Graziani A, Rosker C, Kohlwein SD, Zhu MX, Romanin C, Sattler W, Groschner K, Poteser M. Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy. Biochem J 396: 147–155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res 39: 369–379, 1998 [PubMed] [Google Scholar]
- 7. Ito KM, Sato M, Ushijima K, Nakai M, Ito K. Alterations of endothelium and smooth muscle function in monocrotaline-induced pulmonary hypertensive arteries. Am J Physiol Heart Circ Physiol 279: H1786–H1795, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 114: 912–920, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 10. King AP, Smith P, Heath D. Ultrastructure of rat pulmonary arterioles after neonatal exposure to hypoxia and subsequent relief and treatment with monocrotaline. J Pathol 177: 71–81, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Li S, Song KS, Koh SS, Kikuchi A, Lisanti MP. Baculovirus-based expression of mammalian caveolin in Sf21 insect cells. A model system for the biochemical and morphological study of caveolae biogenesis. J Biol Chem 271: 28647–28654, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 126: 111–126, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maniatis NA, Shinin V, Schraufnagel DE, Okada S, Vogel SM, Malik AB, Minshall RD. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol 294: L865–L873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukhopadhyay S, Xu F, Sehgal PB. Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia, and senescence: live-cell caveolar and cytoplasmic NO imaging. Am J Physiol Heart Circ Physiol 292: H1373–H1389, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282: 16631–16643, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Murata T, Yamawaki H, Hori M, Sato K, Ozaki H, Karaki H. Hypoxia impairs endothelium-dependent relaxation in organ cultured pulmonary artery. Eur J Pharmacol 421: 45–53, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 282: 27436–27446, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem 272: 33416–33421, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Paffett ML, Naik JS, Resta TC, Walker BR. Reduced store-operated Ca2+ entry in pulmonary endothelial cells from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 293: L1135–L1142, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Paffett ML, Riddle MA, Kanagy NL, Resta TC, Walker BR. Altered protein kinase C regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia. J Pharmacol Exp Ther 334: 753–760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Russ RD, Walker BR. Role of nitric oxide in vasopressinergic pulmonary vasodilatation. Am J Physiol Heart Circ Physiol 262: H743–H747, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Schroeder F, Huang H, McIntosh AL, Atshaves BP, Martin GG, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Subcell Biochem 51: 279–318, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 272: 25968–25975, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu H, Weisleder N, Wu P, Cai C, Chen JW. Caveolae/caveolin-1 are important modulators of store-operated calcium entry in Hs578/T breast cancer cells. J Pharmacol Sci 106: 287–294, 2008 [DOI] [PubMed] [Google Scholar]