Abstract
ATP is thought to be released to the extracellular compartment by neurons and astrocytes during neural activation. We examined whether ATP exerts its effect of promoting pial arteriolar dilation (PAD) directly or upon conversion (via ecto-nucleotidase action) to AMP and adenosine. Blockade of extracellular direct ATP to AMP conversion, with ARL-67156, significantly reduced sciatic nerve stimulation-evoked PADs by 68%. We then monitored PADs during suffusions of ATP, ADP, AMP, and adenosine in the presence and absence of the following: 1) the ecto-5′-nucleotidase inhibitor α,β-methylene adenosine 5′-diphosphate (AOPCP), 2) the A2 receptor blocker ZM 241385, 3) the ADP P2Y1 receptor antagonist MRS 2179, and 4) ARL-67156. Vasodilations induced by 1 and 10 μM, but not 100 μM, ATP were markedly attenuated by ZM 241385, AOPCP, and ARL-67156. Substantial loss of reactivity to 100 μM ATP required coapplications of ZM 241385 and MRS 2179. Dilations induced by ADP were blocked by MRS 2179 but were not affected by either ZM 241385 or AOPCP. AMP-elicited dilation was partially inhibited by AOPCP and completely abolished by ZM 241385. Collectively, these and previous results indicate that extracellular ATP-derived adenosine and AMP, via A2 receptors, play key roles in neural activation-evoked PAD. However, at high extracellular ATP levels, some conversion to ADP may occur and contribute to PAD through P2Y1 activation.
Keywords: adenosine, astrocytes, neurovascular coupling, neurovascular unit, pial vessels
although atp is primarily considered to be a source of metabolic energy, accumulating evidence indicates that this intracellular molecule, once released from neurons and astrocytes, plays an important role in cell-to-cell communication. In the brain, ATP participates in a wide variety of nonenergetic physiological signaling processes, such as neurovascular coupling (35, 50), synaptic transmission (37), synaptic plasticity (46), and neuromodulation (36). Increased synaptic activity is often accompanied by both elevated ATP levels in extracellular fluid (ECF) and a rise in Ca2+ in neighboring astrocytes (45). The increased ATP may initially arise from, for example, a presynaptic Ca2+-dependent vesicular release process. That ATP, in turn, can interact with metabotropic purinergic P2Y receptors on nearby astrocytes leading to increased intracellular IP3 and release of Ca2+ from intracellular stores. The elevated Ca2+ can promote cellular efflux of ATP via a number of mechanisms, including vesicular (2) and/or hemichannel-linked processes (11, 13, 22, 24). That signaling cascade may then be further propagated over multiple astrocytes through ATP interactions with P2Y receptors. Communication among astrocytes may also involve other routes, including gap junctions (14).
In addition to its intercellular signaling and neuromodulatory functions, cerebral extracellular ATP can be rapidly hydrolyzed to adenosine through the coupled actions of a number of ecto-nucleotidases located on the surfaces of multiple cells, including astrocytes, neurons, vascular cells, and microglia (54). The relative influence of the different ecto-nucleotidases may vary according to their cellular distribution and the concentration of ATP achieved in the extracellular milieu (54). Ecto-nucleotidases exhibit a variety of forms and functions. Some show distinct apyrase (diphosphohydrolase) roles, whereby ATP is rapidly converted into AMP. Representing this function are ecto-nucleotide pyrophosphatase/phosphodiesterase (e-NPP; particularly e-NPP-1) and ecto-nucleoside triphosphate diphosphohydrolase-1 (e-NTPDase-1). Other ecto-nucleotidases display mainly monohydrolase actions, as exemplified by e-NTPDase-2 [principally ecto-ATPase functions; i.e., ATP to ADP conversion (25)] and ecto-5′-nucleotidase, which mediates AMP to adenosine conversion. The ecto-nucleotidases listed above also display different cellular distributions, with e-NTPDase-2 and ecto-5′-nucleotidase well expressed on astrocytes (48, 53, 54). Furthermore, ecto-5′-nucleotidase is particularly enriched in the leptomeninges (33). On the other hand, e-NPP-1 and e-NTPDase-1 tend to favor vascular elements over glial and neuronal elements (54), whereas vascular expression of ecto-5′-nucleotidase may be limited (18).
Previous findings from our laboratory (49) and others (26, 47) have revealed that somatosensory activation [i.e., sciatic nerve stimulation (SNS)] is associated with a substantial dilation of pial arterioles overlying the hindlimb region of the somatosensory cerebral cortex. That pial arteriolar response occurs despite few, if any, direct contacts with intrinsic neurons. Additional findings indicated that this SNS-evoked vasodilation relied on astrocytic communication involving the glia limitans (49) and was adenosine dependent (27, 35). Further support for the latter can be found in a preliminary report, which provided evidence hinting that ATP hydrolysis products, especially adenosine, play a key role in neurovascular coupling (50). However, others have reported that nonhydrolyzed ATP is capable of eliciting an endothelium-dependent, P2Y receptor-related vasodilation, albeit in isolated cerebral penetrating arterioles (4, 10). Moreover, ADP, which may be formed in association with e-NTPDase-2 actions on ATP, also displays a strong vasodilating influence on cerebral vessels. Thus topical application of ADP promotes pial arteriolar dilation via interactions with P2Y1 receptors that appear to reside both on the glia limitans and pial arteriolar endothelium (52).
In the present study, we first examined whether the adenosine-dependence of SNS-evoked pial arteriolar dilation (26, 35) derives from ATP ecto-hydrolysis. That hydrolytic function was tested using a selective ecto-diphosphohydrolase (i.e., e-NTPDase-1 and e-NPP-1) blocker, ARL-67156. This approach revealed a significant attenuation in the SNS-induced response. Based on this finding, together with evidence supporting key roles for adenosine (26, 35) and the glia limitans (49) [but not ADP (50)] in SNS-evoked pial arteriolar dilations, the following scenario was suggested. Neuronal activation may trigger an interastrocytic signaling process, ultimately resulting in ATP release from the glia limitans into the extracellular compartment (39), and, subsequently, AMP is quickly formed. Then, due to ecto-5′-nucleotidase abundance at the brain surface (33), a rapid generation of adenosine can be anticipated. Therefore, to obtain further evidence in support of the hypothesis that vasodilations arising from ATP hydrolysis products, rather than ATP itself, mediate pial arteriolar dilations during SNS, we used topically applied ATP to mimic the postulated ATP released from the glia limitans. To that end, exogenous ATP and its hydrolytic products (i.e., ADP, AMP, and adenosine) were applied topically, over wide concentration ranges, in the presence and absence of pharmacological blockers of P2Y1 receptors, ecto-diphosphohydrolase activity, adenosine receptors, and ecto-5′-nucleotidase activity.
MATERIALS AND METHODS
Animals.
The study protocol was approved by the Institutional Animal Care and Use Committee. Female Sprague-Dawley rats (250–300 g) were used. After anesthesia induction with isoflurane, rats were intubated and mechanically ventilated. Surgery was performed under continuous anesthesia with 2.0% isoflurane-70% N2O-30% O2. Bilateral femoral arterial and venous catheters were placed for systemic arterial pressure monitoring, arterial blood gas/pH measurement, and drug infusions. Rats were then prepared for placement of a closed cranial window, according to established procedures (52). In brief, a craniotomy (10 mm in diameter) was performed over the midline of the skull, and the underlying dura was carefully removed, keeping the sagittal sinus intact. A cranial window (11 mm diameter) equipped with three ports (inflow, outflow, and intracranial pressure monitoring) was fixed to the skull with cyanoacrylate gel. Isoflurane was discontinued, and a 10 μg/kg fentanyl bolus was given intravenously. The rat was maintained on 70% N2O-30% O2 and fentanyl (25 μg·kg−1·h−1 iv) thereafter. This is an established, validated procedure that ensures adequate analgesia (5, 7, 30). D-tubocurarine was injected to produce muscle paralysis. The space under the window was filled with artificial cerebrospinal fluid (aCSF) that was equilibrated with 10% O2-5% CO2-85% N2. The aCSF solution was suffused at 0.5 ml/min and maintained at a temperature of 37°C. Body temperature was maintained at 37°C with a servocontrol heating pad, and mean arterial blood pressure and intracranial pressure were monitored continuously during the experiments. Arterial blood samples were taken at 60-min intervals for PaO2, PaCO2, and pH analysis using a blood gas/pH analyzer (Gem3000; Instrumentation Laboratories, Lexington, MA). PaO2, PaCO2, and pH were maintained within physiological ranges throughout the study. Pial arterioles were observed using a Nikon microscope equipped with an epi-illumination darkfield system. Images were captured using a digital video camera (CoolSNAP ES; Photometrics, Tucson, AZ), projected on a computer monitor, and stored on a personal computer for later diameter measurements using the MetaMorph software system (Molecular Devices, Downingtown, PA). Vascular reactivity was assessed by measuring the diameters of pial arterioles, with baseline diameters ranging from 30 to 50 μm. Diameter values from three arteriolar segments were obtained and averaged. In all experiments, initial diameter measurements were made at least 1 h post-isoflurane and after 40 min of drug-free aCSF suffusion. Hypercapnia (Pco2 ≈ 70 mmHg) was imposed, over a 3-min period, to test pial arteriolar reactivity. Only those vessels displaying an adequate response to CO2 (reactivity >1.0% diameter increase/mmHg PaCO2 change) were selected for this study. However, only a handful of the rats studied (<2%) failed to achieve this standard.
Group protocols.
The rats were organized into five experimental groups. In the first group, the rats were subjected to somatosensory activation-evoked pial arteriolar dilations. Two subgroups were studied. In both subgroups, drug-free aCSF was suffused under the cranial window for 45 min. This was followed by a 20-s stimulation of the sciatic nerve and measurement of the peak diameter increases in pial arterioles overlying the hindlimb region of the contralateral somatosensory cortex (35, 49). Starting at 20 s before SNS and continuing for 60 s post-SNS, pial arterioles were monitored and the recordings saved for subsequent analysis of time-related diameter changes. In the first subgroup, at a time point when pial arteriolar diameters had returned to their baseline values, following the initial SNS, we initiated a suffusion of the ecto-pyrophosphatase/diphosphohydrolase inhibitor ARL-67156. Thirty minutes later, a second SNS was imposed. The concentration of ARL-67156 used in these experiments was established in pilot experiments, with guidance from published findings. According to the literature, at 50–100 μM, this agent preferentially blocks the conversion of ATP to AMP, via inhibiting e-NTPDase-1 and e-NPP-1 (Ki = 27 μM) but is without effect on the ATP to ADP converting enzyme e-NTPDase-2 (Ki = 1 mM) (8, 12, 21, 29). In preliminary experiments, we tested SNS-evoked pial arteriolar responses in the presence of 0, 10, 50, and 100 μM ARL-67156. Only the 50 and 100 μM doses were associated with an attenuated SNS response (30% and 70% reductions, respectively). Thus, for all subsequent experiments, we used 100 μM ARL-67156. This rather cautious approach was used, since higher ARL-67156 doses might have affected e-NTPDase-2. As such, we concede the possibility that diphosphohydrolase contributions may have been underestimated. For analysis of the saved recordings, the pre- and post-ARL-67156 vascular responses to SNS were each averaged, and the peak responses were used as a measure of ARL-67156 effects. To ensure that ARL-67156 applications were not affecting the level of increased neural activity during SNS, we also monitored cortical somatosensory evoked potentials (SEPs), according to methods described in a recent publication (35). One additional subgroup was included to serve as a time control and to control for the possibility that brief exposure to hypercapnia may be associated with a prolonged enhancement of the hyperemic/vasodilating response to subsequent episodes of somatosensory activation (42). Thus ∼30 min after an initial measurement of the pial arteriolar response to SNS, the rats were tested for CO2 reactivity (see above). After the hypercapnic challenge, pial arteriolar reactivity to SNS was retested at 30-min intervals out to 2 h.
Groups 2–5 were organized according to the nucleotide or nucleoside suffused: 2) ATP, 3) ADP, 4) AMP, and 5) adenosine. The ATP, ADP, and AMP were applied at concentrations of 1, 10, and 100 μM, while adenosine was suffused at 10 and 100 μM. Dose-related pial arteriolar responses to ATP, ADP, AMP, and adenosine were further evaluated in the presence of topical applications of a number of selective pharmacological agents. These included the ecto-5′-nucleotidase inhibitor α,β-methylene adenosine 5′-diphosphate (AOPCP; 300 μM) (except ADP); the A2 receptor blocker ZM 241385 (10 μM); and the P2Y1 antagonist MRS 2179 (10 μM). In addition, ATP responses were examined in the absence and presence of ARL-67156 (100 μM). In published reports, a wide range of AOPCP doses (ranging from low micromolar up to 1 mM) have been used to block 5′-nucleotidase function in brain tissue preparations (for examples, see Refs. 3, 16, 20, 23, 38, and 41). However, the overwhelming majority of studies favor doses in the 50–400 μM range. In preliminary and published studies, we tested AOPCP doses of 100 and 300 μM in our SNS-evoked pial arteriolar dilation model and found nearly identical ∼60% reductions in pial arteriolar reactivity (35). The similarity in the AOPCP effect at the two different doses suggested that, in our model, 300 μM was close to the maximally effective dose. For ZM 241385, the dose used in the present study was also taken from the results of preliminary and published experiments (35), based upon the approximate minimum dose needed to produce a maximal reduction in the pial arteriolar responses to adenosine (10 and 100 μM). However, it should be noted that to achieve a virtually complete blockade of adenosine-induced vasodilation, a 10 μM dose of ZM 241385 was used. That dose likely exceeds its selectivity toward the A2A over the A2B adenosine receptor subtype, but remains selective toward adenylyl cyclase (AC)-activating A2 receptors in relation to AC-inhibitory A1 and A3 adenosine receptors (17, 19, 34). It is well-established that both the A2A and A2B subtypes promote vasodilation via activation of vascular smooth muscle AC (15). Thus an A2 blocker with actions toward both A2 subtypes, like ZM 241385, became the best choice for achieving complete blockade of adenosine-induced dilations. For MRS 2179, we used a dose that has been shown to block completely ADP-induced pial arteriolar dilations (52).
After the initial measurement of CO2 reactivity, ATP, ADP, AMP, or adenosine was suffused into the space under the cranial window (5 min for each concentration). After 10 min of drug-free aCSF suffusion, the baseline diameter generally recovered. Next, an inhibitor was suffused for 30 min, and the nucleotide/nucleoside was then coapplied at sequentially increasing concentrations. Additionally, following measurement of the effects of MRS 2179 on ATP reactivity, the effects of coapplications of MRS 2179 and ZM 241385 (both at 10 μM) on ATP responses were examined. In the ADP suffusion experiments, a combined blockade of A2 and P2Y1 receptors (i.e., ZM 241385 + MRS 2179) was performed following A2 blockade alone.
In our examination of AMP-induced responses, we took into account published reports suggesting that both adenosine and AMP were capable of activating adenosinergic receptors (28, 40). To that end, we addressed whether all, or only part, of the vasodilation elicited by suffusion of AMP was due to its conversion to adenosine via ecto-5′-nucleotidase. For this, two approaches were used. The first involved monitoring pial arteriolar responses to AMP in the presence of AOPCP or ZM 241385 (see above). The second involved examination of AMP reactivity in the presence of adenosine deaminase (ADA; 2 units/ml), by itself, and, subsequently, in the combined presence of ADA and AOPCP, to remove adenosine, leaving only AMP. This was followed by ADA and AOPCP, combined with ZM 241385. The latter was done to confirm the A2-dependency of AMP-induced dilations. For each of the adenine nucleotides examined in the present study (ATP, ADP, and AMP), time control experiments were performed (n ≥ 2) but indicated no temporal variations in the dose-response characteristics of these agents.
Reagents and statistics.
All reagents, except for ZM 241385 (Tocris, Ellisville, MO), were obtained from Sigma (St. Louis, MO). All chemicals, except ZM 241385 and MRS 2179, were directly diluted with aCSF. ZM 241385 and MRS 2179 were first dissolved in DMSO and then diluted 1:1,000 in aCSF before suffusion. In previous studies, this concentration of DMSO was found to have no effect on pial arteriolar diameters (51). All concentrations were expressed as the final molar concentration suffused under the cranial window. Values are presented as means ± SD. Comparisons of arteriolar diameter values within groups were made using one-way repeated-measures ANOVA, combined with a post hoc Tukey analysis. For intergroup comparisons (e.g., ATP responses in the absence and presence of AOPCP vs. ATP responses in the absence and presence of ZM 241385), a one-way ANOVA was used, along with a post hoc Bonferroni test. A P value <0.05 was considered as significant.
RESULTS
Arterial Po2 values were maintained above 100 mmHg in all rats studied; Pco2, pH, and mean arterial blood pressure remained within normal limits (i.e., 32–40 mmHg, 7.35–7.45, and 110–140 mmHg, respectively) over the course of the experiments. Furthermore, no significant differences were observed when comparing hypercapnia-induced pial arteriolar diameter increases in all experimental groups (data not shown).
Pial arteriolar dilation during SNS: effects of ARL-67156.
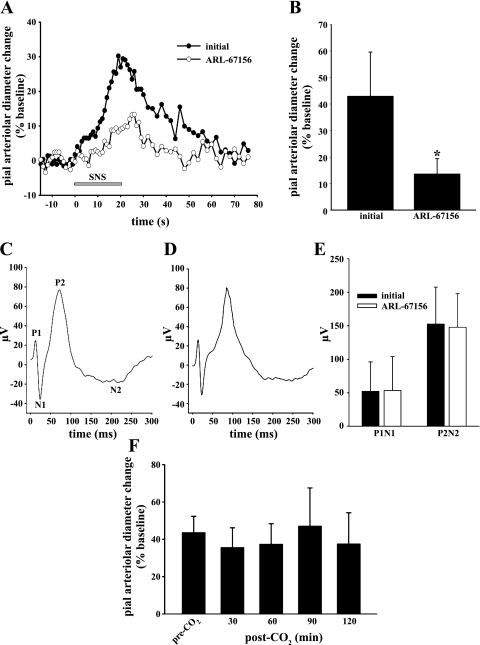
Sciatic nerve stimulation for 20 s elicited pial arteriolar dilations that peaked at 23–30 s following start of stimulation (representative responses in the absence and presence of ARL-67156 depicted in fig. 1A). The initial SNS-evoked increase in pial arteriolar diameter, in the presence of aCSF alone, averaged 42.9% over baseline. In the presence of the ecto-pyrophosphatase/diphosphohydrolase blocker ARL-67156 (100 μM), that response was reduced by 68% (Fig. 1B). The addition of ARL-67156 was found to have no effect on the amplitudes of the SEPs generated during SNS (Fig. 1, C–E). Figure 1F demonstrates the reproducibility of the pial arteriolar response to repeated episodes of SNS. Furthermore, prior exposure to hypercapnia was not associated with any significant changes in the pial arteriolar responses to subsequent sciatic nerve stimulations, applied every 30 min over 2 h (Fig. 1F).
Fig. 1.
Sciatic nerve stimulation (SNS)-related data. A: representative time course of changes in pial arteriolar diameters associated with a 20-s SNS (gray bar). Each curve represents the average response measured in 2 animals, first in the absence (initial; ●) and, subsequently, in the presence of the ecto-pyrophosphatase/diphosphohydrolase blocker ARL-67156 (100 μM; ○). B: effect of ARL-67156 (100 μM) on SNS-evoked pial arteriolar dilations. Values are means ± SD. *P < 0.05 vs. initial; n = 5. Representative SNS-generated somatosensory-evoked potentials (SEPs) recorded from the contralateral cortical surface before (C) and after (D) ARL-67156 application are also shown. E: note that no differences in the SEP peak-to-peak amplitudes were observed, irrespective of whether the P1N1 or the P2N2 amplitude was measured. F: SNS-evoked pial arteriolar diameter percent increases from baseline measured before a CO2 challenge (Pco2, ∼70 mmHg for 3 min) and at 30-min intervals over the 2 h following CO2 exposure. This time control group displayed a remarkably consistent pial arteriolar response to SNS when comparing SNS-evoked pial arteriolar reactivities measured pre- and posthypercapnia. Results in E and F are means ± SD; n = 5.
Effects of ecto-5′-nucleotidase, A2 receptor, P2Y1 receptor, and ecto-diphosphohydrolase blockade on ATP-induced pial arteriolar dilations.
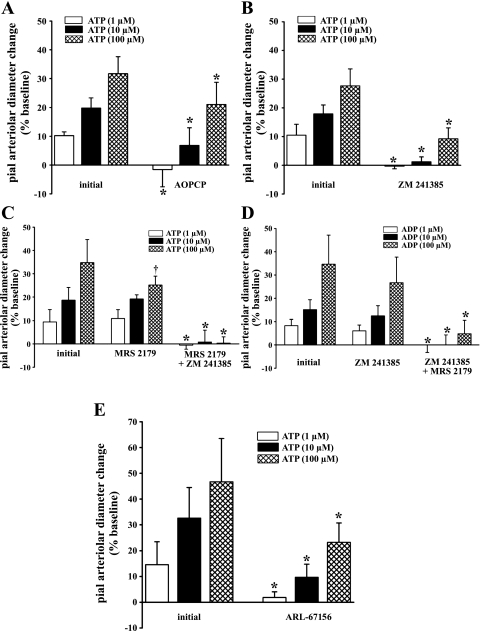
The suffusion of ATP (1, 10, and 100 μM) was accompanied by a substantial dose-dependent pial arteriolar dilation (Fig. 2, A–C; Fig. 2E). Topical application of either the ecto-5′-nucleotidase inhibitor AOPCP (300 μM) or A2 blocker ZM 241385 (10 μM) resulted in a complete loss of pial arteriolar responses to 1 μM ATP and 76% and 94% reductions, respectively, in the pial arteriolar dilations associated with 10 μM ATP (Fig. 2, A and B). At 100 μM ATP, however, a more modest attenuation of the pial arteriolar response was observed, in the presence of AOPCP (34% decrease; Fig. 2A) or ZM 241385 (66% decrease; Fig. 2B). The AOPCP-related reduction in the response to 100 μM ATP was significantly less than the reduction seen in the presence of ZM 241385. Suffusion of the P2Y1 (ADP) antagonist MRS 2179 (10 μM) was associated with a significant 30% decrease in pial arteriolar reactivity to 100 μM ATP but did not affect pial arteriolar dilation induced by ATP at 1 and 10 μM (Fig. 2C). A complete loss of vasodilation to 1, 10, and 100 μM ATP was achieved when combining P2Y1 and A2 receptor blockers (Fig. 2C). These results would appear to indicate that adenosine, derived from ATP ecto-hydrolysis, mediates a large portion of the ATP-induced pial arteriolar relaxation. In particular, with low to moderate extracellular ATP levels (i.e., 1–10 μM), adenosine appears to account for nearly all the ATP vasodilating effect. On the other hand, the incomplete inhibition of the pial arteriolar response to 100 μM ATP, observed in the presence of either ecto-5′-nucleotidase or A2 blockade (Fig. 2, A and B), indicates that another vasodilator, beside adenosine, participates in the ATP response. Supported by the MRS 2179 effects, we suspect that this other vasodilator is ADP. Thus, when extracellular ATP levels are sufficiently elevated, perhaps exceeding the capacity for direct ATP → AMP conversions, we postulate that ADP can accumulate. This may occur through the action of e-NTPDase-2, often labeled as a brain ecto-ATPase (1, 48), in combination with weak ecto-ADPase activity (see Fig. 2D). In addition, a portion of the dilation to ATP could be directly attributed to AMP. This is discussed further below (see discussion). The findings above indicate that most of the pial arteriolar dilating response to 1 and 10 μM ATP is mediated by activation of adenosine A2 receptors. This would seem to indicate the importance of rapid ATP → AMP conversions when ATP is released into the extracellular compartment. Additional confirmation of the key role that ecto-diphosphohydrolase actions play in the dilations evoked by topically applied ATP was obtained in experiments performed in the absence and presence of ARL-67156. Thus pial arteriolar responses to 1 and 10 μM ATP were attenuated by 87% and 70%, respectively, whereas the response to 100 μM ATP was reduced by only 50% (Fig. 2E). These findings are remarkably similar to results observed during A2 receptor blockade (Fig. 2B) and further support the presence, at the cortical surface, of a substantial ecto-nucleotidase activity in the extracellular milieu of pial arterioles and the superficial glia limitans (35, 50).
Fig. 2.
Pial arteriolar responses to the suffusion of 1, 10, and 100 μM ATP in the absence and presence of the ecto-5′-nucleotidase inhibitor α,β-methylene adenosine 5′-diphosphate (AOPCP; 300 μM; A), the A2 receptor blocker ZM 241385 (10 μM; B), the P2Y1 receptor blocker MRS 2179 (10 μM) and MRS 2179 plus the A2 antagonist ZM 241385 (10 μM; C), and the ecto-diphosphohydrolase blocker ARL-67156 (100 μM; E). In D, the dose-related responses to ADP, in the presence or absence of ZM 241385 and ZM 241385 plus MRS 2179, are also shown. Pial arteriolar responses are expressed as the percent change from the baseline diameter value. Values are means ± SD. *P < 0.05 vs. initial; †P < 0.05 vs. AOPCP; n = 5 per group.
Effects of ecto-5′-nucleotidase inhibition and A2 and/or P2Y1 blockade on ADP-induced pial arteriolar dilation.
In accord with multiple published reports (e.g., Ref. 52), topically applied ADP elicited a dose-dependent pial arteriolar dilation. Virtually identical pial arteriolar responses to ADP suffusion were observed in the absence (4.9 ± 2.2% at 1 μM, 12.2 ± 0.7% at 10 μM, and 29.8 ± 1.8% at 100 μM) and the presence (6.2 ± 1.8% at 1 μM, 14.0 ± 1.1% at 10 μM, and 31.5 ± 1.6% at 100 μM) of AOPCP. ZM 241385 suffusion also failed to block dilation induced by ADP (Fig. 2D). However, the pial arteriolar response to ADP was completely eliminated when the P2Y1 blocker MRS 2179 was added (Fig. 2D). These findings confirm previous results from our laboratory indicating that ADP elicits pial arteriolar dilation almost exclusively through P2Y1 activation, without any contributions from adenosine arising from sequential ecto-ADPase and ecto-5′-nucleotidase actions (52).
Effects of ecto-5′-nucleotidase inhibition and A2 blockade on AMP-induced pial arteriolar dilation.
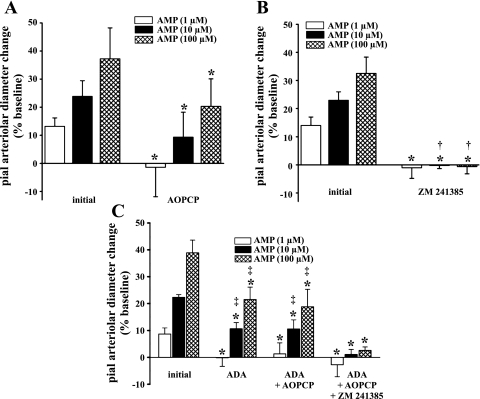
Suffusions of AMP, at 1, 10, and 100 μM, led to dose-dependent pial arteriolar dilations (Fig. 3, A–C). Although the presence of AOPCP was accompanied by significant reductions in AMP-induced responses at all concentrations tested (Fig. 3A), measureable residual responses at higher concentrations of AMP were still observed (65% and 34% reductions at 10 and 100 μM, respectively). On the other hand, the blockade of A2 receptors by ZM 241385 was associated with the complete abolition of AMP-induced pial arteriolar dilations at all AMP concentrations tested (Fig. 3B). To address whether the incomplete loss of AMP-induced vasodilation, associated with AOPCP administration, versus the complete repression seen in the presence of ZM 241385, was due to AMP behaving as an A2 agonist, additional evaluations were conducted. To that end, AMP responses were measured in the presence of adenosine deaminase (ADA), then ADA plus AOPCP (300 μM), to remove all adenosine. The ADA dose used was established in preliminary experiments, where it was found that pial arteriolar dilations elicited by topically applied adenosine (10 and 100 μM) were largely prevented (∼90% reduction, data not shown) in the presence of 2 units/ml ADA. Next, the A2 blocker ZM 241385 (10 μM) was added to the ADA/AOPCP mixture, and AMP responses were once again tested. Pial arteriolar dilations to 10 and 100 μM AMP, in the presence of ADA, were only partially attenuated (by 52% and 45%, respectively) in relation to the initial AMP response (Fig. 3C). When AMP reactivity was tested following the addition of AOPCP to the suffusate containing ADA, no further reductions in AMP reactivity were observed (Fig. 3C). A complete blockade of AMP-induced dilations was achieved only after the addition of ZM 241385 to the previous cocktail (Fig. 3C). These findings strongly suggested the influence of a nonadenosine component in AMP-induced dilations and support the likelihood that AMP, in addition to adenosine, dilates pial arterioles via direct activation of A2 receptors (see Refs. 28 and 40). Another AMP-linked pathway examined by us involved possible ecto-adenylate kinase-mediated generation of ADP. This could occur if AMP levels were elevated in the presence of sufficient ATP (44). Moreover, if one combines AMP with AOPCP application, even greater AMP elevations may be seen, driving the ecto-adenylate kinase reaction toward further ADP formation. However, irrespective of whether AMP was suffused in the absence or presence of AOPCP, no reductions in AMP-induced pial arteriolar dilations were seen during coapplication of MRS 2179, indicating no increased presence of ADP (data not shown).
Fig. 3.
Effect of AOPCP (A) or ZM 241385 (B) on pial arteriolar responses to suffusions of 1, 10, and 100 μM AMP. C: depiction of responses to suffusions of AMP in the absence and presence of adenosine deaminase (ADA; 2 units/ml), ADA + AOPCP (300 μM), and ADA + AOPCP + ZM 241385 (10 μM). Values are means ± SD. *P < 0.05 vs. initial; †P < 0.05 vs. AOPCP; ‡P < 0.05 vs. ADA + AOPCP + ZM 241385; n = 5 per group.
Effects of ecto-5′-nucleotidase inhibition and A2 blockade on adenosine-induced pial arteriolar dilation.
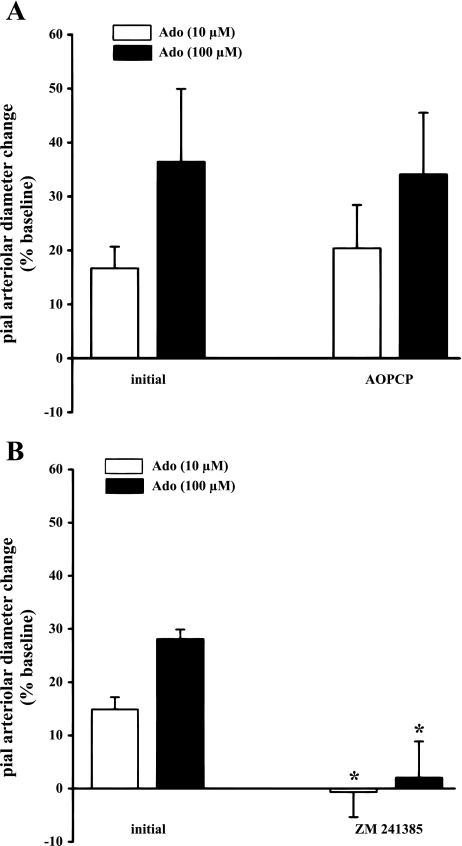
AOPCP lacked any influence on adenosine-elicited pial arteriolar dilation (Fig. 4A). This data further support the selectivity of AOPCP. As expected, A2 blockade by ZM 241385 led to the abolition of adenosine-induced pial arteriolar dilation (Fig. 4B).
Fig. 4.
Effects of AOPCP (300 μM; A) or ZM 241385 (10 μM; B) on pial arteriolar responses induced by suffusions of adenosine (Ado) at 10 and 100 μM. Values are means ± SD. *P < 0.05 vs. initial and AOPCP; n = 5 per group.
DISCUSSION
There were a number of key findings in the present study. First, the ecto-nucleotidase inhibitor ARL-67156, which selectively blocks extracellular ATP to AMP conversions, largely prevented sciatic nerve stimulation-evoked pial arteriolar dilations. This strongly implicated an important role for ATP release, and its extracellular hydrolysis, in the present model of neurovascular coupling. Second, topically applied ATP, which was used to mimic glia limitans ATP release at the brain surface, elicited pial arteriolar dilations that were entirely mediated by products of ATP hydrolysis, rather than ATP itself. Third, ADP contributions to ATP-triggered responses were limited, being evident only at very high ATP levels. Fourth, adenosine, chiefly through interactions with its A2 receptors (32), represented a principal mediator of topical ATP-induced pial arteriolar dilation. Finally, the ATP hydrolysis product AMP also appeared to contribute to ATP-induced vasodilation through adenosine-dependent and -independent actions. The latter effect of AMP likely arose from a direct action toward A2 receptors.
Although the present findings show a key role for AMP/adenosine in ATP-triggered pial arteriolar dilations, it should be emphasized that one cannot generalize these findings to all circumstances involving increased vascular exposure to ATP. Evidence from isolated rat penetrating arterioles indicated that ATP itself can influence cerebral arteriolar tone, through hydrolysis-independent interactions with purinergic receptors residing on smooth muscle and/or endothelium (4, 10). There are any number of factors that could have contributed to the disparate findings when comparing present results with those of Dietrich and coworkers (4, 10). This includes differences related to the arteriolar segment being examined as well as differences associated with the presence or absence of other cellular influences. In the case of the isolated vessel, adenine nucleotide hydrolyzing ecto-enzymes are likely to be less prevalent. This would be particularly true for ecto-nucleotidases that are selectively expressed in astrocytic elements and largely excluded from vascular tissue. One example is ecto-5′-nucleotidase (18, 54). It also merits mention that, in vivo, increased arteriolar exposure to ATP can also occur from the luminal side (6). However, luminal influences were not considered in the present study, since all purine applications were given topically (abluminally). This was done specifically to mimic astrocyte (glia limitans)-derived ATP release at the brain surface, which is thought to be an important component of the astrocyte-dependent dilation of pial arterioles associated with neurovascular coupling in the cerebral cortex (49).
Data obtained in this investigation indicated that hydrolytic products of ATP entirely account for its vasodilating properties during topical applications, with AMP and adenosine predominating. Formation of ADP appeared to contribute very little to that response. Thus only at high micromolar levels of ATP did we observe any attenuation in the vasodilating response to ATP in the presence of the P2Y1 antagonist MRS 2179. However, that effect was modest [∼30% reduction (Fig. 2C)]. Present (Fig. 2D) and previous (52) findings from our laboratory showed that the pial arteriolar vasodilating response to topically applied ADP is completely blocked by MRS 2179 and unaffected by A2 blockade (Fig. 2D). The former appears to be a function of the presence of P2Y1 receptors on the glia limitans and pial arteriolar endothelium (52), whereas the latter may reflect negligible ecto-ADPase activity at the cortical surface. At high micromolar levels of ECF ATP, as might be seen during neural hyperactivity states, like seizure (see ref. 50) or cortical spreading depression (43), hydrolysis of ECF ATP may be influenced by the astrocyte-expressed, e-NTPDase-2 (9, 48, 53, 54), which, given its ecto-ATPase function, may permit some accumulation of ECF ADP. It seems reasonable, therefore, to postulate that ecto-ATPase-derived ADP participation in neurovascular coupling may be limited to circumstances of high neural activity and excessive ATP release.
In subsequent experiments, in addition to further examination of the role of ATP to ADP conversions, we examined the contributions of adenosine, both as a function of ecto-5′-nucleotidase-mediated formation from AMP and as a function of adenosine engagement of its Gs-protein-linked A2 receptors. Pial arteriolar dilations to 1 μM ATP were completely blocked by both the ecto-5′nucleotidase blocker AOPCP and the A2 receptor blocker ZM 241385. At 10 μM ATP, the nearly complete reductions in vasodilating reactivity in the presence of AOPCP and ZM 241385 were not significantly different. These findings are generally consistent with a mechanism whereby moderate elevations in ECF ATP elicit pial arteriolar dilations via ATP conversion to adenosine. However, at the 100 μM ATP concentration, in the presence of either blocker, only partial reductions in pial arteriolar dilations were observed (Fig. 2, A and B), and the effect of ecto-5′-nucleotidase blockade was significantly less than the effect of A2 receptor blockade. The ∼33% residual dilation, in the presence of the A2 blocker ZM 241385, might possibly be linked to ADP. One reason is that the 33% value is similar to the 30% reduction of the 100 μM ATP-induced dilation observed in the presence of the P2Y1 antagonist MRS-2179; more important, that remaining response was eliminated in the presence of a combined P2Y1/A2 blockade (Fig. 2C).
Other factors, besides ADP, must be considered when trying to account for the clear discrepancy between the effects of ecto-5′-nucleotidase versus A2 blockade on the responses to ATP (Fig. 2, A and B). There were a number of strategies used in attempting to resolve this conundrum. The first strategy was to examine whether the discrepancy observed, when comparing AOPCP versus ZM 241385 effects on ATP responses, still occurred when monitoring pial arteriolar responses to topical applications of AMP. In fact, the divergent effects of AOPCP and ZM 241385 were even more pronounced during AMP suffusions (Fig. 3, A and B). Thus we observed that the vascular responses at all concentrations of AMP tested (including 100 μM) were completely blocked by the selective A2 antagonist. However, only a 50–60% blockade was seen at the higher AMP concentrations, when AMP conversion to adenosine was prevented, using AOPCP. This suggests that AMP-induced pial arteriolar dilations entirely involve A2 receptor activation and that adenosine is not the sole ligand interacting with the receptor. In fact, there is evidence in the literature supporting a direct AMP action toward A2 receptors (28, 40). This mechanism was addressed using another strategy, where AMP reactivity was examined in the presence of topical applications of ADA (2 units/ml). As indicated by evidence obtained in pilot studies and published reports, that strategy depletes adenosine, by converting adenosine to the nonvasoactive molecule, inosine (31). Despite the depletion of adenosine, AMP-induced dilations were only attenuated by ∼50% at 10 and 100 μM AMP (Fig. 3C). No added reductions in the AMP response were observed when AOPCP was combined with ADA, further supporting a vasodilating action of AMP that is independent of adenosine formation. The fact that the remaining AMP response could be completely blocked in the presence of ZM 241385 confirmed the A2 dependence of AMP-induced pial arteriolar dilation and that both AMP and adenosine interactions with the A2 receptor were involved. Also, the findings obtained with ADA essentially obviated the possibility that the discrepancy observed when comparing the effects of ecto-5′-nucleotidase versus A2 inhibition relates to incomplete blockade of ecto-5′-nucleotidase by AOPCP. It is unlikely that other AMP-related pathways are involved, namely the adenylate kinase, AMP deaminase, and AMP-activated kinase pathways. One key reason is that there is no adequate scheme involving these pathways that would in any conceivable way lead to adenosine formation. Inasmuch as AMP-induced dilations were completely blocked by A2 inhibition, the only way that the adenylate kinase, AMP deaminase, and AMP-activated kinase pathways could have been involved would have been if products of those reactions were capable of activating A2 receptors. There is no evidence in the literature to support such an effect. One additional concern derives from the possibility that a large molecule, like ADA, may be more diffusion-limited than a smaller molecule, such as AMP, perhaps leading to a scenario where suffused ADA may not gain access to all AMP-responsive ecto-5′-nucleotidase sites. This could result in a less-than-anticipated adenosine depletion. However, ecto-5′-nucleotidase is highly enriched in rat cortical leptomeninges (33). The superficial abundance of a major adenosine-generating enzyme, readily accessible to topically applied ADA, diminishes any concerns related to differential diffusivity.
In conclusion, evidence from previous studies supports a key role for adenosine in the pial arteriolar dilation that accompanies SNS (26, 35). However, the specific source of that adenosine, whether arising from preformed intracellular pools or from extracellular ecto-nucleotidase pathways, has not been specifically determined. The findings in this study strongly suggest that ATP released to the extracellular milieu at the cortical surface, as is likely to occur during somatosensory activation, promotes pial arteriolar dilations that almost entirely involve ATP hydrolysis products. The extracellular origin of the vasoactive products of adenine nucleotide hydrolysis, generated in association with SNS, was established in experiments involving pharmacological blockade of ecto-diphosphohydrolase function. Further evidence was then obtained in experiments using a strategy that mimicked ATP release at the cortical surface-i.e., topical applications of ATP. At low micromolar ATP concentrations, that vasodilation appears to involve rapid ATP conversion to AMP (largely via ecto-pyrophosphatase/diphosphohydrolase actions likely mediated by e-NTPDase-1 and e-NPP-1) and, ultimately, adenosine (through ecto-5′-nucleotidase), with both adenosine and, to a lesser extent, AMP promoting vasodilation via activation of adenosine A2 receptors. At high micromolar extracellular ATP levels, some formation of ADP occurs and contributes to the ATP-triggered pial arteriolar response, albeit via actions involving P2Y1 receptors.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This work was supported by a grant from the American Heart Association (AHA-0635337N), a National Heart, Lung, and Blood Institute Grant (HL-088259), and a Juvenile Diabetes Research Foundation postdoctoral fellowship (JDRF-3-2008-462).
REFERENCES
- 1. Bjelobaba I, Stojiljkovic M, Pekovic S, Dacic S, Lavrnja I, Stojkov D, Rakic L, Nedeljkovic N. Immunohistological determination of ecto-nucleoside triphosphate diphosphohydrolase1 (NTPDase1) and 5′-nucleotidase in rat hippocampus reveals overlapping distribution. Cell Mol Neurobiol 27: 731–743, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Cunha RA, Brendel P, Zimmermann H, Ribeiro JA. Immunologically distinct isoforms of ecto-5′-nucleotidase in nerve terminals of different areas of the rat hippocampus. J Neurochem 74: 334–338, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res 46: 253–264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke 20: 1538–1544, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elsersy H, Sheng H, Lynch JR, Moldovan M, Pearlstein RD, Warner DS. Effects of isoflurane versus fentanyl-nitrous oxide anesthesia on long-term outcome from severe forebrain ischemia in the rat. Anesthesiology 100: 1160–1166, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Farahbakhsh NA. Ectonucleotidases of the rabbit ciliary body nonpigmented epithelium. Invest Ophthalmol Vis Sci 44: 3952–3960, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Grobben B, Anciaux K, Roymans D, Stefan C, Bollen M, Esmans EL, Slegers H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J Neurochem 72: 826–834, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Horiuchi T, Dietrich HH, Tsugane S, Dacey RG. Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am J Physiol Heart Circ Physiol 280: H767–H776, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29: 7092–7097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iqbal J, Vollmayer P, Braun N, Zimmermann H, Muller CE. A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Purinergic Signal 1: 349–358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol 100: 307–317, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kulik TB, Aronhime SN, Echeverry G, Beylin A, Winn HR. The relationship between oxygen and adenosine in astrocytic cultures. Glia 58: 1335–1344, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab 30: 808–815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res 334: 199–217, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Lasley RD, Kristo G, Keith BJ, Mentzer RM., Jr The A2a/A2b receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium. Am J Physiol Heart Circ Physiol 292: H426–H431, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, Limatola C. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology 35: 1550–1559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152: 141–150, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes 10: 251–257, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mackiewicz M, Geiger JD, Pack AI. Simultaneous assessment of ecto- and cytosolic-5′-nucleotidase activities in brain micropunches. J Neurosci Methods 104: 9–18, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Macvicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci 33: 93–102, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Mateo J, Kreda S, Henry CE, Harden TK, Boyer JL. Requirement of Cys399 for processing of the human ecto-ATPase (NTPDase2) and its implications for determination of the activities of splice variants of the enzyme. J Biol Chem 278: 39960–39968, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol 281: H2018–H2027, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Meno JR, Nguyen TS, Jensen EM, Alexander WG, Groysman L, Kung DK, Ngai AC, Britz GW, Winn HR. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J Cereb Blood Flow Metab 25: 775–784, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Moody CJ, Meghji P, Burnstock G. Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur J Pharmacol 97: 47–54, 1984 [DOI] [PubMed] [Google Scholar]
- 29. Muller CE, Iqbal J, Baqi Y, Zimmermann H, Rollich A, Stephan H. Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett 16: 5943–5947, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Nasu I, Yokoo N, Takaoka S, Takata K, Hoshikawa T, Okada M, Miura Y. The dose-dependent effects of isoflurane on outcome from severe forebrain ischemia in the rat. Anesth Analg 103: 413–418, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Ngai AC, Winn HR. Effects of adenosine and its analogues on isolated intracerebral arterioles. Extraluminal and intraluminal application. Circ Res 73: 448–457, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Ohata H, Cao S, Koehler RC. Contribution of adenosine A2A and A2B receptors and heme oxygenase to AMPA-induced dilation of pial arterioles in rats. Am J Physiol Regul Integr Comp Physiol 291: R728–R735, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okada T, Mochizuki T, Huang ZL, Eguchi N, Sugita Y, Urade Y, Hayaishi O. Dominant localization of adenosine deaminase in leptomeninges and involvement of the enzyme in sleep. Biochem Biophys Res Commun 312: 29–34, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 359: 7–10, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Paisansathan C, Xu HL, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and potassium channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol 299: H2009–H2017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflügers Arch 452: 589–597, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Parkinson FE, Ferguson J, Zamzow CR, Xiong W. Gene expression for enzymes and transporters involved in regulating adenosine and inosine levels in rat forebrain neurons, astrocytes and C6 glioma cells. J Neurosci Res 84: 801–808, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Pelligrino DA, Vetri F, Xu HL. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol 22: 229–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ross FM, Brodie MJ, Stone TW. Adenosine monophosphate as a mediator of ATP effects at P1 purinoceptors. Br J Pharmacol 124: 818–824, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato K, Matsuki N, Ohno Y, Nakazawa K. Extracellular ATP reduces optically monitored electrical signals in hippocampal slices through metabolism to adenosine. Eur J Pharmacol 399: 123–129, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Schmitz B, Bottiger BW, Hossmann KA. Brief hypercapnia enhances somatosensory activation of blood flow in rat. J Cereb Blood Flow Metab 16: 1307–1311, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, Ventureyra EC, Thompson CS. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res 1168: 129–138, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sperlagh B, Vizi ES. Extracellular interconversion of nucleotides reveals an ecto-adenylate kinase activity in the rat hippocampus. Neurochem Res 32: 1978–1989, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26: 1378–1385, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci 30: 11870–11882, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vetri F, Menicucci D, Lapi D, Gemignani A, Colantuoni A. Pial arteriolar vasomotion changes during cortical activation in rats. Neuroimage 38: 25–33, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sevigny J, Battastini AM, Robson SC. Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 138: 421–432, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294: H622–H632, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Xu HL, Wolde HM, Gavrilyuk V, Baughman VL, Pelligrino DA. cAMP modulates cGMP-mediated cerebral arteriolar relaxation in vivo. Am J Physiol Heart Circ Physiol 287: H2501–H2509, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol 288: H382–H388, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Zamzow CR, Xiong W, Parkinson FE. Adenosine produced by neurons is metabolized to hypoxanthine by astrocytes. J Neurosci Res 86: 3447–3455, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp 276: 113–128, 2006 [PubMed] [Google Scholar]