Abstract
The α2-isoform of Na,K-ATPase (α2) is thought to play a role in blood pressure regulation, but the specific cell type(s) involved have not been identified. Therefore, it is important to study the role of the α2 in individual cell types in the cardiovascular system. The present study demonstrates the role of vascular smooth muscle α2 in the regulation of cardiovascular hemodynamics. To accomplish this, we developed a mouse model utilizing the Cre/LoxP system to generate a cell type-specific knockout of the α2 in vascular smooth muscle cells using the SM22α Cre. We achieved a 90% reduction in the α2-expression in heart and vascular smooth muscle in the knockout mice. Interestingly, tail-cuff blood pressure analysis reveals that basal systolic blood pressure is unaffected by the knockout of α2 in the knockout mice. However, knockout mice do fail to develop ACTH-induced hypertension, as seen in wild-type mice, following 5 days of treatment with ACTH (Cortrosyn; wild type = 119.0 ± 6.8 mmHg; knockout = 103.0 ± 2.0 mmHg). These results demonstrate that α2-expression in heart and vascular smooth muscle is not essential for regulation of basal systolic blood pressure, but α2 is critical for blood pressure regulation under chronic stress such as ACTH-induced hypertension.
Keywords: adrenocorticotropic hormone, ouabain
na,k-atpase is an integral membrane protein composed of two essential subunits, α and β (29). There are four known isoforms of the catalytic α-subunit (α1, α2, α3, and α4), and each isoform displays a unique tissue distribution and expression pattern suggesting a tissue specific role for the α-subunits (17, 18). The α-subunits contain the highly conserved cardiac glycoside binding site, and in most species, all four α-isoforms are sensitive to inhibition by cardiac glycosides including ouabain. However, the α1-isoform in mice and rats has a low affinity for cardiac glycosides and is therefore resistant to inhibition by these compounds (10, 23, 29, 30).
The electrochemical gradient established by Na,K-ATPase is vital to many cellular functions, including transport of glucose and amino acids into the cell, excitability of muscle and nerves, and regulation of the intracellular concentrations of other ions including Ca2+ (3, 4, 21–23). The Na+ gradient generated by Na,K-ATPase is coupled to the Na/Ca exchanger (NCX). It is widely accepted that inhibition of Na,K-ATPase by cardiac glycosides activates the reverse mode of the NCX, resulting in positive cardiac and smooth muscle cell inotropy. The inotropy, subsequently, increases the force of cardiac myocyte and vascular smooth muscle cell contractility leading to an increase in mean arterial blood pressure (MAP) due to increased cardiac output and total peripheral resistance (5, 6, 17).
In a previous study (17) investigating the role of the α2-isoform of Na,K-ATPase (α2) in vivo, we generated a global genetic knockout of one copy of α2 (α2+/−). Zhang et al. (35) subsequently reported that these α2+/− mice exhibit an increase in their systolic blood pressure (SBP) under basal conditions, characteristic of hypertension. Interestingly, in cardiac myocytes and vascular smooth muscle, α2 represents the minor isoform of Na,K-ATPase, with an α1:α2 expression ratio of ∼2.3:1 (28). Furthermore, current generated by α2 accounts for only ∼20% of total current generated by Na,K-ATPase, indicating that α2 is also the minor functional isoform of Na,K-ATPase expressed in the cardiovascular system (3, 14). Although α2 is a minor isoform of Na,K-ATPase expressed in the cardiovascular system, it is a critical regulator of blood pressure.
The objective of the present study was to examine which cell type(s) expressing the α2 are involved in the regulation of blood pressure. We hypothesized that α2-expression in cardiac myocytes and vascular smooth muscle is responsible for blood pressure regulation. To test this hypothesis, a cell type-specific knockout of α2 in both cardiac myocytes and vascular smooth muscle was developed utilizing the Cre/loxP system. The SM22α Cre was utilized, which has been shown to drive efficient somatic recombination between loxP sites, approaching 100% recombination in aorta, with recombination also detected in cardiac myocytes early in heart development (12, 20).
MATERIALS AND METHODS
Generation of the floxed α2-isoform of the Na,K-ATPase.
A targeting construct was developed in which a 5′-loxP site was introduced in the promoter region of the α2-locus 1,970-bp upstream of the transcriptional start site at the EcoRI restriction site. The insertion of the 5′-loxP site also introduced a BglII restriction site, which is utilized to screen for incorporation of the loxP site by Southern blot analysis. A 3′-neomycin cassette flanked by loxP sites (Flox-Neo) was introduced 100-bp downstream of exon 1, in the first intron of the α2-locus, at the SpeI restriction site. The Flox-Neo cassette contains an EcoRI restriction site, which is utilized to screen for the incorporation of the cassette by Southern blot analysis. The targeting construct also contained a thymidine kinase cassette to allow for negative selection of embryonic stem (ES) cell clones. The targeting construct was transfected into ES cells by electroporation. Successful homologous recombination between the targeting construct and the wild-type allele was determined by Southern blot analysis. The 5′- and 3′-probes utilized in the Southern blot analysis were generated by PCR and are depicted in Fig. 1A. The positive ES cell clones were transiently transfected with Cre-recombinase to excise the neomycin cassette resulting in ES cell clones with three possible outcomes 1) complete excision of the locus, 2) excision of exon 1 (leaving the Neo cassette), or 3) the excision of the Neo cassette alone. A clone positive for the excision of the Neo cassette was identified by Southern blot analysis and was injected into blastocysts to generate chimeric mice. Chimeric mice were bred to black Swiss female mice to generate heterozygous α2WT/Flox mice. Homozygous floxed (α2Flox/Flox) mice were generated by heterozygous mating. Genotyping was determined through allele-specific PCR of DNA isolated from tail biopsies to detect the α2 5′-loxP site using forward primer P1 (5′-GGGCAGGTAGGCGCTGACCCT-3′) and reverse primer P4 (5′-AGTCCTAAGGTGCCCGCTGGC-3′). All experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
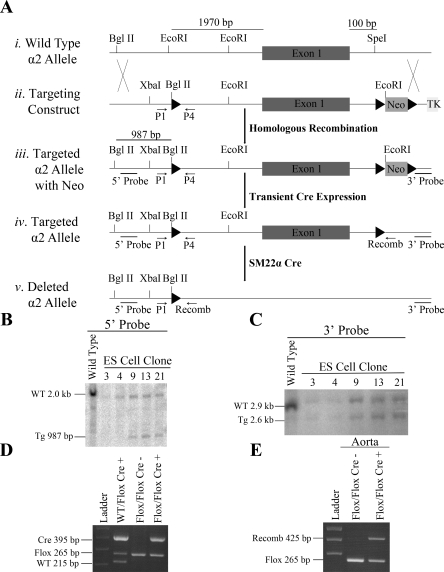
Fig. 1.
Generation of the tissue specific knockout of the α2-isoform of the Na,K-ATPase. A: Cre/LoxP targeting strategy. i: Restriction map depicting the endogenous locus of the wild-type α2 allele. ii: Targeting construct for the α2 allele with the 5′-loxP (black triangles) and 3′-neomycin (Neo) and a thymidine kinase cassette (TK) downstream. iii: Schematic of the targeted α2-allele following successful homologous recombination with the targeting construct. iv: Schematic of the targeted α2 allele following transient Cre expression. v: Schematic of the deleted α2-allele following the tissue specific expression of the SM22α Cre. B: Southern blot analysis of the 5′-loxP site following BglII restriction digests. C: Southern blot analysis of the 3′-loxP site following SpeI restriction digests. D: Genotyping of DNA isolated from tail biopsies. E: Genotyping of DNA isolated from aortas to screen for recombination between the 5′ and 3′ loxP sites. ES, embryonic stem cells.
Generation of smooth muscle α2-isoform of the Na,K-ATPase knockout mice.
α2Flox/Flox mice were mated to heterozygous SM22α Cre animals (Jackson Laboratory, Bar Harbor, ME) to generate the cell type-specific knockout of the α2 in smooth muscle (α2Flox/Flox Cre+). Genotyping was determined through allele-specific PCR to detect the α2 5′-loxP site using forward primer P1 and reverse primer P4 (described above), and recombination between the 5′ and 3′-loxP sites was detected with the forward primer P1 and reverse primer Recomb (5′-CCTGTACTCTTGGGCTAAGGACC-3), and Cre transgene using forward primer (5′-ACCGGTCGATGCAACGAGTG-3′) and reverse primer (5′-GAACCTGGTCGAAATCAGTG-3′).
Whole tissue preparation.
Whole (brain and kidney) or pooled (aorta) tissues were homogenized on ice in 400 μl to 1.5 ml, depending on the tissue, of radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris, 5 mM EDTA pH 7.4, 0.1% SDS, 1% Na-deoxycholate, and 1% Triton-X) containing 1:100 protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) for two 1-min bursts of a Polytron homogenizer. Following homogenization, samples were incubated on ice for 1 h and then centrifuged at <8,000 rpm to remove particulate matter. Protein containing supernatant was removed and stored at −80°C. Protein concentration was determined with the BCA protein assay kit (Thermo Scientific, Rockford, IL).
Crude microsomal preparations.
Microsomal preparations were performed as previously described (8). Briefly, whole hearts were homogenized on ice in 2 ml homogenization buffer (250 mM sucrose, 30 mM imidazole, and 1 mM EDTA) containing 1:100 protease and phosphatase inhibitor cocktails (Sigma-Aldrich) for two 1-min bursts of a Polytron homogenizer. Homogenates were then centrifuged at 5,000 rpm to remove particulate matter. The supernatant was collected and ultracentrifuged at 200,000 g for 1 h. Following ultracentrifugation, the pellet was resuspended in resuspension buffer (1 mM imidazole and 1 mM EDTA pH 7.5) containing 1:100 protease and phosphatase inhibitor cocktails (Sigma-Aldrich) using a Dounce-homogenizer and stored at −80°C. Protein concentration was determined with the BCA protein assay kit (Thermo Scientific).
Western blot analysis.
Western blot analysis was performed by standard methods as previously described (8). Briefly, protein samples were incubated in Laemmli loading buffer (125 mM Tris pH 6.8, 6% SDS, 20% glycerol, 10% β-mercaptoethanol, and 0.1% bromophenal blue) for 30 min at 37°C and separated by electrophoresis in 8% polyacrylamide gels. The separated proteins were transferred to polyvinylidene fluoride membranes and blocked in 5% nonfat dry milk in TBST (150 mM Tris pH 7.4, 100 mM NaCl, and 0.05% Tween 20) at room temperature for 1 h. The blots were incubated in 5% nonfat dry milk in TBST containing primary antibodies overnight at 4°C as follows: α1-isoform monoclonal antibody (1:2,000; α6F; Ref. 31; University of Iowa Developmental Studies Hybrid Bank, Iowa City, IA), α2-polyclonal antibody (1:500; HERED, Dr. Pressley; Ref. 25), and NCX monoclonal antibody (1:1,000; MA3-926; Thermo Scientific). The membranes were incubated in 5% nonfat dry milk in TBST containing 1:10,000 peroxidase-conjugated secondary antibodies (Calbiochem, San Diego, CA) at room temperature for 1 h. The membranes were developed with SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Scientific), and immunoreactivity was visualized using HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ). Equal protein loading was confirmed by reprobing the membranes for GAPDH for whole tissue preparations or calsequestrin for microsomal preparations. In brief, membranes were blocked in 5% nonfat dry milk in TBST at room temperature for 1 h and incubated with primary antibody in 5% nonfat dry milk in TBST at room temperature for 1 h as follows: GAPDH polyclonal antibody (1:6,000; 14C10; Cell Signaling Technology, Beverly, MA) and calsequestrin polyclonal antibody (1:2,000; PA1-913; Thermo Scientific). Secondary antibodies and immunoreactivity visualization were performed as described above. Signal intensities were quantitated by densitometry using ImageQuant Software 5.1 (Molecular Dynamics, Sunnyvale, CA).
Cardiac myocyte isolation.
Ventricular myocytes were isolated from adult mouse hearts as previously described (1). Briefly, hearts were mounted in a Langendorff apparatus and perfused for 5 min at 37°C with a modified Krebs-Henseleit buffer solution (113 mM NaCl, 4.7 mM KCl, 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 1.2 mM MgSO4-7H2O, 12 mM NaHCO3, 10 mM KHCO3, 10 mM HEPES, 30 mM taurine, 10 mM 2,3-butanedione monoxime, and 5.5 mM glucose pH 7.46). The perfusion buffer was then changed to a buffer containing 5 mg Liberase DL (Roche Applied Science, Indianapolis, IN) and 0.14 mg/ml trypsin (Thermo Scientific). Following 15–20 min of perfusion, the ventricles were dissected and dispersed by gentle pipetting. Isolated cardiomyocytes were sieved to remove cell debris, and myocytes were allowed to sediment for 10 min. Isolated cardiac myocytes were visually inspected under a ×20 objective to ensure purity of myocytes; preparations were accepted as highly enriched if 75–80% of the viable cells were beating cardiac myocytes. The pelleted myocytes were then resuspended in 1 ml TriReagent (Molecular Research Center, Cincinnati, OH), and total RNA was isolated according to the manufacturer's recommendations.
Real-time PCR analysis.
Real-time PCR analysis was performed as previously described (26). Briefly, first strand cDNA was synthesized from 2.5 μg of total RNA using the SuperScript III first-stand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations for use with random hexamer primers. Real-time PCR reactions were performed in triplicate using the ABsolute Blue QPCR SYBR Green fluorescein mix (Thermo Scientific) with α2-specific primers (forward 5′-GCTACCCTGTTGCTCAGACTG-3′ and reverse 5′-GGCAGCAGGCGAGTACTCACG-3′) and GAPDH-specific primers (forward 5′-TGACCACAGTCCATGCCATC-3′ and reverse 5′-GACGGACACATTGGGGGTAG-3′). The relative amount of the α2-mRNA was normalized to GAPDH.
Blood pressure analysis.
Tail-cuff measurements of SBP were obtained using the CODA-8 System (Kent Scientific, Torrington, CT) computerized apparatus, as described previously (7). In brief, mice were acclimated to the system for 5 consecutive days immediately followed by 8 days of baseline measurements and 5 days of drug treatment measurements when applicable. Daily blood pressure measurements consisted of 5 acclimation readings followed immediately by 15 SBP measurements, with little time elapse between readings. Data recordings were accepted when SBP was determined by the computer in ≥7 of the 15 measurements and was between 80 and 200 mmHg.
Administration of saline and ACTH.
Mice were administered saline or 375 ng/g body wt Cortrosyn (Amphastar Pharmaceuticals, Rancho Cucamonga, CA), a synthetic peptide of ACTH, every 12 h by subcutaneous injection for 5 days. Tail-cuff measurements of SBP were obtained daily 3 h after administration of the AM dose.
Ouabain administration.
As previously described, mice were administered 300 ng/g body wt ouabain (Sigma-Aldrich) or vehicle (saline), every 24 h by intraperitoneal injection for 3 days (9). Tail-cuff measurements of SBP were obtained daily 3 h after administration of the dose.
Cardiovascular performance.
Cardiovascular measurements were performed in closed-chest mice as previously described (8). Briefly, adult mice were anesthetized with an intraperitoneal injection of 50 μg/g body wt ketamine and 100 μg/g body wt thiobutabarbital (Inactin; Research Biochemical International, Natick, MA). To monitor cardiac performance, a SciSense pressure catheter (SciSense, London, ON, Canada) was advanced into the left ventricle through the right carotid artery. The right femoral artery and vein were cannulated for blood pressure measurements and infusion of drugs. Pressure tracings were recorded and analyzed using PowerLab 4/s and Chart software (ADInstruments, Colorado Springs, CO). Values for heart rate, blood pressure, and left ventricular pressure were measured before and 3 min after intravenous infusion of dobutamine (2, 8, and 32 ng·g body wt−1·min−1) or ouabain (2.5 μg·g body wt−1·min−1). The maximum rate of cardiac contraction (dP/dtmax) and the rate of cardiac contraction at 40 mmHg (dP/dt40) before and after infusion were calculated from the first derivative of the pressure waveforms.
Regional blood flow and vascular resistance.
Regional blood flow and vascular resistance were performed in anesthetized mice as previously described (24). Briefly, adult mice were anesthetized as before, and the femoral artery and vein were cannulated. For carotid blood flow measurements, the right carotid artery was isolated and fitted with a 0.5-PSB perivascular flow probe connected to a TS420 flowmeter (Transonic Systems, Ithaca, NY). Values for blood pressure and carotid blood flow (CBF) were measured before and 5 min after intravenous infusion of phenylephrine (10, 40, and 80 ng·g body wt−1·min−1) and nitro-l-arginine methyl ester (l-NAME; 50 μg·g body wt−1·min−1). Carotid vascular resistance (CVR) was calculated by dividing MAP by mean CBF.
Statistical analysis.
Data are presented as average ± SE, with statistical analysis performed using SigmaStat 3.5 (Systat Software, San Jose, CA). Student t-tests were performed to compare experimental groups (see Figs. 2–3, 7 and Tables 1–2). Two-way ANOVAs for time and treatment with repeated measurements were performed to compare experimental groups (see Figs. 4–6). Differences were considered significant at P < 0.05.
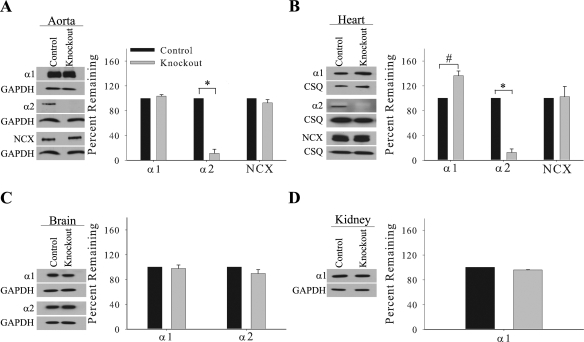
Fig. 2.
Expression profile of the α1- and α2-isoforms of the Na,K-ATPase and Na/Ca exchanger (NCX) in the smooth muscle knockout. Representative immunoblots and relative protein expression of α1, α2, NCX, and GAPDH or calsequestrin (CSQ) in the aorta (A), heart microsomes (B), brain (C), and kidney (D). Relative protein expression is determined by densitometry and normalized to the abundance of GAPDH or calsequestrin. Data represent an arbitrary ratio of the protein expression in knockout mice compared with expression in control mice and are displayed as the percent remaining. Data were obtained from 3 independent experiments and are displayed as average percent remaining ± SE; n = 3. *P < 0.005 and #P < 0.05, statistical difference.
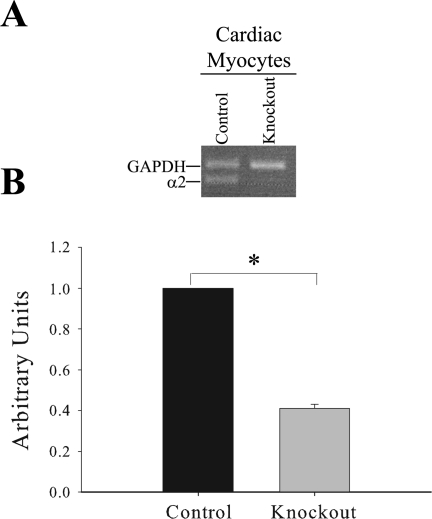
Fig. 3.
mRNA expression of the α2 isoform of the Na,K-ATPase in isolated cardiac myocytes. A: representative qualitative PCR gel from isolated cardiac myocytes using the real-time α2 and GAPDH primers. B: quantitative real-time PCR analysis of mRNA expression of α2 in isolated cardiac myocytes normalized to GAPDH expression. Data represent an arbitrary ratio of mRNA expression of α2 in knockout mice to that expressed in control mice from two independent experiments. Bars represent means ± SE; n = 3. *P = 0.002, statistical difference.
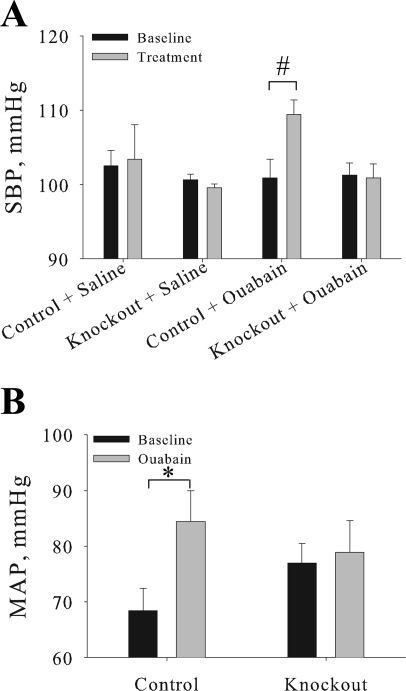
Fig. 7.
Cardiovascular knockout mice fail to develop ouabain-induced hypertension. A: SBP was measured by the tail-cuff method in 3- to 6-mo-old female mice following chronic ouabain (control n = 4; knockout n = 5) or saline (control n = 4; knockout n = 5) treatment. Baseline measurement is the average of 3 consecutive days of basal SBP measurements, and treatment measurement is the average of 3 consecutive days of ouabain or saline treatment. B: MAP was determined in anesthetized control (n = 6) and knockout (n = 6) mice using a pressure transducer advanced into the left ventricle. Measurements were collected at baseline and in response to a ouabain bolus. Values represents means ± SE. #P = 0.02 and *P = 0.04, statistical differences.
Table 1.
Morphometric analysis of male and female mice
| 4 mo |
8 mo |
|||
|---|---|---|---|---|
| Control | Knockout | Control | Knockout | |
| Body, g | 25.3 ± 1.9 | 25.4 ± 1.3 | 36.9 ± 1.6 | 35.6 ± 1.2 |
| Heart, mg | 128.0 ± 7.7 | 122.0 ± 4.3 | 170.6 ± 8.0 | 181.8 ± 9.8 |
| Kidney, mg | 319.8 ± 20.1 | 337.4 ± 22.4 | 423.7 ± 22.4 | 428.2 ± 22.7 |
| Heart-to-body weightt ratio | 5.0 ± 0.1 | 4.9 ± 0.1 | 4.6 ± 0.1 | 5.1 ± 0.2* |
| Kidney-to-body weight ratio | 12.7 ± 0.4 | 13.2 ± 0.4 | 11.5 ± 0.4 | 12.0 ± 0.4 |
Values are means ± SE; n = 5 for 4-mo control, n = 8 for 4-mo knockout, and n = 13 for 8 mo.
P < 0.04.
Table 2.
Cardiovascular knockout mice exhibit normal basal SBP
| SBP, mmHg |
||
|---|---|---|
| Genotype | Male | Female |
| Control | 104.0 ± 2.7 | 104.3 ± 2.2 |
| Knockout | 99.4 ± 3.2 | 102.6 ± 2.7 |
Values are means ± SE; n = 8 for female control, male control, and male knockout and n = 7 for female knockout. SBP, systolic blood pressure.
Fig. 4.
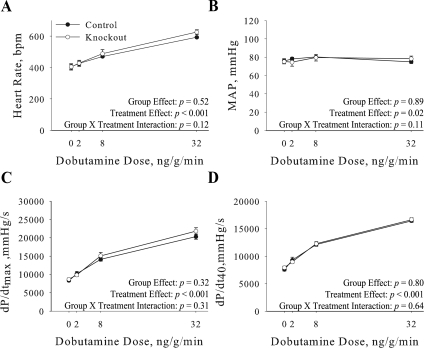
Basal cardiovascular performance. Heart rate (A), mean arterial pressure (MAP; B), maximum rate of cardiac contraction (dP/dtmax; C), and rate of cardiac contraction at 40 mmHg (dP/dt40; D) were determined in anesthetized control (n = 8) and knockout (n = 6) mice using a pressure transducer advanced into the left ventricle. Measurements were collected at baseline (0) and in response to an increasing dose of dobutamine. Values represent means ± SE.
Fig. 5.
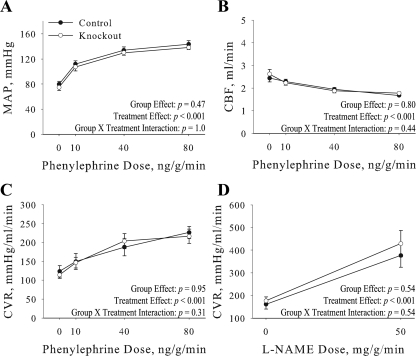
Carotid blood flow and vascular resistance. MAP (A), carotid blood flow (CBF; B), and carotid vascular resistance (CVR; C) were determined in anesthetized control (n = 6) and knockout (n = 8) mice using a flow probe fitted to the right carotid artery. Measurements were collected at baseline (0) and in response to an increasing dose of phenylephrine. CVR was also determined in response to nitro-l-arginine methyl ester (l-NAME; D). Values represent means ± SE.
Fig. 6.
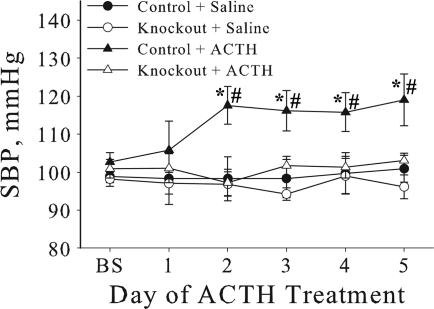
Cardiovascular knockout mice fail to develop ACTH-induced hypertension. Systolic blood pressure (SBP) was measured by the tail-cuff method in 3- to 6-mo-old female mice following ACTH treatment (control n = 6; knockout n = 8) or saline (control n = 6; knockout n = 9). Baseline (BS) measurement is the average of 8 consecutive days of basal SBP measurements, followed by 5 consecutive days of ACTH or saline treatment. Values represent means ± SE. *P < 0.05 and #P < 0.05, statistical difference compared with the corresponding ACTH knockout and saline control, respectively.
RESULTS
Characterization of the floxed α2-isoform of the Na,K-ATPase mice.
To obtain Cre mediated recombination and deletion of α2, the first exon containing the transcriptional start site was flanked by loxP sites utilizing the targeting construct depicted in Fig. 1A. Hundreds of ES cell clones were screened by Southern blot analysis to identify heterozygous ES cell clones incorporating the 5′-loxP site. The representative Southern blot shown in Fig. 1B identifies a 987-bp targeted allele in clones 9, 13, and 21. The positive ES cell clones were further analyzed by Southern blot analysis for incorporation of the 3′ Flox-Neo cassette, and a 2.6-kb targeted allele was identified in clones 9, 13, and 21 (Fig. 1C). Following heterozygous mating, α2Flox/Flox, α2WT/Flox, and α2WT/WT mice were born in normal mendelian ratios. The α2Flox/Flox mice retain normal tissue expression of the α2 (data not shown).
Characterization of the smooth muscle α2-isoform of the Na,K-ATPase knockout.
Homozygous α2Flox/Flox mice were mated to heterozygous SM22α Cre animals to generate the tissue-specific knockout of α2 in smooth muscle (α2Flox/Flox Cre+). The genotypes of the mice were identified by PCR, which identifies a 215-bp wild-type fragment, a 265-bp flox fragment, and 395-bp Cre fragment (Fig. 1D). To confirm that recombination between the 5′- and 3′-loxP sites occurs in the vascular smooth muscle of the α2Flox/Flox Cre+ mice, genomic DNA was isolated from aortas and screened for recombination by PCR. A 425-bp recombination fragment was identified in the aorta (Fig. 1E), indicating the presence of recombination in vascular smooth muscle. In addition, a 265-bp floxed fragment was identified in the aorta resulting from the heterogeneous composition of the tissue, which is composed of vascular smooth muscle, endothelial cells, and connective tissue.
Expression of the α2-isoform of the Na,K-ATPase is reduced in the heart and vascular smooth muscle.
SM22α Cre is highly expressed in vascular smooth muscle, with expression also reported in cardiac myocytes early in heart development (12, 20). Therefore, Southern and Western blot analyses were performed to examine the tissue-specific expression of α2 in the α2Flox/Flox Cre+ (knockout) compared with the α2Flox/Flox Cre− (control) mice. Southern blot analysis on tissue isolated from knockout mice reveals that recombination between the 5′- and 3′-loxP sites is specific to smooth muscle rich tissues including the aorta, bladder, and uterus. However, recombination was also detected in the heart (data not shown). These results are consistent with previous reports (20) demonstrating high expression of the SM22α Cre in smooth muscle of large conduit vessels, as well as resistant vessels, and also in cardiac myocytes early in heart development.
Representative immunoblots and relative expression levels for α1- and α2-isoforms of the Na,K-ATPase and NCX are shown in Fig. 2. In the aorta, the abundance of α2 is significantly reduced by 90% in the knockout (P < 0.005; Fig. 2A). The primary cell type in the aorta is vascular smooth muscle (34); therefore, the reduced expression of α2 in the aorta results from the knockout of α2 in vascular smooth muscle. In addition in the aorta, no significant changes were observed in expression of α1 or NCX in the knockout (Fig. 2A). Similarly, the abundance of α2 in heart microsomes is significantly reduced by 90% in the knockout (P < 0.005; Fig. 2B). In addition, expression of α1 in heart microsomes is significantly upregulated by 40% in the knockout (P < 0.05; Fig. 2B). This is consistent with a previous report (17) in which α2-heterozygous knockout mice exhibit a compensatory upregulation of α1 in the heart. Additionally, no significant change was observed in expression of NCX in heart microsomes (Fig. 2B). Finally, no significant changes were observed in the abundance of α1 or α2 in the brain (Fig. 2C) or in the abundance of α1 in the kidney of the knockout mice (Fig. 2D). These results demonstrate that we have generated a significant reduction in the expression of α2 in heart and vascular smooth muscle and that this reduction is specific to these cell types in the cardiovascular system.
Expression of the α2-isoform of the Na,K-ATPase is reduced in isolated cardiomyocytes.
To determine if the reduced expression of α2 observed in heart microsomes results from leaky expression of the SM22α Cre in cardiac myocytes, we performed real-time PCR analysis on isolated cardiomyocytes. The cardiac myocyte preparations were highly enriched for cardiac myocytes, with little or no smooth muscle cell contamination. To visualize mRNA expression of α2 in cardiac myocyte preparations, qualitative PCR analysis was utilized as shown in Fig. 3A. This reveals very low expression of α2 in the knockout cardiac myocyte preparations. In addition, quantitative real-time PCR analysis demonstrates that mRNA expression of α2 is significantly reduced by 60% in the knockout cardiac myocytes (P = 0.002; Fig. 3B). These results demonstrate that reduced expression of α2 in heart microsomes is due to the combined reduction of α2 expression in cardiac myocytes and vascular smooth muscle, further confirming the specific reduction of α2 expression in these cell types of the cardiovascular system (as demonstrated in Fig. 2).
Mice with the cardiovascular knockout of the α2-isoform of the Na,K-ATPase develop mild hypertrophy.
Morphometric analysis of heart, kidney, and body weight was performed on male and female mice at 4 and 8 mo of age (Table 1). There were no significant differences in the heart-to-body or kidney-to-body weight ratio in knockout compared with control mice at 4 mo of age. However, there was a slight but significant (P < 0.04) increase in the heart-to-body weight ratio in knockout compared with control mice at 8 mo of age, without any changes in the kidney-to-body weight ratio. The slight increase in the heart-to-body weight ratio in the knockout mice at 8 mo of age would indicate the development of a mild hypertrophy; therefore, in all further studies mice were used at 3–6 mo of age before the development of hypertrophy.
Cardiovascular knockout of the α2-isoform of the Na,K-ATPase does not alter basal blood pressure in mice.
Blood pressure was analyzed in conscious resting mice by the tail-cuff method to discern if the cardiovascular-specific knockout of the α2 in cardiac myocytes and vascular smooth muscle is required for the regulation of basal blood pressure. There were no significant differences in basal SBP measurements in female control vs. knockout mice, or in male control vs. knockout mice, as shown in Table 2. Furthermore, there were no significant differences in basal SBP measurements between male and female mice. These results indicated that the cardiovascular-specific knockout of the α2 in cardiac myocytes and vascular smooth muscle does not participate in the regulation of basal blood pressure in either male or female mice; in all additional studies of blood pressure only female mice were utilized.
Knockout of the α2-isoform of the Na,K-ATPase in cardiac myocytes does not alter basal cardiac function.
Cardiac performance was analyzed in intact anesthetized closed chest mice and results are shown in Fig. 4. Under basal conditions there were no significant differences in heart rate (Fig. 4A), MAP (Fig. 4B), dP/dtmax (Fig. 4C), or dP/dt40 (Fig. 4D) in knockout mice. There was also no significant difference in the ability of knockout hearts to maximally contract in response to β-adrenergic stimulation with dobutamine (Fig. 4, A–D). Thus the cardiovascular-specific knockout of the α2 in cardiac myocytes and vascular smooth muscle does not alter basal cardiac performance.
Vascular smooth muscle knockout of the α2-isoform of the Na,K-ATPase does not alter basal vascular function.
To determine the effect of the cardiovascular knockout of the α2 on vascular function, blood flow and vascular resistance were determined in the carotid artery as a representative of vascular function (Fig. 5). Under basal conditions there were no significant differences in MAP (Fig. 5A), CBF (Fig. 5B), and CVR (Fig. 5C) in the knockout mice. There was also no significant difference in the ability of knockout vascular smooth muscle to maximally contract in response to the α-adrenergic receptor agonist phenylephrine (Fig. 5, A–C). To ensure proper endothelial function, mice were treated with l-NAME, a nitric oxidase synthase inhibitor. There was no significant difference in CVR in the knockout in response to l-NAME treatment (Fig. 5D), indicating proper endothelial function in the vasculature of knockout mice. Therefore, the cardiovascular-specific knockout of the α2 in cardiac myocytes and vascular smooth muscle does not affect carotid vascular smooth muscle cell contractility or endothelial function, implying that there are not any vascular abnormalities in the knockout mice.
Mice with the cardiovascular knockout of the α2-isoform of the Na,K-ATPase do not develop ACTH-induced hypertension.
Since the cardiovascular-specific knockout of α2 in cardiac myocytes and vascular smooth muscle resulted in no significant differences in basal cardiovascular hemodynamics as determined by basal SBP, cardiac performance, and carotid vascular resistance, we next determined if α2 regulates SBP in response to ACTH-induced hypertension. Administration of ACTH induces hypertension that is associated with an increase in circulating plasma endogenous cardiac glycosides and has been shown to be dependent on the cardiac glycoside binding site of the α2 (11, 24). Therefore, we assessed the effect of ACTH on SBP in control and knockout mice utilizing the tail-cuff method, since we previously reported a similar elevation of SBP in response to ACTH with both the tail-cuff method and telemetry (11, 24).
As shown in Fig. 6, before ACTH treatment, there was no significant difference in SBP between control and knockout mice, consistent with the data in Table 2. Vehicle control (sc saline injection) had no effect on SBP throughout the 5 days of treatment. In control mice, SBP increased significantly from 102.6 ± 2.5 to 117.6 ± 4.9 mmHg (P < 0.05) within 2 days of ACTH treatment, and pressure remained elevated throughout the course of treatment. In contrast, SBP did not increase in the knockout mice in response to ACTH treatment. These results indicate that α2-expression in cardiac myocytes and vascular smooth muscle is involved in the modulation of SBP in response to stress, such as ACTH-induced hypertension.
Mice with the cardiovascular knockout of the α2-isoform of the Na,K-ATPase do not develop ouabain-induced hypertension.
Cardiovascular knockout animals fail to develop ACTH-induced hypertension in response to increased plasma endogenous cardiac glycosides, consistent with the hypothesis that ACTH elicits an increase in endogenous cardiotonic steroids. Therefore, we assessed the effect of exogenous ouabain on the cardiovascular α2-knockout mice, which has been shown to induce hypertension that is dependent on the cardiac glycoside binding site of the α2 (9).
As shown in Fig. 7A, there was no significant difference in SBP between control and knockout mice at baseline, consistent with the data in Table 2. Vehicle control (ip saline injection) had no effect on SBP. In control mice, SBP significantly increased from 100.9 ± 1.9 to 109.4 ± 1.9 mmHg (P = 0.02) following 3 days of chronic ouabain treatment. In contrast, the SBP was not elevated in the cardiovascular knockout mice in response to ouabain treatment. In addition, acute ouabain treatment resulted in a significant elevation of MAP in the control mice (68.4 ± 4.1 to 84.4 ± 5.6 mmHg), with no elevation of MAP observed in the cardiovascular knockout mice (76.9 ± 3.5 to 78.9 ± 5.8 mmHg; Fig. 7B). These results indicate that α2-expression in cardiac myocytes and vascular smooth muscle is involved in the regulation of SBP in response to ouabain. These results are consistent with the ACTH data in Fig. 6, suggesting that the interaction of an endogenous ouabain with the α2 expressed in cardiac myocytes and vascular smooth muscle is required for modulation of SBP in response to a chronic stress.
DISCUSSION
The present study demonstrates the tissue-specific deletion of the α2-isoform of the Na,K-ATPase in cardiac myocytes and vascular smooth muscle cells in mice using the Cre/loxP system. From quantitative Western blot analysis on isolated aortas obtained from the smooth muscle knockout mice, we estimated an ∼90% reduction in expression of α2 in the aortic vascular smooth muscle. Any remaining α2 expressed in aorta is likely due to the presence of endothelium and nonvascular tissue surrounding the aortic vessel (15) or due to an incomplete knockout of α2 in vascular smooth muscle. In addition, we estimated an ∼90% reduction in expression of α2 in the heart. To determine if the reduced expression of α2 observed in the heart results from leaky expression of the SM22α Cre in cardiac myocytes, qualitative and quantitative PCR was performed on cDNA prepared from isolated cardiac myocytes. We determined that mRNA expression of α2 in isolated knockout cardiac myocytes is reduced by ≥60%, indicating leaky expression of the SM22α Cre and hence deletion of α2 in cardiac myocytes. Taken together, these results demonstrate that we have generated a specific knockout of α2 in the cardiac myocytes and vascular smooth muscle of the cardiovascular system.
Surprisingly, in contrast to the basal hypertension observed with the global germline deletion of one copy of α2 (α2+/−; Ref. 35), the basal blood pressure of the cardiac myocyte and vascular smooth muscle knockout mice is normal. Cardiac and vascular contractility was also unaltered in the knockout mice. Taken together these data indicate that expression of α2 in cardiac myocytes and vascular smooth muscle does not participate in the regulation of basal blood pressure, and further suggest that α2 in another cell type is responsible for the hypertension observed in global α2+/− mice. Previous studies (16, 19, 36) have demonstrated that increased levels of endogenous ouabain in the brain increase activation of the renin-angiotensin system, contributing to the development of hypertension. The increased endogenous ouabain would inhibit the brain α2, resulting in an increased concentration of Na+ in affected cells of the brain. It might be predicted therefore that the α2+/− mice would exhibit an increased brain Na+ concentration, mimicking ouabain inhibition, and therefore exhibit increased activation of renin-angiotensin system leading to hypertension. It is further possible that neuronal expression of α2 in the brain is required for basal regulation and maintenance of blood pressure.
Previous studies (32) demonstrate that in vivo ACTH administration, at doses similar to ours, results in a 1.5-fold increase in the concentration of circulating plasma ACTH in rats. In addition, we have previously reported (10, 11) that these doses result in a 2.5-fold increase in the concentration of circulating plasma endogenous cardiac glycosides in mice and therefore represent a stress on the mice. ACTH treatment results in a significant elevation of MAP, that is associated with changes in cardiac output and total peripheral resistance (13, 27, 32, 33). Interestingly, the cardiac myocyte and vascular smooth muscle knockout mice fail to develop hypertension in response to the stress of ACTH administration, following 5 days of ACTH treatment. These data suggest that expression of α2 in cardiac myocytes and vascular smooth muscle is required for regulation of SBP in response to stress, such as an increased release of endogenous cardiac glycosides in response to ACTH administration.
The increased release of endogenous cardiac glycosides in response to ACTH administration would bind the α2 in cardiac myocytes and vascular smooth muscle in wild-type but not in the knockout mice. The binding of cardiac glycosides to α2 results in two possible outcomes: 1) inhibition of α2 results in an increase in the intracellular concentration of Ca2+ (10, 17), or 2) activation of Src signaling cascades through α2 (2). Either outcome could explain the increase in SBP observed in wild-type mice with ACTH treatment. However, lack of basal hypertension in the cardiac myocyte and vascular smooth muscle knockout mice favors signaling as the foundation for hypertension. The knockout of α2 is expected to mimic ouabain inhibition of the α2 in cardiac myocytes and vascular smooth muscle and therefore alter basal Ca2+ levels. Consequently, it is likely that cardiac glycoside binding to α2 activates Src signaling cascades in the wild-type mice, which serve to regulate blood pressure under stress, such as ACTH administration.
In summary, the present data provide evidence that the cardiovascular expression of α2 in cardiac myocytes and vascular smooth muscle is not required for basal regulation of blood pressure. However, the cardiovascular expression of α2 in cardiac myocytes and vascular smooth muscle was found to play a pivotal role in the modulation of blood pressure in response to ACTH-induced hypertension. These data support the hypothesis that the minor α2-isoform plays a regulatory role in cardiovascular function in response to stress.
GRANTS
This research was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-28573 (to J. B. Lingrel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Robyn Pilcher-Roberts for technical assistance, Dr. Gary E. Shull for help with the preparation of the manuscript, and Maureen Bender for animal husbandry.
REFERENCES
- 1. Al Moamen NJ, Prasad V, Bodi I, Miller ML, Neiman ML, Lasko VM, Alper SL, Wieczorek DF, Lorenz JN, Shull GE. Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure. J Mol Cell Cardiol 50: 137–146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem 276: 46605–46611, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res 73: 92–100, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res 57: 897–912, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 290: R514–R523, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53: 291–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp 27: pii 1291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The α2-isoform of Na,K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the α1- and α2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 290: R524–R528, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA 102: 15845–15850, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-cre, not SMMHC-cre. J Mol Cell Cardiol 41: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gruber KA, Eskridge-Sloop SL, Eldridge JC, Callahan MF. ACTH-induced hypertension in rats: fact or artifact? Am J Physiol Regul Integr Comp Physiol 256: R1308–R1312, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Han F, Tucker AL, Lingrel JB, Despa S, Bers DM. Extracellular potassium dependence of the Na+-K+-ATPase in cardiac myocytes: isoform specificity and effect of phospholemman. Am J Physiol Cell Physiol 297: C699–C705, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99: 7142–7147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang BS, Leenen FH. Brain “ouabain” and angiotensin II in salt-sensitive hypertension in spontaneously hypertensive rats. Hypertension 28: 1005–1012, 1996 [DOI] [PubMed] [Google Scholar]
- 17. James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-cre transgenic mice. Genesis 41: 179–184, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol 72: 395–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lingrel JB, Kuntzweiler T. Na-ATPase. J Biol Chem 269: 19659–196562, 1994 [PubMed] [Google Scholar]
- 23. Lingrel JB, Orlowski J, Shull MM, Price EM. Molecular genetics of Na,K-ATPase. Prog Nucleic Acid Res Mol Biol 38: 37–89, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR, Paul RJ, Lingrel JB. ACTH-induced hypertension is dependent on the ouabain-binding site of the α2-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol 295: H273–H280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pressley TA. Phylogenetic conservation of isoform-specific regions within α-subunit of Na+-K+-ATPase. Am J Physiol Cell Physiol 262: C743–C751, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation 121: 410–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schyvens CG, Mangos GJ, Zhang Y, McKenzie KU, Whitworth JA. Telemetric monitoring of adrenocorticotrophin-induced hypertension in mice. Clin Exp Pharmacol Physiol 28: 758–760, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, Lingrel J, Paul RJ. Na+ pump α2-isoform specifically couples to contractility in vascular smooth muscle: Evidence from gene-targeted neonatal mice. Am J Physiol Cell Physiol 286: C813–C820, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185–220, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Sweadner KJ, Farshi SK. Rat cardiac ventricle has two Na+, K+-ATPases with different affinities for ouabain: Developmental changes in immunologically different catalytic subunits. Proc Natl Acad Sci USA 84: 8404–8407, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem 263: 4347–4354, 1988 [PubMed] [Google Scholar]
- 32. Turner SW, Wen C, Li M, Fraser TB, Whitworth JA. Adrenocorticotrophin dose-response relationships in the rat: Haemodynamic, metabolic and hormonal effects. J Hypertens 16: 593–600, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Wen C, Fraser T, Li M, Turner SW, Whitworth JA. Haemodynamic mechanisms of corticotropin (ACTH)-induced hypertension in the rat. J Hypertens 17: 1715–1723, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res 20: 99–111, 1967 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, White R, Huang BS, Van Huysse J, Leenen FH. High salt intake and the brain renin–angiotensin system in dahl salt-sensitive rats. J Hypertens 19: 89–98, 2001 [DOI] [PubMed] [Google Scholar]