Abstract
Thoracic perivascular adipose tissue (PVAT) is a unique adipose depot that likely influences vascular function and susceptibility to pathogenesis in obesity and the metabolic syndrome. Surprisingly, PVAT has been reported to share characteristics of both brown and white adipose, but a detailed direct comparison to interscapular brown adipose tissue (BAT) has not been performed. Here we show by full genome DNA microarray analysis that global gene expression profiles of PVAT are virtually identical to BAT, with equally high expression of Ucp-1, Cidea, and other genes known to be uniquely or very highly expressed in BAT. PVAT and BAT also displayed nearly identical phenotypes upon immunohistochemical analysis, and electron microscopy confirmed that PVAT contained multilocular lipid droplets and abundant mitochondria. Compared with white adipose tissue (WAT), PVAT and BAT from C57BL6/J mice fed a high-fat diet for 13 wk had markedly lower expression of immune cell-enriched mRNAs, suggesting resistance to obesity-induced inflammation. Indeed, staining of BAT and PVAT for macrophage markers (F4/80 and CD68) in obese mice showed virtually no macrophage infiltration, and FACS analysis of BAT confirmed the presence of very few CD11b+/CD11c+ macrophages in BAT (1.0%) compared with WAT (31%). In summary, murine PVAT from the thoracic aorta is virtually identical to interscapular BAT, is resistant to diet-induced macrophage infiltration, and thus may play an important role in protecting the vascular bed from inflammatory stress.
Keywords: obesity, microarray, macrophages, lipid droplet proteins, diabetes
the burgeoning prevalence of obesity and diabetes throughout the world threatens to accelerate the incidence of associated cardiovascular diseases, including coronary artery disease, hypertension, and congestive heart failure. Although the mechanism by which obesity causes vascular disease is not fully understood, insulin resistance and the metabolic syndrome are thought to play a major role (7, 14, 27, 41). In human obesity as well as mouse models of obesity, enlarged adipocytes in visceral adipose tissue (VAT) become insulin resistant and fail to effectively store excess triglyceride through decreased capacity for lipogenesis and increased lipolysis (6, 7, 14, 24, 40). These changes in adipose function lead to infiltration of immune cells, which are thought to cause chronic low-grade inflammation in obese subjects with the metabolic syndrome (8, 14). Lipid overload in the face of decreased capacity of adipocytes to store triglycerides leads to ectopic lipid deposition in liver and muscle resulting in systemic insulin resistance (14, 22). Prolonged obesity with these associated disorders is thought to be the major cause of β-cell failure and type 2 diabetes in humans.
It is now well established that expansion of VAT, rather than subcutaneous adipose tissue (SAT), confers a high risk for the metabolic syndrome and incident cardiovascular disease (7, 10, 41). Hypotheses for this observation include increased expression of angiotensinogen and complement, a greater rate of monocyte infiltration, and increased IL6 secretion in VAT compared with SAT (42). It has also been postulated that the elevated rates of lipolysis in VAT compared with SAT render the former less able to sequester triglycerides away from liver and muscle (20). These and other important differences between VAT and SAT have stimulated interest in the discovery of additional adipose depots that might have similar or increasingly pathologic functions in obesity. One such adipose depot is perivascular adipose tissue (PVAT), which was recently proposed to be a potential link between diabetes and cardiovascular disease (4, 13, 17, 32, 38). The outermost connective tissue or adventitia surrounding an artery, including PVAT, may be prone to the same inflammation as VAT, and the idea that vascular disease could be impacted and perhaps promoted by factors in the adventitia has long-standing support (25, 30, 35, 36).
Initial studies (4) of PVAT from human coronary arteries surprisingly detected expression of some BAT-specific genes (PRDM16, UCP-1, and CPT1B) to a degree that appeared intermediate to white adipose tissue (WAT) and BAT. These studies suggested PVAT might be an example of the recently described “brite” adipose tissue, white adipose that retains many of its usual characteristics but also displays some expression of BAT genes, including Ucp-1 (31). Interestingly, in rodents, PVAT surrounding the abdominal aorta displays characteristics of WAT, whereas PVAT surrounding the thoracic aorta appears quite different and is known to express some BAT genes (11, 32). Although thoracic PVAT showed similarities to BAT in morphology and expressed Ucp-1, a direct detailed genomic comparison to true interscapular BAT has not yet been made (11, 32). The extent to which thoracic PVAT in mice is more similar to human coronary PVAT, which displays only partial similarity to BAT, or to true interscapular BAT is an important question since mice are frequently used as models of human cardiovascular disease.
We addressed this question by directly comparing genome-wide expression of thoracic PVAT to brown and white adipose depots from mice fed a normal diet (ND) or high-fat diet (HFD) for 13 wk. Remarkably, PVAT and BAT had virtually identical gene expression profiles. Furthermore, compared with the infiltration of macrophages into VAT of obese mice and the increased expression of macrophage-enriched genes in this tissue, thoracic PVAT and BAT were strikingly resistant to inflammation induced by high-fat feeding, as shown by microarray, immunohistochemistry, and FACS analysis. We conclude that PVAT surrounding the murine thoracic aorta is virtually identical to interscapular BAT and, like BAT, is resistant to inflammation under HFD conditions.
MATERIALS AND METHODS
Animal studies.
Male C57BL6/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were fed normal chow until 8 wk of age. Animals were then divided into two groups; one fed normal chow and one fed HFD (45 kcal% fat; D12451; Research Diets) for 13 or 20 wk. Animals were fed ad libitum with free access to water and housed in the University of Massachusetts Medical School (UMASS) Animal Medicine facility with a 12:12-h light-dark cycle. Animals were weighed weekly for the duration of the diet study. Intraperitoneal glucose tolerance testing (GTT) was performed as previously described (33). Area under the curve for the GTT was calculated using the trapezoidal method (39). At the completion of the HFD, mice were fasted for 6 h and then euthanized with CO2 inhalation and bilateral pneumothorax. SAT (inguinal), VAT (epididymal), and BAT (interscapular) were harvested and snap frozen in liquid nitrogen. Blood was drawn via cardiac puncture into heparin-coated tubes, and the circulatory system was perfused with ice-cold PBS. Thoracic PVAT directly adjacent to the lesser curvature of the aortic arch was then harvested and snap frozen in liquid nitrogen. All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee at UMASS Medical School.
Insulin levels.
Blood was collected via cardiac puncture at the time of euthanasia into heparin-coated microcentrifuge tubes and centrifuged at 3,000 g for 15 min. Serum was then decanted and stored at −80°C. Fasting insulin levels were measured in duplicate with 10 μl of serum using a rat/mouse insulin ELISA (Millipore, St. Charles, MO) according to the manufacturers′ instructions.
Quantitative PCR.
Adipose tissue was isolated as previously described, snap frozen in liquid nitrogen, and stored at −80°C. Tissues were homogenized and total RNA was isolated with RNA mini lipid kits (Qiagen, Valencia, CA). Two-hundred fifty nanograms of total RNA were reverse transcribed with the Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). cDNA was diluted 1:5, and 2.5 μl was used in a 12.5-μl reaction volume; each reaction was performed in duplicate. Real-time quantitative (q)PCR was performed with a Bio-Rad C1000 Thermal Cycler and SYBR Green Master Mix (Bio-Rad) using the following cycle parameters: 95.0°C for 3 min, 95.0° for 0:10 min, 60.0°C for 0:15 min, and 72.0°C for 0:30 min for 40 cycles. Expression was normalized to the reference gene 36b4 and expressed as relative to expression in SAT of ND mice using the 2−ΔΔCt method (26). Melt curve analysis and agarose gel electrophoresis were performed to determine the specificity of the PCR reaction products. Primer sequences were as follows: Ucp-1 for AGGCTTCCAGTACCATTAGGT and Ucp-1 rev CTGAGTGAGGCAAAGCTGATTT; Acrp30 for TGTTCCTCTTAATCCTGCCA and Acrp30 rev CCAACCTGCACAAGTTCCCTT; 36b4 for TCCAGGCTTTGGGCATCA and 36b4 rev CTTTATCAGCTGCACATCACTCAGA; Cidea for ATCACAACTGGCCTGGTTACG and Cidea rev TACTACCCGGTGTCCATTTCT; and PPARγ2 for ATGGGTGAAACTCTGGGAG and PPARγ2 rev GTGGTCTTCCATCACGGAGA.
Microarray analysis.
RNA was isolated from SAT, VAT, BAT, and PVAT as previously described. RNA concentrations were determined using a Nanodrop 2000 Spectrophotometer (Thermo Fisher, Wilmington, DE). The RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Only samples with a RNA integrity number >7.5 and normal 18- and 28-s fractions on microfluidic electrophoresis were used. RNA from two mice per tissue and diet was pooled for a total of 250 ng total RNA template for cDNA synthesis and in vitro transcription using the Ambion WT expression kit (Ambion, Carlsbad, CA). Second-strand cDNA was then labeled with the Affymetrix WT terminal labeling kit, and samples were hybridized to Affymetrix Mouse Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA). Gene chip expression array analysis for individual genes was performed as previously described (39), filtering for P < 0.05 and a fold change of >2. Three biological replicate hybridizations per tissue and diet were performed, for a total of 24 hybridizations. Robust multiarray average was adopted in the UMASS Microarray Computational Environment (MACE) to preprocess raw oligonucleotide microarray data. The algorithm was implemented as a function of the R package Affy (2), which is part of the Bioconductor project (12) using the statistical computing language R (R Foundation for Statistical Computing, Vienna, Austria). All statistical calculations are performed using the R statistical computing environment, and the results are stored in a relational database. The preprocessed data are stored as base 2 log transformed real signal numbers and are used for fold-change calculations and statistical tests and to determine summary statistics. Mean signal values and SDs are computed for each gene across triplicate experiments and stored in the database. The fold change of expression of a gene in two experiments is the ratio of mean signal values from these experiments and is always a number greater than one. If the ratio is less than one, the negative value of the inverse ratio is stored as fold change. All downregulated genes therefore have a negative fold change value, and upregulated genes have a positive fold change. In both cases, this value is greater or equal than one.
To determine differential expression of genes in two hybridization experiments, MACE internally conducts a Student's t-test with the expression signal values of the two hybridizations for all genes in the set. The t-test value and test P value are stored in MACE and can be queried through the MACE user interface. The P values stored and displayed as a result of a query are not adjusted for multiple testing. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (9) and are accessible through GEO Series Accession No. GSE28440 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE28440).
Histology and immunohistochemistry.
Adipose tissue samples (n = 3 per group) from normal and HFD animals were fixed in 4% formalin for immunohistochemistry. Briefly, samples were embedded in paraffin, sectioned, and stained with a rat anti-mouse F4/80 (ABd Serotec, Raleigh, NC; 1:50 dilution) or rat anti-mouse CD68 primary antibody (ABd Serotec; 1:40 dilution). Staining was visualized with a horseradish peroxidase-linked rabbit anti-rat secondary antibody. Staining with the secondary antibody alone was performed as a negative control. Images were taken with a Zeiss Microscope and PixeLINK SE Software.
Transmission electron microsopy of adipose tissue samples.
Adipose tissue samples were fixed in 2.5% glutaraldehyde in PBS (pH 7.2). Tissue samples were dissected into 1-mm blocks, and the tissues washed three times and left overnight in fresh buffer. The next morning the samples were postfixed for 1 h in 1% osmium tetroxide. The fixed adipose tissue samples were then washed again in the PBS and dehydrated through a graded series of ethanol to 100% and transferred through two changes of propylene oxide and finally into a 50:50 (vol/vol) mixture of propylene oxide:epoxy resin (SPON 812/Araldite 502) and left overnight to infiltrate. The following morning the adipose tissue samples were processed through two changes of fresh epoxy resin and embedded, allowing the blocks to polymerize 48 h at 70°C.
Blocks of tissue were then selected, cut out, and attached to blank epoxy stubs with a drop of Super Glue. Ultrathin sections were cut on a Reichart-Jung ultramicrotome using a diamond knife. The sections (64-nm thick) were collected and mounted on copper support grids and contrasted with lead citrate and uranyl acetate and examined on a Philips CM 10 transmission electron microscope at 80-Kv accelerating voltage.
FACS analysis.
Mice were euthanized, and BAT and VAT were removed and treated with 4 and 2 mg/ml collagenase, respectively, in 4% BSA in PBS at pH 7.4 for 1 h. Samples were filtered through 200- and 30-μM spectra mesh sequentially and stained with CD31 (BD Biosciences, Franklin Lakes, NJ; 1:1,000 dilution), CD11b (BD Biosciences; 1:200 dilution), and CD11c (BD Biosciences; 1:200 dilution) according to the manufacturer's instructions. Samples were run on a BD LSRII flow cytometer (BD Biosciences) and analyzed in Flow Jo, gating for CD31-negative cells.
Statistical analysis.
All values are shown as means ± SE. For experiments other than the microarray analyses, the Student's t-test for two-tailed distributions with equal variances was used for comparison between two groups; for comparisons greater than ≥3 groups, two-way ANOVA followed by the Bonferroni correction was used. Differences <P ≤ 0.05 were considered significant. Data were entered into Microsoft Excel, and statistical analyses were performed with Graph Pad Prism 5.
RESULTS
Cidea and Ucp-1 are highly expressed in BAT and PVAT independent of obesity.
HFD mice (n = 12) gained more weight than ND mice (n = 12) beginning at 3 wk and remained significantly more obese until the end of the study (30.9 ± 0.75 vs. 42.08 ± 0.78 g; P < 0.001). At the end of 13 wk, HFD mice were more glucose intolerant as shown by an elevated fasting blood glucose concentration (136.4 ± 1.22 vs. 185.8 ± 21.4 mg/dl; P < 0.02), elevated fasting insulin levels (2.1 ± 0.64 vs. 5.4 ± 1.03 ng/ml; P < 0.02), and increased area under the curve for the intraperitoneal GTT (29,352 ± 753 vs. 38,983 ± 1,928 min·mg−1·dl−1; P < 0.01).
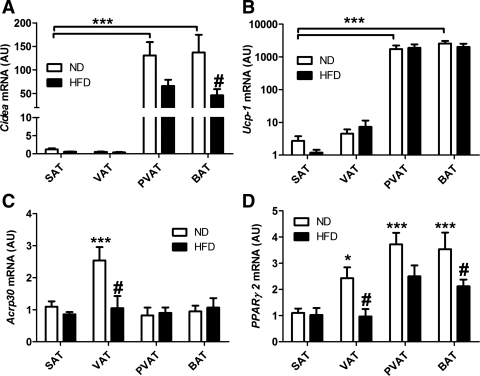
Analysis of mRNA from adipose tissue samples by qPCR showed that Cidea and Ucp-1 were highly expressed in PVAT and BAT compared with SAT. Remarkably, the relative expression between these two depots was similar for both genes (Fig. 1, A and B). Interestingly, Cidea expression was reduced >50% by HFD in both PVAT and BAT; this effect was not observed for Ucp-1 (Fig. 1, A and B). With respect to other markers of adipose differentiation, PPARγ2 expression was slightly greater in VAT, PVAT, and BAT than SAT and significantly reduced by HFD in these depots (Fig. 1D). Adiponectin (Acrp30) expression was significantly greater in VAT and decreased with HFD (Fig. 1C); expression of this gene was similar in all other depots. Therefore, PVAT expresses Cidea and Ucp-1 to an equivalent degree as BAT, suggesting that it may be more similar to BAT than brite adipose tissue. Furthermore, it is responsive to HFD and not different from other adipose depots with respect to makers of adipose differentiation, such as PPARγ2 and Acrp30.
Fig. 1.
Cidea and Ucp-1 are highly expressed in interscapular brown adipose tissue (BAT) and perivascular adipose from the aortic arch (PVAT) independently of obesity. A, B, C, and D: Cidea, Ucp-1, Acrp30, and Pparγ2 expression in normal diet (ND) and high-fat diet (HFD) mice. Quantitative PCR was performed on total RNA isolated from inguinal (SAT), epididymal (VAT), BAT, and PVAT. Expression levels were calculated with the 2−ΔΔCt method using 36b4 as the reference gene and normalized to expression in SAT from ND conditions. Ucp-1 expression is shown in log10 scale. AU, arbitrary units. ND: n = 12 white; HFD: n = 12, black. Results are means ± SE. ***P < 0.001 vs. SAT ND; #P < 0.05. ND vs. HFD of the same fat depot using two-way ANOVA and the Bonferonni correction; P < 0.05 vs. SAT ND.
Mouse thoracic PVAT is morphologically similar to BAT.
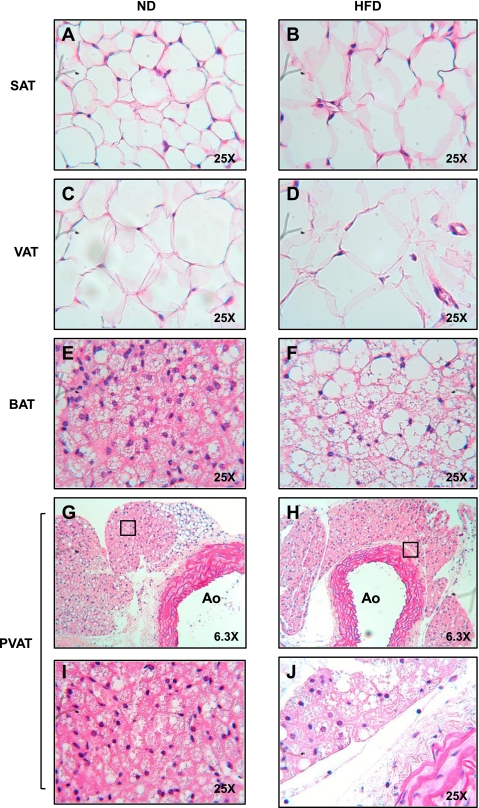
To test the hypothesis that thoracic PVAT is virtually identical to interscapular BAT, we performed microscopic analysis of these adipose depots. As judged by light microscopy on hematoxylin and eosin-stained sections of PVAT and BAT under ND and HFD conditions, PVAT appeared very similar to BAT, with round nuclei, and small, multilocular lipid droplets (Fig. 2G). The majority of PVAT surrounding the thoracic aorta had the appearance of BAT, with small areas of adipose tissue resembling WAT (Fig. 2G). BAT lipid droplet architecture was obviously distorted by 13 wk of HFD, with enlargement and coalescence of lipid droplets (Fig. 2F). Surprisingly, this effect was not apparent in PVAT, although it did occur with a longer duration of HFD (see Fig. 6H).
Fig. 2.
PVAT appears morphologically similar to BAT. Fat was harvested from SAT (A and B), VAT (C and D), interscapular BAT (E and F), and PVAT (G–J) from the lesser curvature of the aortic arch from ND and HFD fed mice and then fixed in formalin. Tissues were stained with hematoxylin and eosin and visualized at ×25. Images G and H are low magnification images (×6.3) of I and J. Ao, aortic lumen.
Fig. 6.
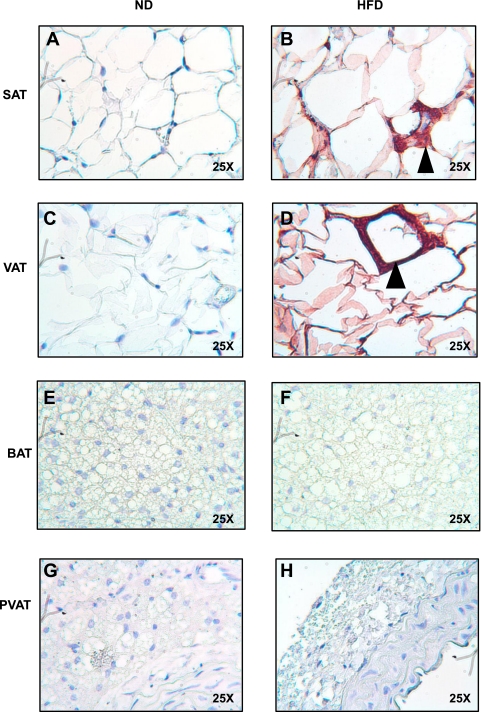
Perivascular and brown adipose tissue are resistant to inflammation after 20 wk of HFD. A second cohort of mice was continued on HFD for 20 wk. SAT (A and B), VAT (C and D), BAT (E and F), and PVAT (G and H) from the aortic arch was harvested from lean and obese mice (n = 3 per group). Samples were fixed in 4% formalin, sectioned, and stained with a rat anti-mouse F4/80 primary antibody (ABd Serotec). Staining was visualized with a HRP linked rabbit anti-rat secondary antibody. Again, abundant macrophages were seen in VAT (D). No macrophages were seen in BAT (F) or PVAT (H) despite distortion and enlargement of lipid droplet morphology (magnification: ×25).
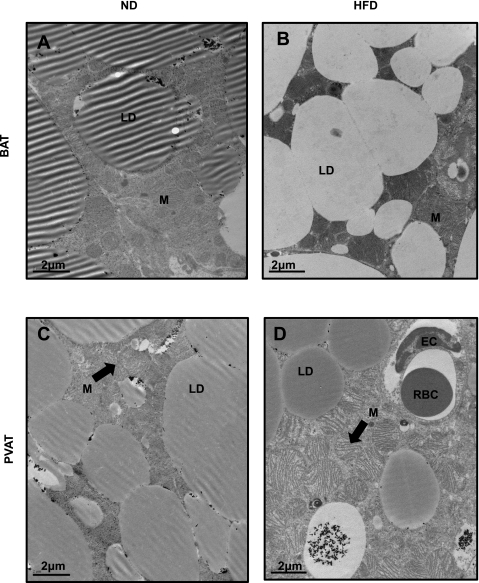
Transmission electron microscopy confirmed the BAT phenotype of thoracic PVAT and detected some interesting differences compared with interscapular BAT (Fig. 3). Both PVAT and BAT from mice fed ND were densely packed with mitochondria and had mulitilocular lipid droplets that were darkly stained with osmium tetroxide (Fig. 3, A and C). Interestingly, lipid droplets in BAT tended to lose their avidity for osmium when obtained from mice after high-fat feeding, mostly appearing white (Fig. 3B). This effect was not observed in PVAT (Fig. 3D). High-fat feeding resulted in significant mitochondrial swelling and unfolding of cristae in PVAT (Fig. 3D), which was not apparent in BAT at this time point but was visible after a longer duration of HFD (data not shown). These observations correlated with changes in gene expression. For example, Fads1 was significantly downregulated (−1.82) in BAT compared with PVAT in HFD conditions. Likewise, a search for alterations in the expression of mitochondrial genes revealed that expression of the proapoptotic gene Bid and mitochondrial solute carrier genes Slc25a35 and Slc25a37 was upregulated in PVAT compared with BAT in HFD conditions; a third member of this latter gene family, the calcium binding carrier protein Aralar1(Slc25a12), was significantly upregulated in BAT compared with PVAT (2.58 FC; P = 0.002; supplemental Table S4). Therefore, thoracic PVAT and BAT appear virtually identical upon analysis by both light and electron microscopy, with the exception of subtle differences in lipid droplet and mitochondrial morphology, which correlate with changes in gene expression.
Fig. 3.
Transmission electron microscopy reveals many similarities between PVAT and BAT. Sections of brown (A and B) and perivascular adipose (C and D) were taken and stained with osmium tetroxide. In ND conditions, brown (A) and perivascular adipose (C) appear very similar with mulitilocular lipid droplets and abundant mitochondria. Two prominent changes were noted in high-fat feeding conditions; lipid droplets in BAT, but not PVAT, lose their avidity for osmium textroxide (B), and mitochondria become swollen with unfolded cristae (D); the latter effect was more prominent in perivascular adipose but also observed in brown adipose after a longer duration of HFD. LD, lipid droplet; M, mitochondria; EC, endothelial cell; RBC, red blood cell. Magnification: ×7900; scale bar = 2 μm.
Microarray analysis confirms PVAT has a characteristic brown adipose gene expression signature.
To validate and expand the above findings, DNA microarrays were used to contrast global differences in gene expression between adipose depots from mice under control and high-fat feeding conditions. Total RNA was hybridized to Affymetrix Mouse Gene 1.0 ST chips, each of which contains probes targeting 28,853 genes. Analysis of the full genome set of gene expression differences among the four adipose depots showed a high similarity of PVAT to BAT and a much greater divergence of gene expression compared with white adipose depots (SAT and VAT; Fig. 4; Table 1; Supplemental Tables S1–4; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website). When comparing PVAT to BAT in ND conditions, only 228 genes demonstrated significantly different expression (Fig. 4; Supplemental Table S3). With regard to the specific genes that were differentially expressed, compared with BAT, PVAT had greater expression of immunoglobulin genes (IgK and Igh-6), complement (Cfh and C7), and mast cell-specific genes (Mcpt4; Supplemental Table S3). In contrast, the vast majority of genes whose expression was greater in BAT than PVAT were related to skeletal muscle differentiation and function (Supplemental Table S3). Interestingly, additional genes expressed to a greater extent in BAT than PVAT were highlighted by the mesodermal developmental genes Tbx15 (3.8-fold), Myf6 (3.2-fold), and Zic1 (2.1-fold; Supplemental Table S3).
Fig. 4.
Microarray analysis reveals that PVAT is more similar to BAT than SAT or VAT. Microarray Computational Environment (MACE) database was queried in ND and HFD conditions for genes with a >2.0 fold change in expression at P < 0.05 level of significance between adipose depots. In ND conditions, PVAT was very similar to BAT, with differential expression of only 228 genes (0.79% of genes); in contrast, expression of 1,229 genes was differentially regulated when comparing PVAT and SAT (4.2% of genes). After high-fat feeding, PVAT became more similar to white adipose, as the number of genes differentially regulated between PVAT and SAT was reduced to 855 (2.9% of genes). White arrows, upregulated; black arrows, downregulated.
Table 1.
Depot-specific expression of select gene categories in normal diet conditions
| PVAT vs. SAT |
PVAT vs. VAT |
PVAT vs. BAT |
|||||
|---|---|---|---|---|---|---|---|
| Symbol | Gene Name | FC | P value | FC | P value | FC | P value |
| Most highly upregulated genes | |||||||
| Ucp1 | uncoupling protein 1 | 21.6 | <0.0010 | 31.2 | <0.001 | 1.0 | 0.85 |
| Elovl3 | elongation of very long chain fatty acids (FEN1/Elo2) | 19.3 | <0.001 | 29.3 | <0.001 | 1.1 | 0.54 |
| Slc27a2 | solute carrier family 27 (fatty acid transporter), member 2 | 17.9 | <0.001 | 17.2 | <0.001 | 1.4 | 0.26 |
| Cox7a1 | cytochrome c oxidase, subunit VIIa 1 | 16.1 | <0.001 | 23.1 | <0.001 | 1.0 | 0.61 |
| Cpt1b | carnitine palmitoyltransferase 1b, muscle | 14.5 | <0.001 | 24.7 | <0.001 | 1.0 | 0.44 |
| Kng2 | kininogen 2 | 14.0 | <0.001 | 12.4 | <0.001 | 1.2 | 0.48 |
| Acot11 | acyl-CoA thioesterase 11 | 12.6 | <0.001 | 15.8 | <0.001 | 1.0 | 0.83 |
| Cidea | cell death-inducing DNA fragmentation factor-like effector A | 12.6 | <0.001 | 31.8 | <0.001 | 1.1 | 0.11 |
| Brown adipose-enriched genes | |||||||
| Ucp1 | uncoupling protein 1 (mitochondrial, proton carrier) | 21.6 | <0.001 | 31.2 | <0.001 | 1.0 | 0.85 |
| Elovl3 | elongation of very long chain fatty acids (SUR4/Elo3, yeast)-like 3 | 19.3 | <0.01 | 29.2 | <0.001 | 1.1 | 0.54 |
| Slc27a2 | solute carrier family 27 (fatty acid transporter), member 2 | 17.8 | <0.001 | 17.1 | <0.001 | 1.4 | 0.26 |
| Cox7a1 | cytochrome c oxidase, subunit VIIa 1 | 16.1 | <0.001 | 23.0 | <0.001 | 1.0 | 0.68 |
| Cpt1b | carnitine palmitoyltransferase 1b, muscle | 14.5 | <0.001 | 24.7 | <0.001 | 1.1 | 0.47 |
| Cidea | cell death-inducing DNA fragmentation factor like effector A | 12.6 | <0.001 | 31.7 | <0.001 | 1.1 | 0.11 |
| Dio2 | deiodinase, iodothyronine, type II | 8.0 | <0.001 | 9.9 | <0.001 | −1.1 | 0.68 |
| Prdm16 | PR domain containing 16 | 1.8 | <0.001 | 2.41 | <0.001 | 1.0 | 0.70 |
| White adipose-enriched genes | |||||||
| Hoxc8 | homeobox C8 | −9.7 | <0.001 | −17.7 | <0.001 | 2.1 | 0.05 |
| Apol7c | apolipoprotein L 7c | −8.2 | <0.01 | −1.6 | 0.21 | 1.2 | 0.50 |
| Dapl1 | death associated protein-like 1 | −6.3 | <0.01 | −2.0 | 0.09 | 1.8 | 0.16 |
| Nnat | Neuronatin | −6.1 | <0.01 | −6.9 | <0.01 | 1.1 | 0.79 |
| Sncg | synuclein, gamma | −4.2 | <0.01 | −5.5 | <0.001 | 1.0 | 0.78 |
| Stap1 | signal transducing adaptor family member 1 | −4.7 | <0.05 | −1.4 | 0.48 | 2.9 | 0.07 |
| Grap2 | GRB2-related adaptor protein 2 | −3.9 | <0.01 | −1.4 | 0.22 | 1.5 | 0.15 |
| Mest | mesoderm specific transcript | −3.8 | <0.01 | −14.2 | <0.001 | 1.0 | 0.74 |
| Immune cell-enriched genes | |||||||
| Il7r | interleukin 7 receptor | −5.7 | <0.01 | −1.3 | 0.47 | 2.4 | 0.13 |
| Ccl5 | chemokine (C-C motif) ligand 5 | −5.6 | <0.05 | −1.3 | 0.37 | 1.6 | 0.48 |
| Cd3g | CD3 antigen, gamma polypeptide | −5.0 | <0.01 | −1.7 | 0.21 | 2.3 | 0.06 |
| Ccl8 | chemokine (C-C motif) ligand 8 | −4.1 | <0.01 | 1.0 | 0.74 | −1.3 | 0.27 |
| Ccr7 | chemokine (C-C motif) receptor 7 | −4.0 | <0.05 | −1.3 | 0.27 | 1.4 | 0.22 |
| Cd3d | CD3 antigen, delta polypeptide | −3.1 | <0.05 | −1.2 | 0.17 | −1.1 | 0.37 |
| Cd68 | CD68 antigen | −2.3 | <0.05 | −1.9 | <0.05 | −1.3 | 0.52 |
| Emr1 | EGF-like module containing, mucin-like, hormone receptor-like 1 | −2.1 | <0.05 | −2.3 | <0.01 | −1.3 | 0.34 |
| Fatty acid biosynthesis and metabolism | |||||||
| Fads3 | fatty acid desaturase 3 | −2.5 | <0.01 | −4.5 | <0.001 | −1.1 | 0.72 |
| Fads1 | fatty acid desaturase 1 | 1.5 | 0.05 | −1.8 | <0.01 | −1.7 | <0.05 |
| Cidec | cell death-inducing DFFA-like effector c | −1.1 | <0.05 | −1.6 | <0.01 | −1.3 | 0.06 |
| Scd1 | stearoyl-coenzyme A desaturase 1 | −1.0 | 0.10 | −1.0 | 0.24 | 1.0 | 0.08 |
| Fasn | fatty acid synthase | −1.5 | 0.12 | −1.5 | 0.05 | 1.0 | 0.96 |
| Gpam | glycerol-3-phosphate acyltransferase, mitochondrial | 2.6 | <0.001 | 1.4 | <0.01 | 1.0 | 0.67 |
| Acss1 | acyl-CoA synthetase short-chain family member 1 | 3.2 | <0.001 | 4.9 | <0.001 | 1.1 | 0.43 |
| Acsl5 | acyl-CoA synthetase long-chain family member 5 | 3.7 | <0.001 | 4.2 | <0.001 | 1.1 | 0.43 |
| Adipokine and related effector genes | |||||||
| Lep | Leptin | −2.7 | <0.05 | −4.6 | <0.01 | −1.3 | 0.36 |
| Retn | Resistin | −1.8 | <0.001 | −2.1 | <0.001 | −1.8 | 0.05 |
| IL6st | interleukin 6 signal transducer | −1.4 | <0.001 | −1.4 | <0.01 | −1.2 | 0.07 |
| Rbp4 | retinol binding protein 4 | −1.4 | <0.05 | −2.3 | <0.001 | −1.0 | 0.84 |
| Adipoq | adiponectin, C1Q and collagen domain containing | −1.3 | <0.05 | −1.6 | <0.01 | −1.1 | 0.26 |
| Tnfaip6 | tumor necrosis factor alpha induced protein 6 | −1.3 | <0.05 | −1.2 | <0.05 | −1.0 | 0.21 |
| Adipor1 | adiponectin receptor 1 | 1.3 | <0.01 | 1.1 | <0.05 | −1.0 | 0.68 |
| Adipor2 | adiponectin receptor 2 | 1.4 | <0.05 | −1.1 | 0.20 | 1.0 | 0.83 |
The eight most highly upregulated genes in perivascular adipose tissue (PVAT) vs. subcutaneous adipose tissue (SAT) in normal diet conditions were ranked in descending order. Expression levels of the same genes in PVAT vs. visceral adipose tissue (VAT) and PVAT vs. brown adipose tissue (BAT) in normal diet conditions were then queried and are entered in the last 2 columns for comparison. Expression of genes from select categories was also queried in normal diet conditions and ranked according in descending order according to expression in SAT. FC, fold change. Significant increases in expression are bold; significant decreases in expression are italicized.
Next, we refined this analysis by comparing gene expression differences in PVAT relative to BAT and SAT from mice fed ND with respect to selected specific gene categories (Table 1). Strikingly, there were no significant differences between PVAT and BAT in the levels of expression of any genes known to be highly expressed in brown adipocytes (Table 1), nor were there any differences between PVAT and BAT under ND conditions in the expression of the majority of specific genes examined, with the exception of Fads1 (Table 1). In contrast to these virtually identical gene expression profiles between PVAT and BAT, highly significant decreases in expression were displayed by brown adipocyte-enriched genes in SAT and VAT compared with PVAT (Table 1). As expected, expression of white adipose-enriched genes was significantly greater in SAT and VAT compared with PVAT (Table 1). In other gene categories, expression of the adipokines Lep, Retn, Rbp4, and Adipoq was higher in SAT and VAT, whereas the receptors for Adipoq (Adipor1/Adipor2) were more highly expressed in PVAT (Table 1). In regards to lipid metabolism genes, those involved in synthesis or storage (Fads1, Fads3, and Cidec) were expressed to a greater extent in SAT and VAT, whereas those functioning in lipid oxidation (Gpam, Acss1, and Acsl5) were more highly expressed in PVAT and BAT (Table 1). Surprisingly, expression of immune cell-enriched genes, such as chemokines (Ccl5 and Ccl8), T-cell receptors (Cd3g, Cd3d), and macrophage markers (Cd68, Emr1) was significantly greater in SAT and VAT compared with PVAT and BAT even under ND conditions (Table 1). Given this dramatic difference in expression of immune cell genes in white compared with brown adipose depots, we compared the effects of high-fat feeding on changes in expression of immune cell-enriched genes in the four adipose depots.
Table 2 shows the fold changes in expression for a series of selected immune cell enriched genes when comparing ND with HFD conditions in PVAT, BAT, SAT, and VAT depots. VAT demonstrated the greatest fold increase in expression of immune cell genes after high-fat feeding (Table 2). Although the other depots exhibited some trends for changes in gene expression between ND and HFD fed mice, none of them met statistical significance (Table 2). Taken together, the data in Fig. 4 and Tables 1 and 2 reveal a remarkably similar global gene expression profile between PVAT and BAT. These two adipose depots show no significant differences in expression in the majority of genes previously shown to be enriched in brown adipocytes, white adipocytes, or immune cells. In addition, the data in Table 2 are particularly revealing in that the levels of expression of immune cell genes, as reflected by their probe set signals, are uniformly higher in both SAT and VAT compared with PVAT and BAT in both ND and HFD conditions. These data imply that the PVAT and BAT depots are relatively resistant to inflammation induced by high-fat feeding compared with WAT depots.
Table 2.
Comparative expression of immune cell-enriched genes in VAT, SAT, PVAT, and BAT in normal and high-fat diet conditions
| SAT |
VAT |
PVAT |
BAT |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | ND | HFD | FC | P | ND | HFD | FC | P | ND | HFD | FC | P | ND | HFD | FC | P |
| Il7r | 2,038 | 1,299 | −1.5 | 0.20 | 268 | 2,779 | 10.4 | <0.001 | 178 | 350 | 1.9 | 0.41 | 62 | 164 | 2.6 | 0.07 |
| Tlr13 | 564 | 531 | −1.1 | 0.87 | 397 | 2,404 | 6.0 | <0.001 | 133 | 343 | 2.6 | 0.20 | 151 | 243 | 1.6 | 0.14 |
| Il1rn | 444 | 499 | 1.1 | 0.56 | 367 | 1,923 | 5.2 | <0.001 | 220 | 403 | 1.8 | 0.16 | 208 | 269 | 1.2 | 0.12 |
| Cd84 | 665 | 574 | −1.2 | 0.54 | 426 | 2,079 | 4.9 | <0.001 | 128 | 281 | 2.2 | 0.28 | 92 | 171 | 1.9 | 0.05 |
| Mpeg1 | 1,527 | 1,292 | −1.2 | 0.58 | 713 | 3,311 | 4.6 | 0.001 | 240 | 555 | 2.3 | 0.22 | 233 | 520 | 2.2 | 0.12 |
| Cd68 | 2,434 | 2,160 | −1.1 | 0.67 | 2,097 | 6,895 | 3.3 | <0.001 | 586 | 1,451 | 2.5 | 0.20 | 500 | 914 | 1.8 | 0.20 |
| Ccl3 | 303 | 329 | 1.1 | 0.61 | 207 | 662 | 3.2 | <0.001 | 111 | 194 | 1.7 | 0.18 | 116 | 182 | 1.6 | 0.18 |
| Emr1 | 1,247 | 1,177 | −1.1 | 0.84 | 1,276 | 3,800 | 3.0 | <0.001 | 283 | 604 | 2.1 | 0.23 | 242 | 475 | 2.0 | 0.11 |
| Ccl2 | 536 | 556 | 1.0 | 0.83 | 546 | 1,174 | 2.1 | <0.05 | 158 | 225 | 1.4 | 0.24 | 138 | 185 | 1.3 | 0.22 |
| Ccl9 | 1,457 | 1,691 | 1.1 | 0.56 | 1,529 | 3,262 | 2.1 | <0.01 | 347 | 780 | 2.2 | 0.16 | 249 | 467 | 1.8 | 0.11 |
| Tnfrsf1b | 911 | 735 | −1.2 | 0.34 | 682 | 1,183 | 1.7 | <0.01 | 226 | 304 | 1.3 | 0.29 | 184 | 256 | 1.4 | 0.05 |
Querying the Microarray Computational Environment (MACE) dataset for genes with the greatest fold increase in expression from normal diet (ND) to high-fat diet (HFD) conditions showed that VAT had the greatest increase in expression of inflammatory genes. The same probe sets showed nonsignificant increases in SAT and much smaller signals in PVAT and BAT. Italicized data are for genes that were significantly upregulated.
Thoracic PVAT and interscapular BAT are relatively resistant to macrophage infiltration under HFD conditions.
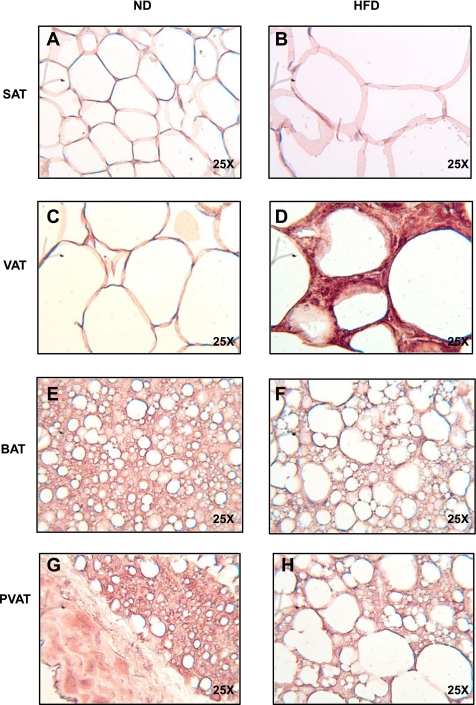
To directly test the extent of macrophage infiltration into BAT and PVAT, we immunostained sections of SAT, VAT, BAT, and PVAT from the thoracic aorta for the macrophage marker F4/80 (Fig. 5). Feeding mice a HFD for 13 wk resulted in significant macrophage infiltration in VAT and SAT, as expected (Fig. 5, B and D). In contrast, little or no F4/80 staining was detected in BAT or PVAT from these same mice (Fig. 5, F and H). To test whether a longer duration of HFD would result in inflammation of BAT or PVAT, we continued HFD for 20 wk in a second cohort of animals (Fig. 6). Again, visceral fat showed abundant macrophage infiltration (Fig. 6D), but despite marked enlargement and coalescence of lipid droplets in BAT and PVAT, macrophages were absent (Fig. 6, F and H). The same results were obtained after staining BAT and VAT from animals fed a HFD for 20 wk with a second macrophage marker, CD68 (Data not shown).
Fig. 5.
PVAT and BAT are resistant to inflammation after 13 wk of HFD. SAT (A and B), VAT (C and D), BAT (E and F), and PVAT (G and H) from the aortic arch was harvested from lean and obese mice (n = 3 per group). Samples were fixed in 4% formalin, sectioned, and stained with a rat anti-mouse F4/80 primary antibody (ABd Serotec). Staining was visualized with a horseradish-peroxidase-linked rabbit anti-rat secondary antibody. Abundant macrophages were seen predominantly in VAT, but also SAT, forming crown-like structures (arrowheads). No macrophages were seen in BAT or PVAT (magnification: ×25).
FACS analysis confirms prolonged HFD in mice results in little macrophage infiltration of BAT compared with VAT.
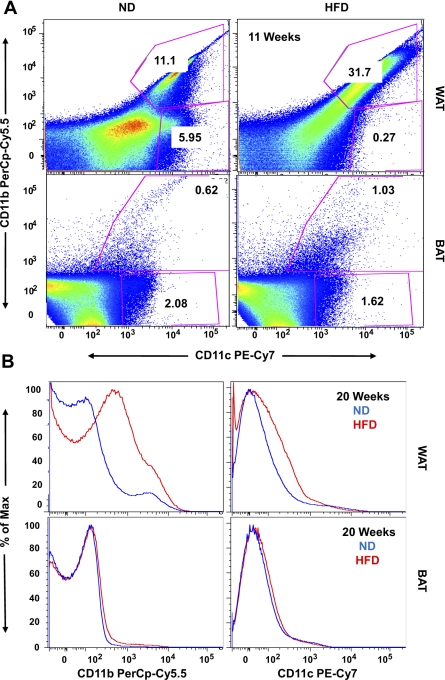
To better characterize the levels of macrophage accumulation in VAT vs. BAT, we performed FACS analysis on the stromal vascular fraction of collagenase-digested adipose tissue from VAT and BAT in ND and HFD conditions. CD68 and F4/80 markers define total macrophage populations in adipose tissue but do not differentiate between cells at various levels of activation (43, 45, 46). Therefore, after exclusion of CD31+ endothelial cells from the stromal vascular fraction, we chose to doubly stain cells for CD11b and CD11c, the latter being a marker of classical (M1) macrophage differentiation (45). Eleven weeks of HFD resulted in a striking and significant enrichment of CD11b+/CD11c+ macrophages in VAT compared with that observed for BAT (31.7 vs. 1.03%; Fig. 7A). As we failed to observe macrophage recruitment into BAT with 11 wk of HFD, we extended the duration of the HFD to 20 wk (Fig. 7B). This prolonged HFD duration resulted in a marked, significant increase in CD11b+/CD11c+ macrophages in VAT, while BAT showed no enrichment of this cell population. Unfortunately, we were unable to perform FACS on PVAT due to the small quantity of this tissue in the aortic arch. However, the results of FACS analysis confirm that VAT is enriched with M1 macrophages after 11 and 20 wk of HFD, while BAT was resistant to this effect.
Fig. 7.
BAT is resistant to inflammation after 11 and 20 wk of HFD. A: after 11 wk of HFD, the stromal vascular fraction was isolated from VAT and BAT and stained with CD31-PE, CD11b-PerCp Cy5.5, and CD11c-PE Cy7. Samples were analyzed on a LSRII flow cytometer, gating for CD31-negative cells. High-fat feeding resulted in a significant increase in the percentage of CD11b- and CD11c-positive cells in the SVF of visceral but not brown adipose (31.7 vs. 1.03%). B: similar results were obtained after 20 wk of HFD. Results represent 3 independent experiments.
DISCUSSION
A major finding of the present studies is that PVAT from the murine thoracic aorta displays a gene expression pattern that is nearly identical to interscapular BAT (Fig. 4; Table 1; Supplemental Tables S3 and S4). The similarity of gene expression between PVAT and BAT includes identical expression levels of the brown adipocyte genes Cidea, Ucp-1, Dio2, and Prdm16. Only 0.79% of genes represented on chip were differentially regulated between PVAT and BAT in ND conditions at the stringency level used. Interestingly, immunoglobulin genes were expressed to a greater extent in PVAT; this has also been demonstrated in human coronary PVAT (28). We hypothesize that this due to the presence of vascular-associated lymphoid tissue (1), although we have been unable to visualize such tissue using B-cell-specific markers (data not shown).
In contrast to these subtle differences between PVAT and BAT, major differences between PVAT and WAT were observed in the expression of genes previously shown to be highly and selectively expressed in BAT or WAT (Table 1). Our data thus clarify and definitively substantiate previous indications that mouse thoracic PVAT is similar to BAT (3, 11, 32). Importantly, these previous studies demonstrate that PVAT can have characteristics of both BAT and WAT but this dichotomy largely depends on the anatomical context, that surrounding the abdominal aorta resembling WAT and that surrounding the thoracic aorta BAT (11, 32). Our studies directly comparing thoracic PVAT gene expression (Table 1; Supplemental Tables S1–S4; Fig. 4) and histological appearance (Fig. 2) to mouse interscapular BAT show unequivocally that these two adipose depots are virtually identical. This appears different from human coronary PVAT, which demonstrates an intermediate phenotype between white and brown adipose (4).
After 13 wk of high-fat feeding, VAT displayed the expected dramatic increase in expression of many immune cell-enriched genes, while thoracic PVAT and BAT did not. This was somewhat surprising because some previous reports indicated that increased infiltration of macrophages and T cells into both PVAT (32, 38, 42) and BAT (16, 18) could be detected in mouse models. Xu et al. (46) reported that genetic and diet-induced obesity resulted in chronic inflammation in WAT but not BAT. Thus the extent of BAT inflammation may greatly depend upon the mouse strain and conditions used. Our data are consistent with the results of Xu et al., suggesting BAT is comparatively resistant to inflammation in C57BL6/J mice after HFD feeding.
In contrast to our present findings of very low inflammation of PVAT in mice after 13 wk of HFD, some investigators have proposed that PVAT is an intrinsically proinflammatory depot (4, 17, 28). For example, humans with established coronary artery disease have higher mRNA and protein levels of IL-1β, IL-6, MCP-1, and TNF-α in epicardial adipose than in paired SAT samples. More recently, Chatterjee et al. (4) published data regarding human coronary PVAT and murine thoracic PVAT, the same anatomic fat depot we have studied here. In control mice, relative mRNA expression of Acrp30, Pparγ, and Fabp4 was lower in PVAT than SAT. With 2 wk of HFD feeding, expression of these adipocyte-specific genes further declined, while expression of the proinflammatory genes Lep and Mip1α increased (4). The authors concluded that compared with SAT, PVAT was poorly differentiated and intrinsically proinflammatory and hence possibly an etiologic factor in the development of vascular disease (4).
Our findings are in contrast to the above (4) in that expression of Acrp30 and Pparγ2 was not different in thoracic PVAT compared with other fat depots. A possible explanation for the difference in expression of Acrp30 and Pparγ2 between the two studies is that there may be diet- or age-dependent changes in the expression of these two genes; Chatterjee et al. (4) used a shorter duration HFD and younger mice. For example, it may be that younger and older mice than those studied herein (21 wk) are prone to inflammation of PVAT. Two studies (32, 38) that have reported obesity associated inflammation of abdominal and femoral PVAT used 40- and 22-wk-old mice, respectively. Therefore, it is likely that many factors including age, strain, diet, and specific anatomic location influence whether or not PVAT becomes inflamed. Finally, although Chatterjee et al. (4) did not report expression of BAT genes in their mouse model, their failure to observe significant expression of immune cell-specific genes Cd68 and Cd3 by qPCR does concur with our results.
Importantly, both we and Chatterjee et al. (4) have studied PVAT from the thoracic aortic arch, as this a segment of the aorta prone to atherosclerosis in both humans and mice. Although PVAT was originally hypothesized to signal in a “vasocrine” fashion to influence vascular tone (47), it has subsequently been implicated in adventitial inflammation, which might promote atherosclerosis (4, 17, 28, 42, 47); our study was designed to test these latter hypotheses.
The overall evidence from the literature combined with our present work suggests that PVAT can have different characteristics depending on its anatomical location. Thus BAT-like adipose surrounds the thoracic aorta and WAT surrounds the abdominal aorta (11, 32). As such, we suggest that PVAT surrounding the abdominal aorta, like its visceral fat counterpart, is prone to the dysregulated adipocyte biology of obesity and subsequent inflammation. This idea has been verified by Takaoka et al. (38) who demonstrated that PVAT surrounding the femoral artery is WAT that has beneficial properties in lean conditions that are mediated by the paracrine effects of adiponectin (Acrp30). In obese states, inflammation and macrophage infiltration of PVAT surrounding the femoral artery result in decreased adiponectin secretion and increased Tnfα expression, both of which facilitate pathological neointimal hyperplasia in response to vessel injury (38). In humans, neointimal hyperplasia contributes to the pathophysiology of coronary artery disease. For example, diabetes and obesity are associated with increased rates of in-stent restenosis following percutaneous intervention due to neointimal hyperplasia (29). A scenario in which white PVAT is beneficial in lean conditions, but becomes dysfunctional in obese conditions, is analogous to the current model of obesity-induced insulin resistance (14). These and other reports support the concept that PVAT with white adipose features conforms to this model (13, 32, 38, 44). The important contribution of our work is that brown PVAT surrounding the thoracic aorta appears to be resistant to obesity-induced inflammation and hence may offer protection from the associated changes to the arterial adventitia.
The finding that PVAT and BAT display little or macrophage infiltration under HFD conditions, while WAT does, raises the interesting question: what is unique to WAT that causes infiltration of macrophages? A recent study by Kosteli et. al (22) may shed insight into this important question. Using caloric restriction of previously HFD fed mice, the authors showed that rates of adipocyte lipolysis correlate with macrophage infiltration. These studies suggest that the chronic elevation in WAT lipolysis observed in obesity causes increased release of fatty acids, which serve to stimulate macrophage infiltration (22). This hypothesis offers a potential explanation for why BAT and BAT-like PVAT fail to attract immune cells. The unique function of BAT and PVAT to rapidly metabolize fatty acids via high capacity for β-oxidation likely results in relatively low rates of local free fatty acid release from the cells compared with WAT (48). A second possibility is that the fatty acid species released by BAT and PVAT may also afford an anti-inflammatory effect. The ratio of saturated to polyunsaturated fatty acids has been shown to increase in the VAT and abdominal PVAT of high-fructose fed rats (34). Thus under these conditions WAT may release relatively higher levels of saturated fatty acids, which may be more active in stimulating macrophage chemotaxis (23, 37). Finally, abdominal or white PVAT may secrete proinflammatory adipokines or cytokines. We have found that although white adipose (SAT and VAT) has greater expression of adiponectin, which has anti-inflammatory effects, it also has greater mRNA expression of adipokines that correlate with vascular disease (Rbp4 and Resistin; Table 1; Refs. 5, 19). Furthermore, abdominal PVAT has been shown to secrete greater amounts of the chemokine MCP-1 than thoracic PVAT; our data also confirm that Mcp-1(Ccl-2) mRNA is increased in VAT but not thoracic PVAT after high-fat feeding (Table 2; Ref. 32). The comparatively greater diameter of white adipocytes in VAT and abdominal PVAT may be one factor responsible for increased Mcp-1 transcription (15, 21).
In summary, results from this present study demonstrate that PVAT surrounding the thoracic aorta is effectively BAT, as shown by light and electron microscopy, and full genome expression analysis. Thoracic PVAT and interscapular BAT are resistant to inflammation induced by 13 and 20 wk of HFD, as shown by reduced expression of immune cell-enriched genes, immunohistochemistry with macrophage markers, and FACS analysis for activated macrophages. This work provides an important mandate to study expression of BAT genes in human PVAT from patients with and without vascular disease. In light of the recent discovery of functional BAT in adult humans, and given the known beneficial metabolic and herein described anti-inflammatory properties of BAT, promotion of a BAT phenotype in the perivascular niche may have important effects in preventing vascular diseases such as hypertension and atherosclerosis.
GRANTS
We thank Hardy Kornfeld and the Millennium MD/PhD Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the Morphology Core Facility and the Genomics Core Facility of the UMASS Medical School Diabetes and Endocrinology Research Center (DK-32520) for assistance with histology and microarray analyses, respectively. These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-30898 and DK-60837 (to M. P. Czech) and American Heart Association Predoctoral Fellowship Grant No. 11PRE5000000 (to T. P. Fitzgibbons).
REFERENCES
- 1. Bobryshev YV, Lord RS. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis 154: 15–21, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Association of adiponectin, resistin, and vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol 31: 944–949, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Czech MP, Richardson DK, Smith CJ. Biochemical basis of fat cell insulin resistance in obese rodents and man. Metabolism 26: 1057–1078, 1977 [DOI] [PubMed] [Google Scholar]
- 7. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53: 1270–1287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Guilherme AVJ, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Cell Biol 9: 367–377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2276–2283, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hageman RS, Wagener A, Hantschel C, Svenson KL, Churchill GA, Brockmann GA. High-fat diet leads to tissue-specific changes reflecting risk factors for diseases in DBA/2J mice. Physiol Genomics 42: 55–66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henrichot EJAC, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 25: 2594–2599, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci USA 107: 240–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care 32: 733–735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S57–63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 74: 1479–1487, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120: 3466–3479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23: 201–229, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Li G, Chen YF, Kelpke SS, Oparil S, Thompson JA. Estrogen attenuates integrin-beta(3)-dependent adventitial fibroblast migration after inhibition of osteopontin production in vascular smooth muscle cells. Circulation 101: 2949–2955, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension 53: 577–584, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Mazurek TZL, Zalweski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein HJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108: 2460–2466, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Nikolsky E, Kosinski E, Mishkel GJ, Kimmelstiel C, McGarry TF, Jr, Mehran R, Leon MB, Russell ME, Ellis SG, Stone GW. Impact of obesity on revascularization and restenosis rates after bare-metal and drug-eluting stent implantation (from the TAXUS-IV trial). Am J Cardiol 95: 709–715, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104: 2228–2235, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest 116: 125–136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rebolledo A, Rebolledo OR, Marra CA, Garcia ME, Roldan Palomo AR, Rimorini L, Gagliardino JJ. Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc Diabetol 9: 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 89: 1111–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94: 1655–1664, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 27: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Takaoka MND, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Perivadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res 105: 906–911, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Tang X, Guilherme A, Chakladar A, Powelka AM, Konda S, Virbasius JV, Nicoloro SM, Straubhaar J, Czech MP. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc Natl Acad Sci USA 103: 2087–2092, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144: 5159–5165, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Vela DBM, Madjid M, Burke A, Naghavi M, Willerson JT, Casscells SW, Litovsky S. The role of periadventitial fat in atherosclerosis: an adipose subset with potential diagnostic and therapeutic implications. Arch Pathol Lab Med 131: 481–487, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Withers SB, Agabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol 31: 908–913, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 30: 186–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signaling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 35: 49–56, 2003 [DOI] [PubMed] [Google Scholar]