Abstract
This study determined the effect of ANG-(1–7) on salt-induced suppression of endothelium-dependent vasodilatation in the mesenteric arteries of male Sprague-Dawley rats. Chronic intravenous infusion of ANG-(1–7), oral administration of the nonpeptide mas receptor agonist AVE-0991, and acute preincubation of the arteries with ANG-(1–7) and AVE-0991 all restored vasodilator responses to both ACh and histamine that were absent in the arteries of rats fed a high-salt (4% NaCl) diet. The protective effects of ANG-(1–7) and AVE-0991 were inhibited by acute or chronic administration of the mas receptor antagonist A-779, the ANG II type 2 (AT2) receptor blocker PD-123319, or N-nitro-l-arginine methyl ester, but not the ANG II type 1 receptor antagonist losartan. Preincubation with the antioxidant tempol or the nitric oxide (NO) donor diethylenetriamine NONOate and acute and chronic administration of the AT2 receptor agonist CGP-42112 mimicked the protective effect of ANG-(1–7) to restore vascular relaxation. Acute preincubation with ANG-(1–7) and chronic infusion of ANG-(1–7) ameliorated the elevated superoxide levels in rats fed a high-salt diet, but the expression of Cu/Zn SOD and Mn SOD enzyme proteins in the vessel wall was unaffected by ANG-(1–7) infusion. These results indicate that both acute and chronic systemic administration of ANG-(1–7) or AVE-0991 restore endothelium-dependent vascular relaxation in salt-fed Sprague-Dawley rats by reducing vascular oxidant stress and enhancing NO availability via mas and AT2 receptors. These findings suggest a therapeutic potential for mas/AT2 receptor activation in preventing the vascular oxidant stress and endothelial dysfunction associated with elevated dietary salt intake.
Keywords: renin-angiotensin system, Sprague-Dawley rat
the small peptide ANG-(1–7), representing the antihypertensive arm of the renin-angiotensin system, antagonizes many of the actions of ANG II (13). Most of the beneficial actions of ANG-(1–7) are mediated by its specific receptor (mas) (43), which is distinct from ANG II type 1 (AT1) and type 2 (AT2) receptor subtypes. The actions of ANG-(1–7) can be mimicked by the nonpeptide mas agonist AVE-0991 (42) and can be inhibited by the selective ANG-(1–7) antagonist A-779 (40). A number of studies in the literature (8, 11, 32, 35, 48) have indicated that AT1 and AT2 receptors may contribute to the physiological effects of mas receptor activation by ANG-(1–7) via cross talk or receptor transactivation, although the contribution (or lack of contribution) of AT1 and AT2 receptors to the effects of mas receptor activation by ANG-(1–7) varies widely among different physiological responses.
Salt-induced suppression of ANG II below normal physiological levels causes impaired vascular relaxation, elevated superoxide levels in blood vessels, reduced nitric oxide (NO) availability, and endothelial dysfunction in normotensive animals (25, 27, 33, 49, 57). Because ANG-(1–7) receptor activation has been shown to activate endothelial NO synthase and stimulate endothelial NO release (12, 18, 39, 53), it is possible that mas receptor activation may be beneficial under conditions of impaired endothelial function, such as those existing in normotensive salt-insensitive animals fed a high-salt (HS) diet (11, 25, 28, 57) and in many different forms of hypertension.
The goal of the present study was to determine whether acute in vitro application or chronic administration of ANG-(1–7) or the pharmacological mas receptor agonist AVE-0991 would ameliorate the endothelial dysfunction and restore the impaired relaxation of mesenteric resistance arteries that occurs during elevated salt intake in normotensive Sprague-Dawley rats (57). In the case of acute ANG-(1–7) or AVE-0991 pretreatment, we also investigated whether the improvement of endothelial function was due to the increased endothelial NO release and whether the effects of mas receptor activation can be mimicked by the SOD mimetic tempol or a NO donor [diethylenetriamine NONOate (DETA-NONOate)], both of which increase the bioavailability of NO (45, 57).
Because existing studies (6, 8, 11, 23, 26, 32, 34, 35, 46, 48) have reached widely varying conclusions regarding the role of AT1 and AT2 receptors in mediating the response of diverse physiological systems to ANG-(1–7), we also determined whether any effect of ANG-(1–7) or AVE-0991 to restore vascular relaxation and endothelial function in Sprague-Dawley rats fed a HS diet is mediated solely via the mas receptor or whether AT1 or AT2 receptors contribute to the effects of ANG-(1–7). The ability of AT2 receptor activation to restore endothelium-dependent vasodilation in salt-fed rats was also tested by acute and chronic administration of the AT2 receptor agonist CGP-42112. In a final series of experiments, we determined whether any restoration of vasodilation by ANG-(1–7) in salt-fed Sprague-Dawley rats may be mediated by changes in vascular superoxide levels, which affect the availability of NO in the vessel, and whether chronic infusion of ANG-(1–7) upregulates the expression of Cu/Zn SOD or Mn SOD in the arterial wall.
MATERIALS AND METHODS
Experimental animals.
The present study used 8- to 10-wk-old male Sprague-Dawley rats fed either a low-salt (LS; 0.4% NaCl) or HS (4% NaCl) diet for 3–6 days.
For chronic infusion experiments, animals were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg), acepromazine (2.5 mg/kg), and anased (10 mg/kg). A chronic catheter was tunneled from the midscapular region and installed in the femoral vein for chronic intravenous infusion using standard aseptic surgery procedures. During the recovery period, rats were kept on a LS diet and were continuously infused with isotonic saline (0.9% NaCl). Separate groups of rats were kept either on the LS diet or switched to the HS diet 6 days before the experiments while receiving a continuous infusion (0.5 ml/h) of either 1) isotonic saline vehicle; 2) CGP-42112 (3.3 μM·kg−1·h−1); or 3) ANG-(1–7) (289.2 nM·kg−1·h−1) with or without the mas receptor antagonist A-779 [(d-Ala7)-ANG-(1–7); 29 μM·kg−1·h−1], the AT1 receptor blocker losartan (2.6 mM·kg−1·h−1), or the AT2 receptor inhibitor PD-123319 (407.2 μM·kg−1·h·) for the last 3 days. The infusion dose of ANG-(1–7) used in the present study is equimolar to the ANG II infusion used in other studies (11, 50). Separate groups of Sprague-Dawley rats fed a LS diet or switched to a HS diet for 6 days were treated with the orally active mas receptor agonist AVE-0991 (10−7 M) in the drinking water for the last 3 days. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Blood pressure measurement and evaluation of mesenteric vascular reactivity.
On the experimental days, rats were anesthetized by either an intraperitoneal injection (noninfused animals) or an intravenous administration (infused animals) of the anesthetic cocktail (see above) through the infusion lines, and blood pressure was measured by direct cannulation of the carotid artery. Mesenteric resistance arteries (∼300-μm diameter) feeding the small intestine were isolated and cannulated in a tissue culture myograph system (Danish Myo-Technology, Aarhus, Denmark). Arteries were incubated at 37°C and 75-mmHg intraluminal pressure for 60 min while the vessel was continuously perfused and superfused with physiological salt solution (PSS) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture. The PSS used in these experiments had the following composition (in mM) 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, and 0.03 Na2-EDTA.
Outer and inner vessel diameters were measured using video microscopy techniques similar to those used in previous studies (14, 28). Wall thickness [(outer diameter − inner diameter)/2] and the wall-to-lumen ratio [(outer diameter − inner diameter)/inner diameter] were also calculated. To assess vascular relaxation responses, arteries were preconstricted with an EC50 concentration of norepinephrine (4 μM), and their responses to vasodilator agonists were evaluated by adding increasing concentrations of ACh (10−10–10−5 M) or histamine (10−10–10−3 M) [with or without N-nitro-l-arginine methyl ester (l-NAME; 10−4 M)] or the NO donor DETA-NONOate (10−6–10−3 M) to the tissue bath. In another series of experiments, arteries were preincubated with 1 μM ANG-(1–7) or AVE-0991 [with or without l-NAME (10−4 M), losartan (10−5 M), A-779 (10−5 M), PD-123319 (10−5 M)], CGP-42112 (10−6 M), tempol (10−4 M), or DETA-NONOate (10−4 M) for 30 min followed by a complete washout, after which responses to ACh were evaluated as described above.
Western blot analysis for Cu/Zn SOD and Mn SOD.
To evaluate Cu/Zn SOD and Mn SOD enzyme expression, mesenteric resistance arteries supplying the small intestine were isolated and homogenized in 200 μl of a solution containing 250 mM sucrose, 1 mM EDTA, 1 mM KH2PO4, 10 mM K2HPO4 (pH 7.7), and 1.5 μl phosphatase and protease inhibitor cocktails. Tissue debris and nuclear fragments were removed by centrifugation at 12,000 g for 20 min at 4°C. The protein concentration was determined using a Bradford protein assay (Thermo Scientific, Rockford, IL), and 10–35 μg of each sample were loaded onto a 4–20% SDS-polyacrylamide gel and separated by electrophoresis. Samples were transferred to a nitrocellulose membrane (0.45 μm) and blocked in Tris-buffered saline-Tween 20 (TBST; 10 mM Tris, 150 mM NaCl, and 0.1% Tween 20) containing 10% nonfat dried milk at room temperature for 2 h. Membranes were then incubated with a 1:1,000 dilution of rabbit anti-Cu/Zn SOD primary antibody (ADI-SOD-101, Enzo Life Sciences, Plymouth Meeting, PA) or a 1:8,000 dilution of rabbit anti-Mn SOD primary antibody (SOD-111, Assay Design, Ann Arbor, MI) at 4°C overnight. The following day, membranes were washed with TBST and incubated with horseradish peroxidase-conjugated goat anti-rabbit (sc-2004) secondary antibodies for Cu/Zn SOD (1:6,000 dilution) or Mn SOD (1:8,000 dilution) for 2 h. After incubation with the secondary antibodies, membranes were washed, and protein bands were visualized using chemiluminescence (Super Signal, Pierce, Rockford IL). Membranes were exposed to Blue Lite Autorad film (F-9024–8x10, GeneMate, Bioexpress, Kaysville, UT) and developed in a Kodak X-OMAT 2000A developer (Eastman Kodak, Rochester, NY). Densitometry values (in pixels) were obtained using UnScanIT 6.1 software (Silk Scientific, Orem, UT). The expression of bands of the target protein for each animal was expressed as a percentage of the β-actin loading control and normalized as 100% for each control group, i.e., LS diet for HS diet-fed animals and HS diet with saline infusion for HS diet-fed animals with ANG-(1–7) infusion.
Dihydroethidium staining and imaging.
Arteries from LS or HS diet-fed rats [with or without preincubation with 10−6 M ANG-(1–7) (see above)] and from HS-fed rats receiving either isotonic saline or ANG-(1–7) infusion were equilibrated in the tissue culture myograph system for 60 min. Vessels were then incubated with dihydroethidium (DHE) at a final concentration of 50 μM for 30 min, after which the residual DHE was washed out with PSS. Vessels were removed from the chambers and embedded in Tissue Tek. Blocks were frozen at −18°C, and 6-μm sections were taken with a cryostat. Sections were placed on microscope slides, coverslipped with Fluoro Gel mounting medium (Electron Microscopy Sciences, Hatfield, PA), and stored in the dark until analysis. Bright-field and fluorescent images of multiple cross sections of each individual mesenteric artery were taken with a ×20 lens using a Nikon Eclipse TS100 microscope with a QImaging Retiga-2000R digital camera (Surrey, BC, Canada) and 540-nm excitation and 605-nm emission filters (Chroma Technology, Bellows Falls, VT). Cross sections were analyzed using Metamorph imaging and analysis software (Universal Imaging, Downington, PA). Fluorescent intensity in the vessel wall was quantified as fluorescence intensity per pixel for each sample.
Drugs and reagents.
All chemicals used in this study were purchased from Sigma (St. Louis, MO) except for A-779 (Bachem,Torrance, CA), PD-123319 and CGP-42112 (Tocris, Ellisville, MO), and DETA-NONOate (Cayman, Ann Arbor, MI). AVE-0991 was a generous gift from Sanofi-Aventis (Frankfurt, Germany).
Statistical analysis.
Diameter changes were calculated as the difference from the diameter measured after preconstriction with an EC50 concentration of norephinephrine in the tissue bath. Data are expressed as means ± SE. Differences between individual groups were evaluated using Student's t-test for comparisons of two groups or ANOVA with a post hoc Dunnett test for comparisons of more than two experimental groups. In the case of dose-dependent responses, one-way repeated-measures ANOVA was used and Dunnett's pair-wise multiple comparison procedure was applied to test for significant differences from the preconstricted diameter. Statistical significance was taken as P < 0.05.
RESULTS
Experimental animal groups and arterial diameters.
To conserve resources, arteries used in many of these experiments were obtained from rats used in another study (11). Dietary salt content, ANG-(1–7) infusion (either alone or in combination with inhibitors of mas, AT1, or AT2 receptors), CGP-42112 infusion, and AVE-0991 treatment had no significant effect on body weight or arterial blood pressure in any of the experimental groups (Table 1). In saline-infused rats, blood pressure values at the point where the arteries were isolated for study were ∼10 mmHg less compared with the noninfused groups, most likely due to the direct intravenous application of the anesthetics through the infusion line versus intraperitoneal injection (see materials and methods). None of the chronic infusion treatments [ANG-(1–7) with or without inhibitors or CGP-42112] produced a significant change in anesthetized blood pressure.
Table 1.
General characteristics of the various experimental animal groups of Sprague-Dawley rats in the present study and the corresponding vessel diameters
| Control Diameter Without l-NAME |
Control Diameter With l-NAME |
|||||
|---|---|---|---|---|---|---|
| Body Weight, g | Blood Pressure, mmHg | Diameter, μm | %Control | Diameter, μm | %Control | |
| Low-salt diet | 295.2 ± 4.0 (n = 49) | 114.8 ± 2.1 (n = 39) | 293.6 ± 5.4 | 80.1 ± 1.0 (n = 25) | 292.8 ± 7.8 | 77.9 ± 1.3 (n = 17) |
| Low-salt diet + preincubation | ||||||
| ANG-(1-7) | 309.2 ± 6.3 | 77.3 ± 1.3 (n = 16) | ||||
| High-salt diet | 298.0 ± 2.8 (n = 108) | 111.8 ± 1.6 (n = 75) | 298.0 ± 5.0 | 82.8 ± 0.8 (n = 25) | 300.0 ± 6.5 | 78.5 ± 1.5 (n = 17) |
| High-salt diet + preincubation | ||||||
| ANG-(1-7) | 306.8 ± 4.4 | 76.5 ± 1.3 (n = 16) | ||||
| ANG-(1-7) + A-779 | 308.1 ± 5.1 | 79.7 ± 2.0 (n = 8) | ||||
| ANG-(1-7) + losartan | 294.4 ± 6.1 | 80.0 ± 1.7 (n = 8) | ||||
| ANG-(1-7) + PD-123319 | 300.6 ± 10.7 | 81.1 ± 1.9 (n = 8) | ||||
| ANG-(1-7) + l-NAME | 303.4 ± 10.1 | 80.6 ± 1.1 (n = 8) | ||||
| AVE-0991 | 298.8 ± 7.6 | 78.8 ± 1.1 (n = 8) | ||||
| AVE-0991 + A-779 | 316.4 ± 11.7 | 80.9 ± 1.6 (n = 8) | ||||
| AVE-0991 + losartan | 323.0 ± 9.5 | 78.9 ± 1.0 (n = 8) | ||||
| AVE-0991 + PD-123319 | 304.8 ± 5.8 | 79.3 ± 2.0 (n = 8) | ||||
| AVE-0991 + l-NAME | 326.9 ± 11.0 | 79.3 ± 1.7 (n = 8) | ||||
| CGP-42112 | 325.8 ± 16.0 | 79.9 ± 0.8 (n = 8) | ||||
| Tempol | 316.8 ± 8.1 | 80.5 ± 0.8 (n = 8) | ||||
| DETA-NONOate | 313.6 ± 5.2 | 78.0 ± 1.2 (n = 8) | ||||
| AVE-0991 treatment | ||||||
| Low-salt diet | 304.5 ± 5.0 (n = 8) | 114.1 ± 3.7 (n = 8) | 302.3 ± 8.6 | 78.8 ± 1.6 (n = 8) | ||
| High-salt diet | 308.4 ± 5.1 (n = 8) | 112.6 ± 3.0 (n = 7) | 300.6 ± 8.0 | 80.0 ± 1.2 (n = 8) | ||
| Low-salt diet + infusion | ||||||
| Saline | 279.2 ± 6.6 (n = 25) | 101.2 ± 2.9 (n = 22) | 318.9 ± 5.3 | 79.4 ± 1.0 (n = 16) | 310.9 ± 5.4 | 77.1 ± 1.2 (n = 16) |
| ANG-(1-7) | 267.0 ± 3.0 (n = 32) | 103.8 ± 2.4 (n = 29) | 306.5 ± 5.5 | 79.8 ± 0.8 (n = 16) | 304.0 ± 6.4 | 76.5 ± 1.4 (n = 16) |
| High-salt diet + infusion | ||||||
| Saline | 274.4 ± 3.4 (n = 39) | 103.7 ± 2.9 (n = 25) | 308.1 ± 6.5 | 78.5 ± 1.5 (n = 16) | 294.9 ± 5.9 | 76.7 ± 1.1 (n = 16) |
| ANG-(1-7) | 280.3 ± 3.9 (n = 43) | 103.2 ± 2.6 (n = 33) | 309.2 ± 6.0 | 79.5 ± 1.5 (n = 16) | 307.1 ± 5.0 | 75.7 ± 1.0 (n = 16) |
| ANG-(1-7) + A-779 | 285.1 ± 6.1 (n = 8) | 110.7 ± 4.1 (n = 7) | 314.2 ± 6.3 | 82.1 ± 1.6 (n = 8) | ||
| ANG-(1-7) + losartan | 278.2 ± 3.8 (n = 11) | 91.4 ± 7.2 (n = 11) | 309.2 ± 8.2 | 79.8 ± 2.0 (n = 8) | ||
| ANG-(1-7) + PD-123319 | 278.5 ± 6.6 (n = 10) | 99.6 ± 3.2 (n = 10) | 302.6 ± 12.2 | 78.3 ± 1.6 (n = 8) | ||
| CGP-42112 | 294.5 ± 4.4 (n = 8) | 98.1 ± 3.2 (n = 8) | 322.9 ± 8.9 | 80.8 ± 1.4 (n = 8) | 302.3 ± 8.1 | 80.0 ± 2.8 (n = 8) |
Values are means ± SE; n, no. of rats. Body weights and arterial blood pressures of all Sprague-Dawley rats are shown. Control diameters of mesenteric arteries and degrees of their preconstriction with EC50 norepinephrine (%control diameter) used in the different experimental groups in this study are also shown. l-NAME, N-nitro-l-arginine methyl ester; DETA-NONOate, diethylenetriamine NONOate.
Control diameters of the isolated arteries from Sprague-Dawley rats determined after the incubation period in PSS or after preincubation of the vessels with ANG-(1–7) or AVE-0991 (either alone or in combination with l-NAME, mas, AT1, or AT2 receptor antagonists), CGP-42112, DETA-NONOate, or tempol were not different across the groups (Table 1). Wall thickness and the wall-to-lumen ratio were unaffected by dietary salt content (LS vs. HS diet) or by ANG-(1–7) versus saline infusion in Sprague-Dawley rats fed a HS diet (data not shown), showing that no significant remodeling occurs in the mesenteric arterial wall due to elevated dietary salt content or chronic infusion of ANG-(1–7) in this rat strain.
Effect of HS diet and chronic ANG-(1–7) infusion on responses to vasodilator stimuli.
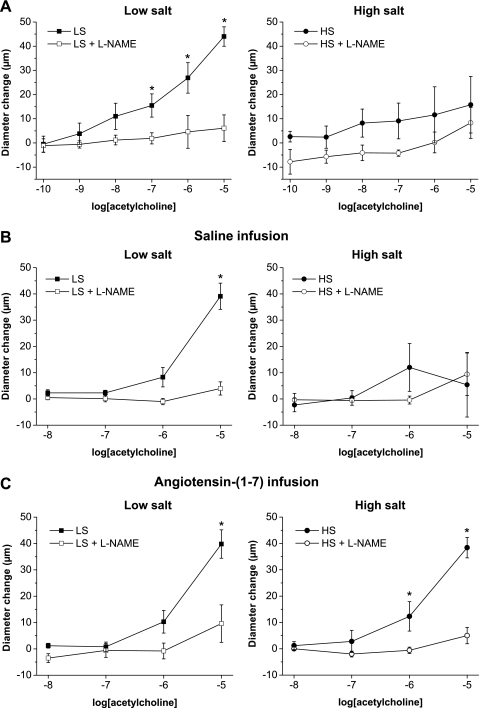
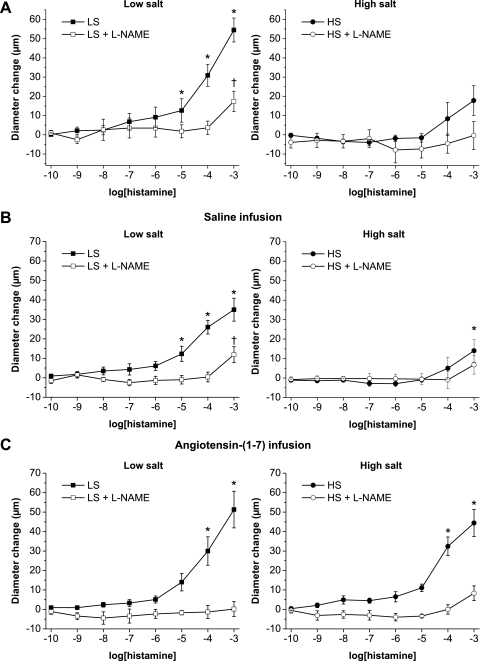
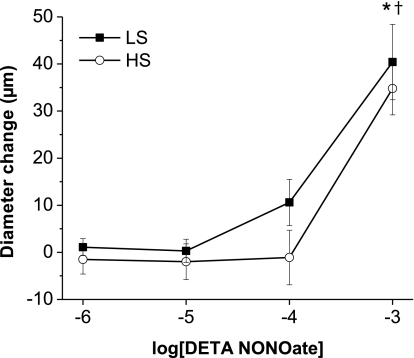
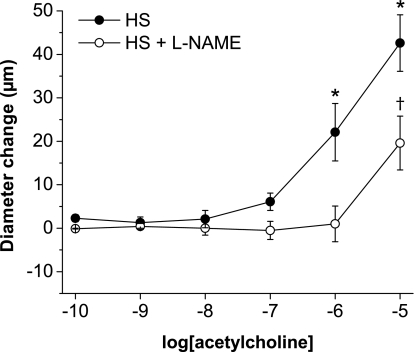
Consistent with earlier findings in multiple vessel types (11, 25, 27, 28, 33, 49, 57), l-NAME-sensitive vasodilation in response to ACh (Fig. 1A) and histamine (Fig. 2A) were absent in mesenteric arteries from Sprague-Dawley rats fed a HS diet. Similar to noninfused rats, vasodilator responses to ACh (Fig. 1B) and histamine (Fig. 2B) were present in arteries of saline-infused rats fed a LS diet and were absent in arteries of saline-infused rats fed a HS diet. However, vessels from Sprague-Dawley rats fed either a LS or HS diet dilated in a similar fashion in response to the NO donor DETA-NONOate (Fig. 3), showing that the arteries of those animals are still sensitive to the vasodilator effects of NO, independent of salt diet.
Fig. 1.
Effect of chronic saline infusion, chronic ANG-(1–7) infusion, and high-salt (HS) diet on ACh concentration-response curves: diameter changes to increasing concentrations of ACh (10−10–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from control (noninfused; A), saline-infused (B), or ANG-(1–7) (289.2 nM·kg−1·h−1)-infused (C) Sprague-Dawley rats fed either a low-salt (LS) diet (left; n = 8) or a HS diet (right; n = 8–9). *P < 0.05 vs. precontracted control diameters in the absence of 10−4 M N-nitro-l-arginine methyl ester (l-NAME).
Fig. 2.
Effect of chronic saline infusion, chronic ANG-(1–7) infusion, and HS diet on histamine concentration-response curves: diameter changes to increasing concentrations of histamine (10−10–10−3 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from control (noninfused; A), saline-infused (B), or ANG-(1–7) (289.2 nM·kg−1·h−1)-infused (C) Sprague-Dawley rats fed either a LS diet (left; n = 8) or a HS diet (right; n = 8). *P < 0.05 vs. precontracted control diameters of vessels in the absence of 10−4 M l-NAME; †P < 0.05 vs. precontracted control diameters of vessels in the presence of 10−4 M l-NAME.
Fig. 3.
Effect of a HS diet on diethylenetriamine NONOate (DETA-NONOate) concentration-response curves: diameter changes to increasing concentrations of the nitric oxide (NO) donor DETA-NONOate (10−6–10−3 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from rats fed either a LS diet (n = 8) or a HS diet (n = 8). *P < 0.05 vs. precontracted diameters of vessels from LS diet-fed rats; †P < 0.05 vs. precontracted diameters of vessels from HS diet-fed rats.
Vasodilator responses to ACh (Fig. 1C) and histamine (Fig. 2C) were unaffected by ANG-(1–7) infusion in rats fed a LS diet compared with control and saline-infused rats fed a LS diet. In rats fed a HS diet, ANG-(1–7) infusion restored the impaired arterial dilation in response to both ACh (Fig. 1C) and histamine (Fig. 2C) compared with control or saline-infused rats fed a HS diet. Similar to all three groups of rats fed a LS diet, the restored dilations in response to ACh (Fig. 1C) and histamine (Fig. 2C) in ANG-(1–7)-infused Sprague-Dawley rats fed a HS diet were eliminated by l-NAME, demonstrating that they were mediated by NO.
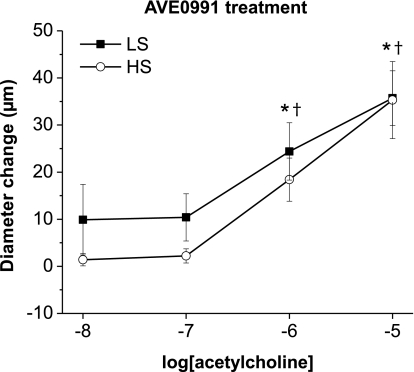
Effect of orally administered AVE-0991 on ACh-induced dilation in arteries of rats fed a HS diet.
As shown in Fig. 4, chronic administration of the orally active mas receptor agonist AVE-0991 in Sprague-Dawley rats fed a HS diet also restored the ACh-induced dilations to values similar to those seen in arteries from rats fed a LS diet. The restoration of vasodilator responses to ACh by oral administration of AVE-0991 was similar to the protective effect of chronic intravenous infusion of ANG-(1–7) (Fig. 1C), providing further support for the hypothesis that mas receptor activation can ameliorate the severe endothelial dysfunction that occurs in response to elevated dietary salt intake in Sprague-Dawley rats fed a LS diet.
Fig. 4.
Effect of orally administered AVE-0991 and salt diet on ACh-induced dilation: diameter changes to increasing concentrations of ACh (10−8–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from Sprague-Dawley rats fed either a LS diet (n = 8) or HS diet (n = 8) treated with 10−7 M AVE-0991 in drinking water. *P < 0.05 vs. precontracted control diameters of vessels from LS diet-fed rats; †P < 0.05 vs. precontracted control diameters of vessels from HS diet-fed rats.
Effect of mas, AT1, and AT2 receptor blockade on the restoration of vasodilator responses by ANG-(1–7) infusion in rats fed a HS diet.
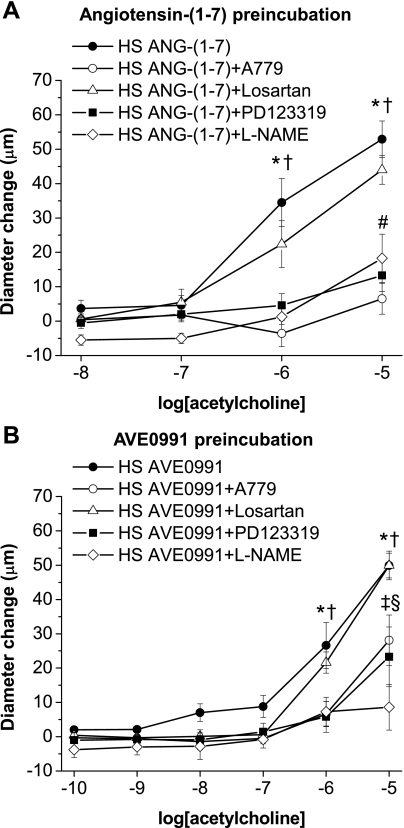
The protective effect of chronic ANG-(1–7) infusion to restore ACh-induced dilation of mesenteric arteries of rats fed a HS diet was blocked by coinfusion of either the mas receptor antagonist A-779 or the AT2 receptor blocker PD-123319, but not the AT1 antagonist losartan (Fig. 5). Taken together, these findings show that mas and AT2 receptors (but not the AT1 receptor) contribute to the protective effect of ANG-(1–7) to restore ACh-induced dilation in these vessels from HS diet-fed Sprague-Dawley rats.
Fig. 5.
Effect of ANG II type 1 (AT1), ANG II type 2 (AT2), and mas receptor inhibition on the restored response to ACh in HS diet-fed rats with chronic ANG-(1–7) infusion: diameter changes to increasing concentrations of ACh (10−8–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from Sprague-Dawley rats fed a HS diet and infused with ANG-(1–7) (289.2 nM·kg−1·h−1) in combination with mas (A-779; 29 μM·kg−1·h−1), AT1 (losartan; 2.6 mM·kg−1·h−1), or AT2 (PD-123319; 407.2 μM·kg−1·h−1) receptor inhibitors. n = 8 rats/group. *P < 0.05 vs. precontracted control diameters of vessels with ANG-(1–7) + losartan; † P < 0.05 vs. precontracted control diameters of vessels with ANG-(1–7) + A-779.
Effect of chronic CGP-42112 infusion on ACh-induced dilation in arteries of rats fed a HS diet.
In light of the finding that AT2 receptor blockade (as well as mas receptor blockade) prevented the restoration of vasodilator responses to chronic ANG-(1–7) infusion in HS diet-fed rats, we also determined whether chronic AT2 receptor activation with CGP-42112 infusion can improve the response of the arteries to ACh. Similar to chronic ANG-(1–7) infusion (Fig. 1C), intravenous infusion of the AT2 agonist CGP-42112 improved the vasodilator response to ACh in mesenteric arteries from rats fed a HS diet, and this effect was inhibited by l-NAME (Fig. 6).
Fig. 6.
Effect of chronic AT2 receptor activation on ACh-induced dilation in HS diet-fed rats: diameter changes to increasing concentrations of ACh (10−10–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from Sprague-Dawley rats fed a HS diet and infused with the AT2 receptor activator CGP-42112 (3.3 μM·kg−1·h−1). n = 8 rats/group. *P < 0.05 vs. precontracted control diameters of vessels in the absence of l-NAME; †P < 0.05 vs. precontracted control diameters of vessels in the presence of l-NAME.
Effect of acute treatment with ANG-(1–7) or AVE-0991 on the response to vasodilator agonists.
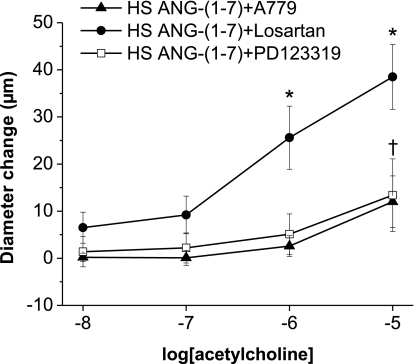
ACh and histamine responses were also measured after acute preincubation of the vessels for 30 min with 10−6 M ANG-(1–7) (Fig. 7A) or AVE-0991 (Fig. 7B) either alone or in combination with A-779, losartan, PD-123319, or l-NAME. Similar to chronic intravenous infusion of ANG-(1–7), preincubation with either ANG-(1–7) or AVE-0991 (followed by washout) also restored vasodilator responses to ACh (Fig. 7, A and B) and histamine (not shown) in arteries from Sprague-Dawley rats fed a HS diet. The ability of acute preincubation with ANG-(1–7) or AVE-0991 to restore ACh-induced dilation was inhibited by A-779, PD-123319, and l-NAME, but not by losartan (Fig. 7, A and B), demonstrating that both ANG-(1–7) and AVE-0991 have acute and direct actions that are mediated via mas/AT2 receptors to improve NO-dependent endothelial function in HS diet-fed Sprague-Dawley rats (in addition to their long-term beneficial effects during chronic administration of either of the agonists).
Fig. 7.
Effect of acute preincubation with ANG-(1–7) or AVE-0991 (with or without l-NAME) on ACh-induced responses in arteries of rats fed a HS diet: diameter changes to increasing concentrations of ACh (10−10–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from Sprague Dawley rats fed a HS diet. Arteries were pretreated for 30 min with 10−6 M ANG-(1–7) (A) or 10−6 M AVE-0991 alone or in combination with 10−5 M mas (A-779), AT1 (losartan), or AT2 (PD-123319) receptor inhibitors or 10−4 M of the NO synthase inhibitor l-NAME (B). Symbols indicate P < 0.05 vs. precontracted diameters of vessels preincubated with ANG-(1–7) or AVE-0991 alone (*) or with losartan (†), A-779 (‡), PD-123319 (§), or l-NAME (#) in the tissue bath.
Effect of acute treatment with CGP-42112, tempol, and DETA-NONOate on the response to vasodilator agonists.
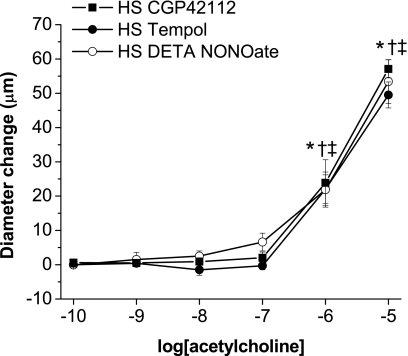
Preincubation with the AT2 agonist CGP-42112 also mimicked the effect of acute ANG-(1–7) and AVE-0991 (Fig. 8), supporting an involvement of the AT2 receptor in the restoration of endothelial dilatations by ANG-(1–7) or AVE-0991. To evaluate the role of oxidant stress in contributing to impaired vascular relaxation and to test whether NO liberated by ANG-(1–7) and AVE-0991 during the preincubation period might be able to restore endothelium-dependent dilatation, arteries from HS diet-fed rats were also preincubated for 30 min with either the NO donor DETA-NONOate (10−4 M) or the SOD mimetic tempol (10−4 M). Preincubation with either DETA-NONOate or tempol also mimicked the effect of ANG-(1–7) and AVE-0991 (Fig. 8) indicating that endothelium-dependent vasodilation can be restored either by increasing NO availability exogenously or by scavenging superoxide radicals before the administration of ACh.
Fig. 8.
Effect of acute preincubation with CGP-42112, tempol, or DETA-NONOate on ACh-induced responses suppressed by a HS diet: diameter changes to increasing concentrations of ACh (10−10–10−5 M) in norepinephrine (4 μM)-precontracted mesenteric arteries from Sprague-Dawley rats fed a HS diet. Arteries were pretreated for 30 min with 10−6 M CGP-42112, tempol, or DETA-NONOate. n = 8 rats/group. Symbols indicate P < 0.05 vs. precontracted diameters of vessels preincubated with CGP-42112 (*), tempol (†), or DETA-NONOate (‡) in the superfusate.
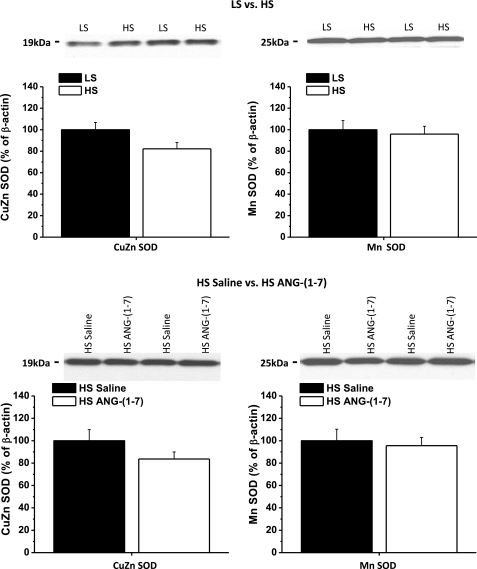
Effect of a HS diet and ANG-(1–7) infusion on Cu/Zn SOD and Mn SOD protein expression.
Figure 9 shows the effect of a HS diet and ANG-(1–7) infusion on the expression of Cu/Zn SOD and Mn SOD in mesenteric resistance arteries. The HS diet tended to reduce the expression of Cu/Zn SOD, although this was not significant (P = 0.07) in the present study. Mn SOD protein expression was unaffected by the HS diet. ANG-(1–7) infusion, which restores vasodilator responses in HS diet-fed animals, did not affect the expression of either of the SOD isoforms, indicating that chronic mas/AT2 receptor activation is not accompanied by upregulation of these two antioxidant enzymes.
Fig. 9.
Effect of a HS diet and ANG-(1–7) infusion on the expression of Cu/Zn SOD and Mn SOD: expression of Cu/Zn SOD and Mn SOD protein in mesenteric arteries of Sprague-Dawley rats and the effect of a HS diet (n = 7) and ANG-(1–7) (289.2 nM·kg−1·h−1, n = 8) infusion, as evaluated by Western blot analysis. Results were normalized to either LS diet-fed (n = 7) or HS diet-fed saline-infused (n = 8) controls (100%). Representative blots are shown as insets.
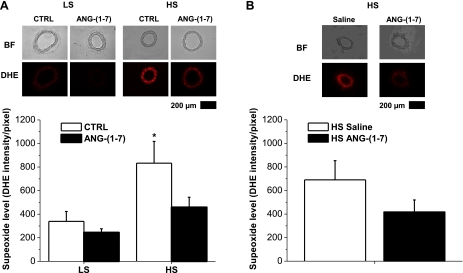
Effect of a HS diet, acute ANG-(1–7) pretreatment, and chronic ANG-(1–7) infusion on DHE fluorescence.
The HS diet significantly increased DHE fluorescence as an indicator of vascular superoxide levels compared with LS diet-fed controls (Fig. 10A). Acute incubation with ANG-(1–7) caused a significant reduction of oxidative stress in arteries of HS diet-fed rats compared with changes in arteries of the LS diet-fed group (Fig. 10A). Chronic ANG-(1–7) infusion (Fig. 10B) also appeared to ameliorate oxidant stress (∼40% decrease of the DHE signal) in arteries of HS diet-fed rats, although, in contrast to an earlier study (11), this difference was not significant in the present experiments.
Fig. 10.
Effect of a HS diet, acute ANG-(1–7) preincubation, and chronic ANG-(1–7) infusion on dihydroethidium (DHE) fluorescence: DHE fluorescence levels in mesenteric arteries from Sprague-Dawley rats fed either a LS diet (n = 7) or HS diet (n = 7) with or without (CTRL) 10−6 M ANG-(1–7) preincubation (A) and HS diet with chronic intravenous infusion of saline (n = 7) or 289.2 nM·kg−1·h−1 ANG-(1–7) (n = 6; B). Representative images are shown as bright field (BF) or DHE for each experimental group. *Significant difference (P < 0.05) from LS diet-fed control rats.
DISCUSSION
A long-term clinical study (51) has shown that salt-sensitive individuals are not only more prone to develop hypertension than salt-resistant persons but also have a significantly higher mortality rate, even if they remain normotensive. In a recent review, Widlansky et al. (52) noted that the presence of endothelial dysfunction in peripheral arteries is a powerful predictor of adverse cardiovascular events in humans, such as myocardial infarction, stroke, and death. Consistent with multiple reports of the deleterious effects of elevated dietary salt intake in human (3, 21, 31) and animal studies (11, 25, 27, 28, 33, 49, 57), Tzemos et al. (47) recently reported that elevated dietary salt intake also leads to impaired endothelial function in healthy young normotensive humans.
In recent years, interest has been growing in the role of the small peptide ANG-(1–7) as a counterregulatory mechanism to offset the deleterious effects of supraphysiological levels of the vasoconstrictor and pro-oxidant peptide ANG II (13, 20). Sampaio et al. (39) showed that ANG-(1–7) activates endothelial NO synthase and releases NO in human aortic endothelial cells having constitutive mas receptor expression, and Faria-Silva et al. (12) showed that mas receptor stimulation with either ANG-(1–7) or AVE-0991 improves endothelial function and potentiates the hypotensive effect of ACh by stimulating NO release in normotensive Wistar rats. These findings suggest that mas receptor agonists like AVE-0991 (42) or oral formulations of ANG-(1–7) [e.g., hydroxypropyl-β-cyclodextrin (HPβCD)/ANG-(1–7)] (29) may have therapeutic benefits in pathological conditions characterized by endothelial dysfunction and emphasizes the importance of elucidating the actions of mas receptor activation in the presence of abnormal endothelial function.
In normotensive Wistar rats, acute infusion of ANG-(1–7) elevates cardiac output, reduces total peripheral resistance, and decreases regional vascular resistance, including that in the hemodynamically important mesenteric vascular bed (38). However, the effects of dietary salt intake on vascular control by ANG-(1–7) are unclear, particularly at the level of the resistance arteries. For example, in hypertensive models, Iyer et al. (20) reported that infusion of either the mas receptor antagonist A-779 or neutralizing antibodies to ANG-(1–7) caused a large increase in blood pressure in salt-depleted spontaneously hypertensive rats and (mRen-2)27 renin transgenic rats (when plasma ANG II levels are high) but not in animals maintained on a normal salt diet. In contrast to those findings, Roks et al. (35) reported radically different effects of ex vivo ANG-(1–7) administration on maximal ANG II-induced vasoconstriction in rat aortic rings in the presence of NO inhibition. In that study, ANG-(1–7) reduced maximum ANG II-induced vasoconstriction in aortic rings of male Wistar rats fed a normal or HS diet, but this noncompetitive antagonism of ANG-(1–7) was absent when the animals were fed a LS diet. Comparison of the disparate findings of Iyer et al. (20) and Roks et al. (35) emphasizes the importance of clarifying the vascular effects of ANG-(1–7) and mas receptor agonists during elevated dietary salt intake, especially in light of the salt-induced vascular dysfunction that occurs in normotensive rats (11, 25, 27, 28, 49, 57), normotensive mice (33), healthy human volunteers (48) and in various salt-sensitive and salt-insensitive forms of hypertension.
Recent studies have shown that a HS not only suppresses plasma ANG II levels (16, 56) but also eliminates or dramatically reduces vascular relaxation in response to ACh and many other vasodilator stimuli in multiple vessel types from different species without an increase in blood pressure (25, 27, 33, 49, 50, 57). This impairment of vascular relaxation is accompanied by increased oxidative stress (24, 25, 57, 58), reduced NO release (24, 56–58), and impaired endothelial Ca2+ signaling (56).
In the present study, we found that mas receptor activation in response to either chronic ANG-(1–7) infusion, chronic oral administration of AVE-0991, or acute incubation with ANG-(1–7) or AVE-0991 restored the severely impaired vascular relaxation in response to ACh and histamine in small mesenteric arteries of normotensive Sprague-Dawley rats fed a HS diet. These results are consistent with the earlier reports of Sampaio et al. (39), Faria Silva et al. (12), and Xu et al. (54) indicating that mas receptor activation improves endothelial cell function. Taken together, these findings suggest that mas receptor activation may be beneficial under conditions of impaired endothelial function, such as those occurring with elevated dietary salt intake (25, 27, 28, 33, 49, 50, 56, 57). An important implication of our findings is that the potential beneficial effects of ANG-(1–7) and therapeutic drugs that activate the mas receptor [e.g., AVE-0991 or HPβCD/ANG-(1–7)] do not depend on elevated ANG II levels but exert other protective effects to improve vascular function.
In these experiments, the significantly higher oxidative stress level in mesenteric arteries of rats fed a HS diet was reduced by ∼40% by chronic systemic infusion of ANG-(1–7) and by ∼45% by acute incubation of arteries from salt-fed animals with ANG-(1–7). In addition to reducing vascular superoxide levels, both these treatments restored NO-mediated dilatations to ACh and histamine. These findings are consistent with the results of a previous study (11) showing that chronic ANG-(1–7) infusion causes a significant reduction in superoxide levels and restores vasodilator responses to ACh in middle cerebral arteries of normotensive Sprague Dawley rats fed a HS diet. As such, the results of this study indicate that the beneficial effect of chronic mas receptor activation may be attributed, in part or as a whole, to a reduction in oxidant stress in the vessel wall, which would also increase NO availability.
The exact mechanisms by which acute and chronic administration of ANG-(1–7) lead to reductions in superoxide levels in mesenteric arteries of HS diet-fed rats remain to be determined. The ability of acute exposure to ANG-(1–7) and AVE-0991 [in addition to chronic infusion of ANG-(1–7)] to reduce vascular superoxide levels and our Western blots of Cu/Zn SOD and MnSOD in HS diet-fed rats receiving ANG-(1–7) infusion indicate that the antioxidant effect of mas receptor activation does not depend on the upregulation of antioxidant enzyme expression at the protein level. This is contrary to the protective effects of chronic low-dose ANG II infusion to prevent the downregulation of Cu/Zn SOD in cerebral arteries of HS diet-fed rats (30). Instead, the findings of the present study suggest that the protective effect of ANG-(1–7) to restore vascular relaxation and reduce superoxide levels in rats fed a HS diet may be due to a number of factors, including the liberation of NO (18, 53) in amounts sufficient to quench superoxide radicals, reductions in NADPH oxidase activity (5, 9), and/or acute increases in the activity of antioxidant enzymes such as SOD (54).
As noted above, ANG-(1–7) has been proposed as an important buffer of the cardiovascular effects of ANG II (13, 20, 38). Various studies in the literature have reported that the effects of ANG-(1–7) or AVE-0991 on different physiological systems are mediated either via its unique (mas) receptor alone (6, 26, 34, 46) or involve AT1 (8, 18, 23, 32, 34, 48, 53) and/or AT2 (8, 18, 34, 35, 48, 53) receptors acting in conjunction with the mas receptor. Therefore, an important question is whether the protective action of ANG-(1–7) and AVE-0991 to restore endothelium-dependent vasodilation in salt-fed animals is unique to the mas receptor or whether other ANG II receptors may contribute to the protective effects of ANG-(1–7) and preincubation with AVE-0991 to restore vascular relaxation in salt-fed rats. In the present study, we found that blockade of either the mas receptor (with A-779) or the AT2 receptor (with PD-123319) dramatically reduced the ability of both acute and chronic ANG-(1–7) to restore vascular relaxation to ACh in salt-fed rats. This observation is consistent with the results of existing studies (8, 34, 35, 48) indicating that the AT2 receptor plays a role in the response of some physiological systems, including NO release (18, 53), to mas receptor activation by ANG-(1–7).
A number of studies (11, 28, 30, 49, 50, 56, 57) have shown that the detrimental effects of a HS diet on vascular function can be prevented by chronic intravenous infusion of a subpressor dose of ANG II and that the protective effect of low-dose ANG II infusion is mediated via activation of the AT1 receptor (11, 50). An important finding in the present study is that the protective effect of ANG-(1–7) to restore endothelium-dependent vascular relaxation to ACh in salt-fed rats is unaffected by AT1 receptor blockade with losartan and is therefore not mediated by the AT1 receptor for ANG II. Our finding that AT2 receptor blockade with PD-123319 blocks the restoration of vascular relaxation by ANG-(1–7) infusion also contrasts with the results of an earlier study (50) showing that the protective effect of low-dose ANG II infusion to restore vascular relaxation in skeletal muscle resistance arteries of Sprague-Dawley rats is unaffected by AT2 receptor blockade with PD-123319. Thus, the mechanism of action of ANG-(1–7) to restore vasodilatation is independent of the AT1 receptor and is distinct from the protective effect of low-dose ANG II infusion to restore vascular relaxation in salt-fed animals in an AT1 receptor-sensitive fashion (50, 56). Overall, these observations indicate that the ability of ANG-(1–7) to restore vascular relaxation in salt-fed rats is a therapeutic effect to offset the impaired vascular relaxation caused by increased oxidant stress, reduced NO availability, and downregulation of antioxidant defense mechanisms (11, 24, 25, 27, 30, 33, 49, 50, 56–58) that arise from chronic exposure to low levels of circulating ANG II in the plasma. In contrast, the protective effect of low-dose ANG II infusion to restore vascular relaxation and endothelial function in HS diet-fed rats is mediated by offsetting the physiological response of salt-induced ANG II suppression (28, 49, 50, 58).
The precise mechanism by which the AT2 receptor contributes to the actions of ANG-(1–7) to alleviate endothelial dysfunction in salt-fed rats remains to be determined. Rowe and colleagues (36) reported that, in the brain, ANG-(1–7) binds AT1 receptors ∼150-fold less effectively and AT2 receptors 6,000-fold less effectively than ANG II. The AT2 antagonist PD-123319 is unable to displace ANG-(1–7) binding to the mas receptor in cells transfected with the mas receptor, showing that there is little or no recognition of PD-123319 by the mas receptor (43). Another study (41) has shown that A-779 has minimal effects on ANG II binding and signaling through either AT1 or AT2 receptors. The high specificity of the receptor antagonists for their individual receptors and the low binding affinity of ANG-(1–7) for either the AT1 or AT2 receptor suggest that there is receptor cross talk or dimerization between mas and AT2 receptors. The latter hypothesis is supported by a number of studies in the literature (7, 8, 22, 34) suggesting that the mas receptor either forms functional dimers with ANG II receptors or that there are functional interactions such as receptor cross talk or a permissive role for AT1 and/or AT2 receptors at downstream points in the mas receptor pathway.
The AT2 receptor has been reported to have antioxidant functions in obese Zucker rats (37), and studies in the literature (1, 2, 4, 15, 17, 19, 44, 55) have shown that AT2 receptor activation increases NO and cGMP levels. These findings suggest that the beneficial effect of ANG-(1–7) to restore vascular relaxation via the AT2 receptor may be related to the ability of this interaction to increase NO and cGMP levels in the cells. This hypothesis is supported by our observation that acute preincubation of the arteries with either CGP-42112 or DETA-NONOate or chronic infusion of CGP-42112 restored vascular relaxation in response to ACh in arteries of HS diet-fed rats.
Perspectives.
The present study shows that, in addition to its direct vasodilator effect in different vascular beds, ANG-(1–7) has another beneficial action, namely, to reverse the deleterious effects of a HS diet on vascular relaxation by improving endothelial function. The ability of ANG-(1–7) to ameliorate salt-induced endothelial dysfunction was mimicked by the nonpeptide mas receptor agonist AVE-0991 and appears to be mediated via the combined actions of the mas receptor and AT2 receptor.
ANG-(1–7) not only dilates blood vessels (13, 32) as a part of the depressor arm of the renin-angiotensin system but, according to the novel findings in the present study, has another beneficial action, namely, to reverse the negative consequences of a HS diet on vascular relaxation by restoring NO-dependent vasodilatation and reducing vascular oxidant stress. Another important implication of the present study, which is worthy of further investigation, is that a HS diet may contribute to salt-induced endothelial dysfunction via suppression of ANG-(1–7) levels, in addition to salt-induced ANG II suppression (28, 49, 50).
The ability of mas receptor activation to restore endothelium-dependent vasodilatation reported in the present study is especially significant in light of the ability of a HS diet to abrogate vessel responses to a striking variety of vasodilator stimuli, including ACh and histamine (Figs. 1 and 2), the stable prostacyclin analog iloprost, reduced Po2, and cholera toxin (27, 28, 49, 50, 56–58), and growing evidence that an elevated dietary salt intake can lead to endothelial dysfunction in normotensive humans (10, 47). The ability of ANG-(1–7) and AVE-0991 to restore endothelium-dependent vascular relaxation in HS diet-fed rats suggests that cardiovascular drugs that activate the mas receptor for ANG-(1–7) could be of substantial benefit in alleviating the endothelial dysfunction that arises from elevated dietary salt intake, especially in salt-sensitive form of hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65289, HL-72920, and HL-092026 and by American Heart Association Midwest Affiliate Postdoctoral Grant 0920116G (to G. Raffai) and Predoctoral Grant 0815532G (M. J. Durand) Fellowships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Sarah Balus, Lynn Dondlinger, and Katherine Fredrich for the surgical expertise used to chronically instrument the animals and for running Western blot assays in this study.
REFERENCES
- 1. Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension 42: 600–604, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Andresen BT, Shome K, Jackson EK, Romero GG. AT2 receptors cross talk with AT1 receptors through a nitric oxide- and RhoA-dependent mechanism resulting in decreased phospholipase D activity. Am J Physiol Renal Physiol 288: F763–F770, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Aviv A. Salt and hypertension: the debate that begs the bigger question. Arch Intern Med 161: 507–510, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Baranov D, Armstead WM. Nitric oxide contributes to AT2 but not AT1 angiotensin II receptor-mediated vasodilatation of porcine pial arteries and arterioles. Eur J Pharmacol 525: 112–116, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Brosnihan KB, Li P, Tallant EA, Ferrario CM. Angiotensin-(1–7): a novel vasodilator of the coronary circulation. Biol Res 31: 227–234, 1998 [PubMed] [Google Scholar]
- 7. Canals M, Jenkins L, Kellett E, Milligan G. Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem 281: 16757–16767, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1–7) prevents diabetes-induced attenuation in PPAR-γ and catalase activities. Eur J Pharmacol 638: 108–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1–7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 299: H1024–H1033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1–7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension 46: 948–952, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced Po2. Am J Physiol Heart Circ Physiol 267: H706–H715, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension 31: 349–355, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1317–R1323, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 290: F1430–F1436, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 37: 72–76, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hiyoshi H, Yayama K, Takano M, Okamoto H. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension 45: 967–973, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Iyer SN, Averill DB, Chappell MC, Yamada K, Allred AJ, Ferrario CM. Contribution of angiotensin-(1–7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension 36: 417–422, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis 49: 59–75, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lara LS, Correa JS, Lavelle AB, Lopes AG, Caruso-Neves C. The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cβ-protein kinase C pathway is involved in activation of proximal tubule Na+-ATPase activity by angiotensin(1–7) in pig kidneys. Exp Physiol 93: 639–647, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H395–H402, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension 29: 394–400, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension 33: 686–688, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Marques FD, Ferreira AJ, Sinisterra RD, Jacoby BA, Sousa FB, Caliari MV, Silva GA, Melo MB, Nadu AP, Souza LE, Irigoyen MC, Almeida AP, Santos RA. An oral formulation of angiotensin-(1–7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension 57: 477–483, 2011 [DOI] [PubMed] [Google Scholar]
- 30. McEwen ST, Schmidt JR, Somberg L, de la Cruz L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 16: 220–234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke 35: 1543–1547, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB. Characterization of angiotensin-(1–7) receptor subtype in mesenteric arteries. Peptides 24: 455–462, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M, Bleich M, Santos RA. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension 44: 490–496, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Roks AJ, Nijholt J, van Buiten A, van Gilst WH, de Zeeuw D, Henning RH. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1–7) on angiotensin II. J Hypertens 22: 2355–2361, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Rowe BP, Saylor DL, Speth RC, Absher DR. Angiotensin-(1–7) binding at angiotensin II receptors in the rat brain. Regul Pept 56: 139–146, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol 300: F700–F706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol 284: H1985–H1994, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Sampaio WO, Souza dos Santos RA, Faria-Silva R, Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen JC. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen JC. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Santos RA, Ferreira AJ. Pharmacological effects of AVE 0991, a nonpeptide angiotensin-(1–7) receptor agonist. Cardiovasc Drug Rev 24: 239–246, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33: 1237–1242, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283: H353–H363, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Tirapelli CR, Fukada SY, de Godoy MA, de Oliveira AM. Analysis of the mechanisms underlying the vasorelaxant action of angiotensin II in the isolated rat carotid. Life Sci 78: 2676–2682, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960–966, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol Heart Circ Physiol 278: H500–H506, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 280: H2196–H2202, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1–7) on the endothelium. Hypertension 40: 847–852, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension 51: 574–580, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Yayama K, Hiyoshi H, Imazu D, Okamoto H. Angiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptor. Hypertension 48: 958–964, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 291: H929–H938, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 286: H575–H583, 2004 [DOI] [PubMed] [Google Scholar]