Abstract
Pathological conditions such as diabetes, insulin resistance, and obesity are characterized by elevated plasma and myocardial lipid levels and have been reported to exacerbate the progression of heart failure (HF). Alterations in cardiomyocyte Ca2+ regulatory properties and myofilament proteins have also been implicated in contractile dysfunction in HF. However, our prior studies reported that high saturated fat (SAT) feeding improves in vivo myocardial contractile function, thereby exerting a cardioprotective effect in HF. Therefore, we hypothesized that SAT feeding improves contractile function by altering Ca2+ regulatory properties and myofilament protein expression in HF. Male Wistar rats underwent coronary artery ligation (HF) or sham surgery (SH) and were fed normal chow (SHNC and HFNC groups) or a SAT diet (SHSAT and HFSAT groups) for 8 wk. Contractile properties were measured in vivo [echocardiography and left ventricular (LV) cannulation] and in isolated LV cardiomyocytes. In vivo measures of contractility (peak LV +dP/dt and −dP/dt) were depressed in the HFNC versus SHNC group but improved in the HFSAT group. Isolated cardiomyocytes from both HF groups were hypertrophied and had decreased percent cell shortening and a prolonged time to half-decay of the Ca2+ transient versus the SH group; however, SAT feeding reduced in vivo myocyte hypertrophy in the HFSAT group only. The peak velocity of cell shortening was reduced in the HFNC group but not the HFSAT group and was positively correlated with in vivo contractile function (peak LV +dP/dt). The HFNC group demonstrated a myosin heavy chain (MHC) isoform switch from fast MHC-α to slow MHC-β, which was prevented in the HFSAT group. Alterations in Ca2+ transients, L-type Ca2+ currents, and protein expression of sarco(endo)plasmic reticulum Ca2+-ATPase and phosphorylated phospholamban could not account for the changes in the in vivo contractile properties. In conclusion, the cardioprotective effects associated with SAT feeding in HF may occur at the level of the isolated cardiomyocyte, specifically involving changes in myofilament function but not sarcoplasmic reticulum Ca2+ regulatory properties.
Keywords: contractile function, calcium, dietary fat
heart failure (HF) is a progressive disorder often associated with hypertension, obesity, insulin resistance, and diabetes (17a). At the whole heart level, HF is characterized by deteriorating left ventricular (LV) function and at the cellular level, by decreased cell shortening, a prolonged relaxation time, and blunted responsiveness to β-adrenergic stimuli. In consideration of the comorbidities associated with HF (e.g., obesity, hypertension, and diabetes), dietary guidelines have traditionally recommended a low-fat/high-carbohydrate diet for coronary artery disease patients (19), although these recommendations are currently under revision (16, 42). Nonetheless, high dietary fat, particularly saturated fat, has long been implicated with myocardial lipid accumulation, contractile and mitochondrial dysfunction, apoptotic cell death, enhanced ventricular remodeling, and cardiac hypertrophy (35, 37).
Given the heart's limited capacity to store lipids, coupled with a decrease in the β-oxidation of fatty acids in HF, it has been suggested that excessive fat intake may increase the propensity for lipotoxicity (22, 23, 35), ultimately leading to contractile dysfunction. In contrast to the proposed lipotoxic effects on contractile function, we have previously reported no further progression of HF/LV dysfunction in rats fed a high-saturated fat (SAT) diet postinfarction compared with rats fed normal chow (NC). Rather, HF animals fed a SAT diet showed improvements in LV contractility (as assessed by peak LV +dP/dt) (34), LV maximal power (4), and mitochondrial function despite elevations in myocardial triglyceride (TG) and ceramide content (34). These results demonstrate that the administration of a high-fat diet immediately after coronary ligation surgery may ameliorate the deterioration in myocardial contractility typically associated with the progression of HF/LV dysfunction. However, the mechanism by which a high-fat diet exerts this cardioprotective effect and improves LV contractility in HF has yet to be determined.
At the level of cardiomyocytes, Ca2+ plays a critical role in myocyte contractility. Ca2+-handling properties are regulated by several proteins, including L-type Ca2+ channels, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), and phospholamban (PLB), all of which participate in coordinating Ca2+ movement between cellular compartments (3). Likewise, changes in Ca2+ regulation contribute to the contractile dysfunction associated with HF. In HF, the sarcoplasmic reticulum (SR) Ca2+ pool is often depleted due to downregulated SERCA activity, a decreased SERCA-to-PLB ratio, decreased PLB phosphorylation, and increased SR Ca2+ leak, resulting in decreased Ca2+ transients and impaired cardiomyocyte contractility (3). Similarly, at the myofilament level, the composition and phosphorylation of contractile proteins also impact contractility. The relative abundance of myosin heavy chain (MHC) α- and β-isoforms correlates with in vivo contractile function (41). Specifically, an increase in MHC-β contributes to decreased in vivo contractile function as a result of lower intrinsic ATPase activity and reduced myofilament shortening velocity (46). Likewise, the phosphorylation of other myofilament proteins, e.g., myosin-binding protein C (MyBP-C), troponin I, and myosin regulatory light chain (MRLC), have also been shown to alter Ca2+ sensitivity and/or cross-bridge kinetics, impacting both in vitro and in vivo contractility (31, 39). Alterations in the ratio of MHC isoforms and the phosphorylation status of other myofilament proteins have been implicated in the contractile dysfunction characteristic of HF in a variety of animal models (21, 25, 31, 39, 40). However, the mechanism(s) by which dietary lipids impact the Ca2+ regulation of contractile function and myofilament protein composition in HF has not been investigated.
Prior studies from our laboratory have established that SAT feeding is associated with improvements in global contractile function in coronary ligation-induced HF/LV dysfunction; however, a clear mechanism of action has yet to be identified. Given that cardiomyocyte contractility is largely dependent on Ca2+-handling properties and myofilament protein composition, this study examined the impact of high-fat diet feeding on the Ca2+ regulation of contractile properties and myofilament protein expression in isolated cardiomyocytes from rats with ligation-induced HF/LV dysfunction. We hypothesized that in infarcted rat hearts, improvements in intrinsic contractile performance associated with high dietary fat are the result of changes in Ca2+ regulatory properties and/or myofilament protein expression. Taken together, these results will contribute to our understanding of the cardioprotective effects of high-fat diet feeding in postinfarcted hearts.
MATERIALS AND METHODS
Experimental model.
This study was conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. The model used in this study has been previously described (34). Male Wistar rats (300–350 g) were anesthetized with isoflurane (1.5–2.0%) and ventilated during infarction surgery. HF/LV dysfunction was induced by ligation of the left main coronary artery as previously described (27). After ligation (HF) or sham surgery (SH), rats were randomly assigned a dietary group and fed ad libitum either a NC diet (SHNC and HFNC groups; 14% fat, 26% protein, and 60% carbohydrate) or a SAT diet [SHSAT and HFSAT groups; 60% fat (25% palmitic, 33% stearic, and 33% oleic acid), 20% protein, and 20% complex carbohydrates] (Research Diets, New Brunswick, NJ) for 8 wk.
Plasma metabolic substrates.
Glucose, free fatty acids (FFA), and TG concentrations in plasma were measured using enzymatic spectrophotometric kits (Wako Chemicals, Richmond, VA) (32).
Echocardiography.
Myocardial function was evaluated by echocardiography 7 wk postligation. A Sequoia C256 system (Siemens Medical, Malvern, PA) with a 15-MHz linear array transducer was used as previously described (27) with anesthetized rats (1.5–2.0% isoflurane). Two-dimensional, two-dimensional guided M-mode, and Doppler echocardiographic analyses of aortic and transmitral flows were performed via parasternal and foreshortened apical windows. End-diastolic and end-systolic dimensions were measured, and the ejection fraction was calculated as previously described (27) (n = 10–11). The criteria for a successful coronary artery ligation were an ejection fraction below 70% (based on the average ejection fraction of ∼90% in SH animals) and clear evidence of a scar (necrotic and thinned LV wall below the ligation suture).
Hemodynamic measurements.
In vivo LV contractile properties were assessed 8 wk after coronary artery ligation surgery (n = 11–14). Rats were anesthetized (1.5–2.0% isoflurane), intubated, and ventilated. A Millar 3.5-Fr microtip pressure transducer catheter was introduced via the right carotid artery as previously described (34). End points related to heart rate, LV maximum pressure, and peak rates of LV pressure rise and fall (+dP/dt and −dP/dt) were determined with the aid of PVAN/Chart 5 software (Millar Instruments, Houston, TX). Systemic vascular resistance was calculated as maximal pressure/cardiac output.
Histological assessment of cardiac morphology.
A separate set of hearts was harvested (n = 5–6) for the histological evaluation of myocyte and infarct size. All hearts were perfused with cardioplegic buffer [containing (in mM) 110 NaCl, 16 MgCl2, 16 KCl, 10 NaHCO3, 5 dextrose, and 1.2 CaCl2] to ensure a relaxation state and then formalin fixed and paraffin embedded. The base, mid-LV, and apex of the hearts were sliced into 6-μm-thick horizontal sections (∼1/4 of the distance from the apex to the base of the heart) using a slicer matrix. For infarct size measurements, samples from the apex were stained with Masson's trichrome (Sigma, St. Louis, MO). Infarct size was determined by quantifying the area of the entire LV (including the septum) and the area defined by the scar (evidenced by the thinned area in blue) using MetaMorph software (Molecular Devices, Sunnyvale, CA), and the scar size was expressed as a percentage of the total LV area. Due to the extreme thinning of the wall in the area of the infarction, the calculation of area of infarction underestimates the extent of the infarction in terms of actual surface area. For cross-sectional area measurements, all three locations of samples were stained with fluorescein conjugated wheat germ agglutinin (Invitrogen, Carlsbad, CA), which recognizes cell membranes, and 4′,6-diamidino-2-phenylindole (Invitrogen), which recognizes nuclei. The cross-sectional area of the myocytes was determined by measuring the area of the cells with the aid of MetaMorph software (Molecular Devices).
Cardiomyocyte isolation.
LV cardiomyocytes were isolated as previously described with modifications (2). Rats were anesthetized (1.5% isoflurane), heparin (200 units) was injected intravenously, and hearts were quickly excised and chilled in ice-cold Ca2+-free Tyrode buffer containing (in mM) 136 NaCl, 5.4 KCl, 1 MgCl2, 10 HEPES, 1.2 NaH2PO4(H2O), 5.6 d-glucose, 2 l-glutamine, and 5 taurine (pH 7.4). The coronary arteries were perfused at 37°C via the aorta with Tyrode buffer containing 2 mM CaCl2, followed by Ca2+-free Tyrode buffer and then Ca2+-free Tyrode buffer containing collagenase type II (Worthington, Lakewood, NJ). The entire LV from SH rats and the nonscar LV tissue from HF rats were removed and minced in modified Kraft-Brühe solution containing (in mM) 110 potassium glutamate, 10 KH2PO4, 25 KCl, 2 MgSO4, 20 taurine, 5 creatine, 0.5 EGTA, 20 glucose, and 5 HEPES (pH 7.4). Cells were filtered and resuspended in Ca2+-free Tyrode buffer containing 0.5 mg/ml BSA. Ca2+ was reintroduced in a graded fashion to a final concentration of 1 mM. Cells were used within 4 h after isolation. Cardiomyocyte isolation procedures typically yielded ∼2 million cells with ∼50% viability.
In vitro cardiomyocyte shortening and Ca2+ transients.
Cardiomyocyte shortening and Ca2+ transients were measured as previously described with modifications (33). Cardiomyocytes were field stimulated at 1 Hz using a Grass stimulator (Astro-Med, West Warwick, RI). A video edge detector (Crescent Electronics, Windsor, ON, Canada) with 60-Hz temporal resolution was used for cell shortening measurements. Cell shortening (absolute shortening/maximum relaxation cell length, presented as percent cell shortening) and peak velocity of cell shortening and relaxation (−dL/dt and +dL/dt) were calculated (n = 117–137). For Ca2+ transient measurements, cells were incubated with 1 μM fura-2 AM (Invitrogen) at room temperature for 15 min; this concentration of fura-2 had no effect on cell shortening. The remaining dye was washed out twice after 10 min of gravity cell settling. Fura-2 was excited at 340 and 380 nm through a computer-controlled high-speed random access monochromator, and the fluorescent signals were detected at 510 nm by an analog/photon counting photomultiplier detector with background fluorescence measured with an unloaded myocyte from the same treatment group beforehand and automatically subtracted by FeliX32 software (Photon Technology, Birmingham, NJ). Ca2+ transients were recorded with a sampling frequency of 120 Hz and expressed as the ratio of fluorescence at 340- to 380-nm wavelength (n = 130–168). Only myocytes with clear edges that remained viable throughout the entire recording were included. All experiments were performed at 37°C under continuous buffer flow conditions. All data were analyzed using Matlab.
Protein expression by Western blot analysis.
Nonscar LV tissue (n = 6–8) was homogenized in Mammalian Protein Extraction Reagent buffer (Thermo Scientific, Lake Barrington, IL) containing protease and phosphotase inhibitor cocktails (Sigma). Protein concentrations were quantified by BCA protein assay (Thermo Scientific). Fifteen micrograms of protein were loaded onto 7.5% Tris·HCl gels (Bio-Rad, Hercules, CA) for SERCA2. Forty-five micrograms of protein were loaded onto 18% Tris·HCl gels for phosphorylated (p-)Ser16/Thr17 PLB (p-PLB16 and p-PLB17) as well as PLB. Heat shock chaperone 70 (HSC70) was chosen to serve as a loading control. Gels were run at 200 V for 1 h and then transferred to polyvinylidene difluoride membranes at 100 V for 45 min. Membranes were incubated overnight with 1:2,000 anti-SERCA2 (MA3-919, Thermo Scientific), 1:1,000 anti-PLB (05-205, Millipore, Billerica, MA), 1:1,000 anti-p-PLB16 (07-052, Millipore), 1:5,000 anti-p-PLB17 (A010-13, Badrilla, Leeds, UK), and 1:10,000 HSC70 (sc-7298, Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubations with the appropriate secondary antibodies. Membranes were incubated with chemiluminescence reagents (Thermo Scientific) and exposed to films. Densitometry of bands was determined using ImageJ (NIH). A standardizing sample was run on each gel to allow for normalizing densitometry across individual gels.
L-type Ca2+ current measurements.
Ca2+ currents (ICa) were acquired using whole cell patch-clamp techniques as previously described (5) with modifications. After baseline ICa was acquired, 1 μM isoproterenol (Iso) was applied, and the β-adrenergic response was recorded at steady state. The maximal β-adrenergic response at 4 min after exposure was analyzed (n = 6–9). All experiments were performed under continuous flow conditions at room temperature, and the ICa amplitude was normalized to myocyte capacitance (in pA/pF). Clampfit and Origin software (OriginLab, Northampton, MA) were used for electrophysiological data analysis.
MHC protein expression.
Protein contents of cardiac MHC-α and MHC-β were determined via gel electrophoresis as previously described with modifications (47). Nonscar LV tissue (n = 5) was homogenized in RIPA buffer. The protein concentration was quantified by BCA protein assay (Thermo Scientific). Ten micrograms of protein were loaded onto a 6% acrylamide gel cross-linked with N-N′-diallyltartardiamide. (See Ref. 47 for complete gel and buffer recipes.) Gels were run at 10-mA constant current for 19 h at 4°C. Bands were detected by silver staining (Bio-Rad) per the manufacturer's protocol. The densitometry was analyzed using the GelBandFitter analysis program as previously described (26).
Myofilament protein phosphorylation and expression.
LV tissue (n = 6) was harvested, and myofilament proteins were purified using a previously described protocol (20). Ten micrograms of protein were loaded onto a 4–12% XT Bis-Tris gel (Bio-Rad), followed by Pro-Q Diamond Phosphoprotein Gel staining (Invitrogen) per the manufacturer's protocol. The same gel was then stained with Coomassie blue for total protein expression. Densitometry of bands was performed using ImageJ (NIH). The degree of protein phosphorylation was quantified as Pro-Q density/Coomassie blue density.
Statistics.
All statistical analyses were performed using SigmaStat software. Differences were determined using two-way ANOVA followed by Bonferroni post hoc analysis for multiple pairwise comparisons unless otherwise noted. Significance was established at P < 0.05. Data are expressed as means ± SE.
RESULTS
Body weight and metabolic substrates.
High-fat diet feeding was associated with increased body weight in the SHSAT group but not in the HFSAT group, despite there being no significant difference in total caloric intake between groups (SHNC group: 574 ± 28 kcal/wk, SHSAT group: 645 ± 19 kcal/wk, HFNC group: 576 ± 25 kcal/wk, and HFSAT group: 617 ± 29 kcal/wk, P = 0.069). Although body weight in the HFSAT group was significantly lower than in the SHSAT group, coronary artery ligation surgery had no impact on body weight between the two HF groups (SHNC group: 509 ± 16 g, SHSAT group: 561 ± 11 g, HFNC group: 489 ± 12 g, and HFSAT group: 506 ± 15 g).
Plasma FFAs and TGs were increased with SAT feeding in both SHSAT and HFSAT groups. Plasma glucose was increased in both surgical (HFNC and HFSAT) groups compared with their SH controls (Table 1).
Table 1.
Plasma substrates, echocardiography, and hemodynamic function
| SHNC | SHSAT | HFNC | HFSAT | |
|---|---|---|---|---|
| Plasma substrates | ||||
| Glucose, mg/dl | 243 ± 16 | 261 ± 10 | 321 ± 19* | 279 ± 9* |
| Free fatty acids, μmol/ml | 0.14 ± 0.02 | 0.25 ± 0.01† | 0.18 ± 0.03 | 0.26 ± 0.03† |
| Triglycerides, mg/ml | 0.53 ± 0.05 | 0.87 ± 0.12† | 0.48 ± 0.09 | 0.68 ± 0.08† |
| Echocardiography | ||||
| End-diastolic area, cm2 | 1.03 ± 0.04 | 1.02 ± 0.03 | 1.18 ± 0.03‡ | 1.25 ± 0.05‡ |
| End-systolic area, cm2 | 0.43 ± 0.02 | 0.43 ± 0.02 | 0.73 ± 0.03‡ | 0.82 ± 0.06‡ |
| Ejection fraction, % | 90.1 ± 0.5 | 90.8 ± 1.2 | 58.2 ± 2.4‡ | 56.3 ± 3.1‡ |
| Hemodynamic function | ||||
| Maximum LV pressure, mmHg | 135 ± 3 | 129 ± 3 | 123 ± 4 | 132 ± 3 |
| LV +dP/dt, mmHg/s | 8,900 ± 477 | 8,751 ± 315 | 6,168 ± 121‡ | 7,633 ± 379‡§ |
| LV −dP/dt, mmHg/s | −9,045 ± 570 | −8,615 ± 369 | −5,686 ± 172‡ | −6,866 ± 400‡§ |
Values are means ± SE for plasma substrates (n = 8–10), echocardiography (n = 10–11), and hemodynamic results (n = 11–14). Animals were divided into the following groups: sham surgery (SH) with a normal chow (NC) diet (SHNC), SH with a high-saturated fat (SAT) diet (SHSAT), heart failure (HF) with a NC diet (HFNC), and HF with a SAT diet (HFSAT). LV, left ventricular.
P < 0.05, main effect for surgery;
P < 0.05, main effect for diet;
P < 0.05, HF vs. SH within diet;
P < 0.05, diet effect within HF.
Cardiac morphology.
Representative color images of infarct size at the apex of the LV are shown in Fig. 1A. Infarct size was not different between the HFNC and HFSAT groups (Fig. 1B). Representative fluorescent images of LV horizontal sections are shown in Fig. 1C. The cross-sectional area of the myocytes indicated hypertrophy in both HF groups compared with SH groups. Although high-fat diet feeding was associated with mild hypertrophy in the SHSAT group, the degree of hypertrophy was significantly decreased in the HFSAT group compared with the HFNC group (Fig. 1D).
Fig. 1.
Histological assessment of infarct size and myocyte cross-sectional area. Animals were divided into the following groups: sham surgery (SH) with a normal chow (NC) diet (SHNC), SH with a high-saturated fat (SAT) diet (SHSAT), heart failure (HF) with a NC diet (HFNC), and HF with a SAT diet (HFSAT). A: representative color images of stained apical cardiac slices with fibrotic tissue in blue and the remaining (viable) myocardium in red. B: infarct size expressed as a percentage of the total left ventricular (LV) area (n = 5–6). Due to the extreme thinning of the wall in the area of the infarction, the calculation of area of infarction underestimates the extent of the infarction in terms of actual surface area. C: representative fluorescent images at the apex of the LV with membranes shown in green and nuclei shown in blue. D: average of the cross-sectional area of the LV cardiomyocytes (n = 70/section/animal) from all sections (base, midventricular, and apex) of the heart (n = 5–6). *P < 0.05, HF vs. SH groups within diet; †P < 0.05, diet effect within SH or HF groups.
Echocardiography and hemodynamic function.
The progression of contractile dysfunction and LV remodeling was assessed by echocardiography at 7 wk and by direct LV cannulation at 8 wk after ligation. End-systolic and end-diastolic areas were increased in both HF groups compared with SH groups, indicative of LV remodeling subsequent to ligation surgery (Table 1). In addition, ejection fractions, an indirect measure of LV contractile function, were decreased in both HF groups compared with SH groups (Table 1). There was no evidence of LV dysfunction or remodeling due to high-fat diet feeding in the SH groups.
Hemodynamic assessment via direct LV cannulation confirmed that myocardial contractility was depressed in the HFNC group compared with the SHNC group, as measured by peak LV +dP/dt and −dP/dt (Table 1). However, peak LV +dP/dt and −dP/dt were significantly improved with high-fat diet feeding in the HFSAT group compared with the HFNC group. High-fat diet feeding in the SH groups did not alter peak LV +dP/dt or −dP/dt (Table 1). Heart rate (SHNC group: 324 ± 9 beats/min, SHSAT group: 332 ± 6 beats/min, HFNC group: 318 ± 6 beats/min, and HFSAT group: 329 ± 6 beats/min), maximum LV pressure (Table 1), and systemic vascular resistance (SHNC group: 1.28 ± 0.13 mmHg·ml−1·min−1, SHSAT group: 1.19 ± 0.09 mmHg·ml−1·min−1, HFNC group: 1.41 ± 0.18 mmHg·ml−1·min−1, and HFSAT group: 1.47 ± 0.15 mmHg·ml−1·min−1) were not different between groups.
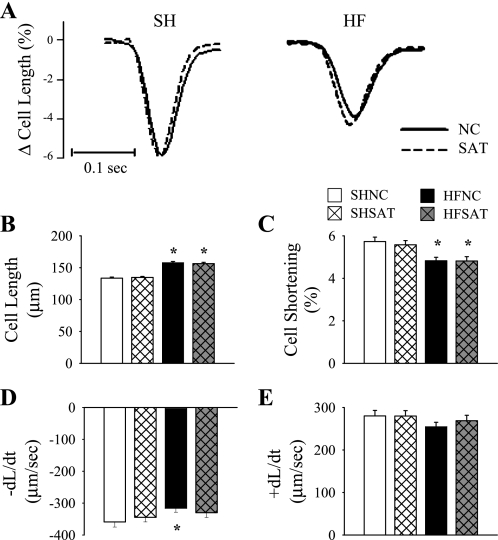
Cell shortening.
To assess whether improvements in contractile function in the HFSAT group were related to individual cardiomyocyte contractility, unloaded cell shortening was assessed in freshly isolated LV cardiomyocytes that had been field stimulated at 1 Hz. Representative recordings of cell shortening are shown in Fig. 2A. LV cardiomyocytes from HF hearts were hypertrophied (∼17% longer) compared with SH hearts (Fig. 2B). Cell shortening (expressed as a percentage after being normalized to cell length; Fig. 2C) was decreased in both HF groups compared with SH groups. Interestingly, −dL/dt was decreased in the HFNC group compared with the SHNC group but was not different in the HFSAT group (Fig. 2D). In contrast, +dL/dt was not different between groups (Fig. 2E).
Fig. 2.
LV cardiomyocyte shortening. A: representative tracings of cell shortening at 1 Hz averaged from 8 peaks from the same cell from each group. B–E: cell length (B), percent cell shortening (C), and velocity of cell shortening (−dL/dt; D) and velocity of relaxation (+dL/dt; E) were measured on fresh isolated LV cardiomyocytes at 1 Hz. n = 117–137. *P < 0.05 HF vs. SH groups within diet.
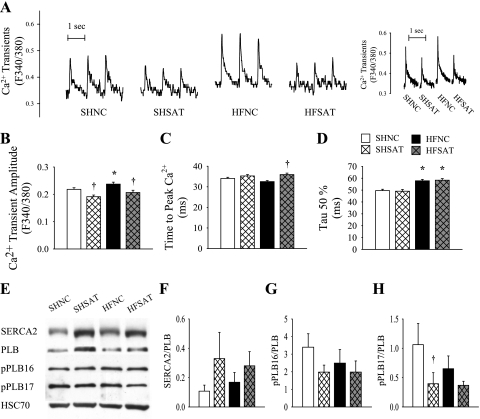
Ca2+ transients.
Representative Ca2+ transients are shown in Fig. 3A. Ca2+ transient measurements with fura-2-loaded LV cardiomyocytes revealed an unexpected trend relative to our measurements of in vivo contractile function. The mean amplitude of Ca2+ transients was increased in the HFNC group compared with the SHNC group and was decreased in both SH and HF groups fed the SAT diet (Fig. 3B). Although the time to peak Ca2+ was prolonged in the HFSAT group compared with the HFNC group (Fig. 3C), the time to half Ca2+ decay (τ50%) was prolonged in both HF groups compared with SH groups (Fig. 3D).
Fig. 3.
LV cardiomyocyte Ca2+ regulation. A: representative raw tracings of Ca2+ transients at 1 Hz (left) and tracings averaged from 10 peaks from the same cell from each group. B–D: Ca2+ transients (B), time to peak Ca2+ (C), and time to half Ca2+ decay (τ50%; D) were measured in fresh isolated LV cardiomyocytes loaded with fura-2 at 1 Hz. n = 130–168. E: representative bands of sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2), phospholamban (PLB), phosphoryated (p-)PLB at Ser16 (p-PLB16), p-PLB at Thr17 (p-PLB17), and heat shock chaperone 70 (HSC70). Protein expression was quantified using Western blot analysis. F: ratio of SERCA2 to PLB (both proteins normalized to HSC70). G: ratio of p-PLB16 to PLB. H: ratio of p-PLB17 to PLB. n = 6–8. *P < 0.05 HF vs. SH groups within diet; †P < 0.05 diet effect within SH or HF groups.
Ca2+ regulatory protein expression.
Representative Western blots for proteins involved in Ca2+ regulation are shown in Fig. 3E. SERCA2 normalized to our loading control HSC70 was significantly increased in the HFSAT group compared with the HFNC group (SHNC group: 0.14 ± 0.05, SHSAT group: 0.46 ± 0.13, HFNC group: 0.24 ± 0.07, and HFSAT group: 0.61 ± 0.21), but the PLB-to-HSC70 ratio was not different (SHNC group: 2.25 ± 0.69, SHSAT group: 3.59 ± 0.98, HFNC group: 2.26 ± 0.88, and HFSAT group: 2.52 ± 0.57). The ratio of SERCA2 to total PLB (both proteins normalized to HSC70; Fig. 3F) and p-PLB16 phosphorylation of PLB (Fig. 3G) were not different between groups. However, the p-PLB17-to-PLB ratio was downregulated in the SHSAT group compared with the SHNC group (Fig. 3H).
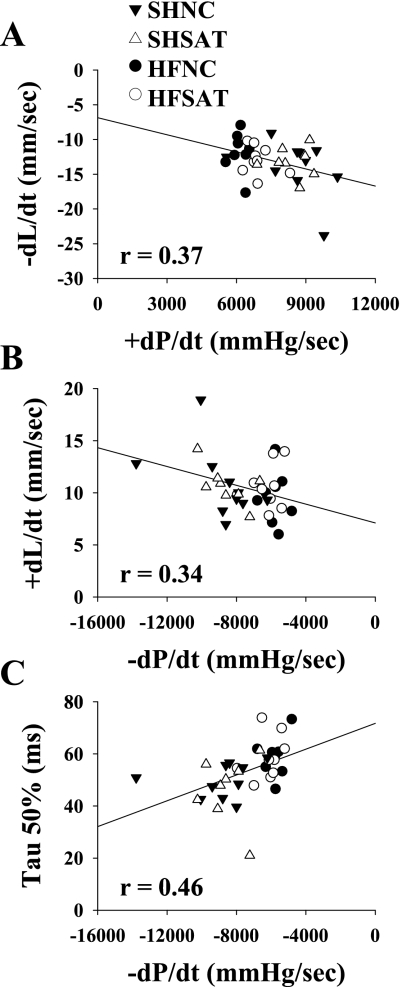
Correlations between in vivo and in vitro function.
A significant correlation was reported between in vivo and in vitro measures of systolic (cardiomyocyte −dL/dt and peak LV +dP/dt, r = 0.37, P < 0.05) and diastolic (cardiomyocyte +dL/dt and peak LV −dP/dt, r = 0.34, P < 0.05) contractility (Fig. 4, A and B). The changes reported in peak LV −dP/dt also correlated with cardiomyocyte τ50% (r = 0.46, P < 0.05; Fig. 4C). However, no relationship was found between functional changes in unloaded isolated cardiomyocytes (e.g., percent cell shortening and −dL/dt) and Ca2+ measurements (e.g., Ca2+ transients and time to peak Ca2+).
Fig. 4.
Correlations between in vivo and in vitro functional measurements. A–C: scatterplots showing significant correlations between LV cardiomyocyte −dL/dt and peak LV +dP/dt (A), LV cardiomyocyte +dL/dt and peak LV −dP/dt (B), and LV cardiomyocyte τ50% and peak LV −dP/dt (C). This analysis was performed using 1 data point/animal comparing in vivo and in vitro responses from the same animal.
ICa.
L-type ICa was measured using whole cell voltage-clamp techniques and normalized to cell size. Peak ICa at −10 V was analyzed. There was no effect of ligation surgery or diet at baseline (Fig. 5A) or with Iso stimulation (Fig. 5B). ICa was larger with Iso stimulation compared with baseline in the HFNC group by Student's t-test (P < 0.05).
Fig. 5.
L-type Ca2+ currents (ICa). ICa was measured on fresh isolated LV cardiomyocytes using whole cell voltage-clamp techniques in the absence (A) or presence (B) of 1 μM isoproterenol (Iso). n = 6–9.
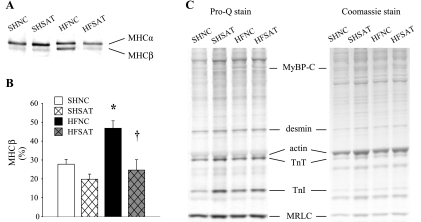
Myofilament protein composition and phosphorylation.
A representative gel silver stained for MHC is shown in Fig. 6A. (Bands for each protein were from the same gel, just reorganized according to group order.) The MHC isoform switch from α to β was evident only in the HFNC group. The slow isoform MHC-β content was elevated (both as a ratio of β to α and as a percentage; Fig. 6B) in the HFNC group but was unchanged in the HFSAT group. A representative Pro-Q-stained gel for myofilament protein phosphorylation and a Coomassie blue-stained gel for myofilament protein expression are shown in Fig. 6C. The degree of phosphorylation of MyBP-C, desmin, troponin T, troponin I, and MRLC were not different between groups.
Fig. 6.
Myofilament protein composition and phosphorylation. A: representative bands of myosin heavy chain (MHC) α- and β-isoforms from the same gel. Protein expression was quantified using electrophoresis followed by silver staining. B: MHC-β expressed as a percentage of total MHC. n = 5. C: representative Pro-Q- and Coomassie blue-stained gels. TnT, troponin T; TnI, troponin I. *P < 0.05, HF vs. SH groups within diet; †P < 0.05, diet effect within HF groups.
DISCUSSION
The results of this study extend our previous findings and demonstrate that the initiation of SAT diet feeding after the induction of mild-moderate HF improves cardiomyocyte contractility, specifically, −dL/dt, and is associated with decreased MHC-β content. Changes in isolated ventricular myocyte systolic (−dL/dt) and diastolic (+dL/dt and τ50%) function and reductions in in vivo myocyte hypertrophy correspond to improvements in in vivo myocardial contractile function (peak +dP/dt and −dP/dt) but not changes in Ca2+ regulatory properties. Ca2+ transients revealed an opposite trend to in vivo myocardial contractile function but were not due to changes in protein expression of SERCA and PLB or the phosphorylation of PLB. In summary, these data suggest that the improvement in myocardial contractile function associated with SAT diet feeding in HF occurs primarily at the level of myofilament composition and function.
An important goal of this study was to relate in vivo contractile function and cellular/molecular changes associated with high-fat diet feeding. Consistent with our previously published studies, in vivo contractile function (specifically, peak LV +dP/dt and −dP/dt) was significantly improved with high-fat diet feeding in HF. Ventricular cardiomyocytes from both HF groups had decreased fractional shortening compared with SH groups. However, −dL/dt was depressed in the HFNC group but not in the HFSAT group, a result that correlated with in vivo LV contractility as assessed by peak LV +dP/dt. Furthermore, in vivo diastolic function (peak LV −dP/dt) correlated with cardiomyocyte diastolic properties (+dL/dt and τ50%). These results suggest that in vitro myocyte function reflects the improvements reported in in vivo contractile function.
Results similar to those presented here have been reported by Howarth et al. (15) in a diabetic-prone mouse model. In their study (15), ventricular myocytes isolated from high-fat diet-fed animals had improved contractility and shortened relaxation times. Likewise, high palmitate levels resulted in accelerated Ca2+ transients and increased cell shortening in obese, insulin-resistant ob/ob mice (8). These authors speculated that increased fatty acid oxidation typically associated with obesity and type 2 diabetes may result in increased ATP production, thereby promoting better SR Ca2+ handling and improved contractile function. Whether myocytes from failing hearts can restore their metabolic flexibility and adapt to an increase in fatty acids, thereby reverting to an adult phenotype where fatty acids remain the primary energy source, is a current subject of debate (18, 43). In terms of clinical relevance, these results appear to be contradictory to the traditional diet-heart hypothesis that relates dietary fat to coronary heart disease. However, this diet-heart hypothesis has been under increasing scrutiny by the medical community, and dietary recommendations for patients with heart disease are undergoing considerable revisions (16, 42).
A marked switch from MHC-α to MHC-β was observed in the HFNC group, but this switch was prevented in HF animals fed the SAT diet. A MHC isoform switch is a key determinant of shortening velocity (which is primarily due to the intrinsic ATPase activity of fast α-MHC compared with slower β-MHC); this isoform shift translates to a decrease in contractility (44) and myocyte power output (13) and has been well documented in HF (21, 25, 40, 45). The increased expression of MHC-β in the HFNC group could account for the decreased cell shortening velocity observed in isolated cardiomyocytes. In contrast, the SAT diet prevented the increased expression of MHC-β and maintained normal cell shortening velocity. Okere et al. (30) also reported that a high-fat diet prevented a Mhc-α to Mhc-β isoform switch that was accompanied by reductions in LV remodeling and contractile dysfunction in hypertension-induced hypertrophy. In addition, increased glucose availability has been reported to promote the Mhc-α to Mhc-β isoform switch, whereas high fatty acid levels blocked the changes in myosin isoforms (41, 49) considered a hallmark for a progressive decline in cardiac function. Thus, there may be a metabolic link that functions to protect the heart under pathological conditions through alterations in gene and protein expression involved in regulating contractile function; however, a potential cardioprotective effect may be unique to our model of mild-moderate HF/LV dysfunction. Evaluation of this hypothesis in this and other HF models of varying severity requires further study.
Other studies (29, 30) have reported that a reduction in cardiac growth and LV hypertrophy is associated with a high fat diet-induced attenuation in contractile dysfunction and inductions of molecular markers of hypertrophy, e.g., MHC-β. Our results support this concept given that in vivo myocyte hypertrophy (cross-sectional area) was reduced in our HFSAT group compared with our HFNC group. A low-carbohydrate/high-fat diet has been associated with a reduced stimulation of the insulin signaling pathway, resulting in decreased cardiac growth, contractile dysfunction, and gene expression in response to hypertension (37). Interestingly, we (7) recently reported that HF animals fed a SAT diet exhibited preserved myocardial contractile function under conditions of myocardial insulin resistance and alterations in insulin signaling, specifically, diminished activation of Akt and increased total glycogen synthase kinase 3β. Similarly, a high-fat diet has also been reported to downregulate IGF-I (6), a factor previously implicated in upregulating MHC-β (12) and increasing cardiac hypertrophy (24). Although plasma IGF-I levels were not different (data not shown), it remains to be determined whether tissue IGF-I is decreased in a manner similar to our reports of insulin resistance accompanied by decreased insulin signaling. A concomitant downregulation of the insulin/IGF-I signaling pathway could account for changes in physiological and molecular markers of hypertrophy that, in turn, contribute to improvements in myocardial contractile function.
At the level of the SR, the Ca2+ transient amplitude was increased in the HFNC group compared with the SHNC group and was decreased in both SAT groups. Differences in Ca2+-handling proteins, specifically, the expression of SERCA2, PLB, and phosphorylation of PLB, cannot account for the reported differences in Ca2+ transients, suggesting that other Ca2+-handling proteins could be involved. These are intriguing findings in that they contradict our in vivo LV contractility and in vitro cell shortening velocity results. However, conflicting reports of Ca2+ transients and total SERCA and total/phosphorylated PLB have been reported in the same model of ligation-induced HF (1, 9, 10, 14, 28, 50). While the increased Ca2+ transients in the HFNC group might represent a compensatory response for the loss of viable myocardial tissue after infarction, it is contradictory to a decrease in myocyte shortening velocity. Furthermore, no correlations were reported between functional changes in isolated cardiomyocytes and Ca2+ measures. These results demonstrate that Ca2+ regulation of isolated cardiomyocyte contractile function may be dissociated from global in vivo function. In our model, factors such as alterations at the level of the myofilaments and/or an increased exposure to cellular lipids may affect the contractile function of isolated cardiomyocytes.
ICa was not different between HF and SH groups, a result confirming previous reports in the literature (14, 38, 51). Given that ICa is a function of many factors (e.g., number of functional channels, open probability, and availability), one explanation for this result is a combined effect of decreased channel protein expression and increased open probability, as reported in human HF (36) and canine tachycardia-induced HF (11). However, ICa was also not different with high-fat diet feeding. Long-chain fatty acids have been reported to directly activate voltage-dependent Ca2+ channels, resulting in Ca2+ overload during ischemia, possibly through modifications of the lipid-protein membrane interface (17). However, baseline Ca2+ concentrations were not different with HF or high-fat diet feeding (data not shown). Our results do suggest, however, that defects in ICa do not account for the observed differences in cardiomyocyte contractile function.
In this study, we focused on correlating in vivo cardiac function with in vitro cellular and molecular changes. We reported some discrepancies between in vivo hemodynamic and in vitro cardiomyocyte functional measurements that might be explained by structural differences (e.g., cardiomyocyte from the remote vs. border zone of the viable myocardium and alterations at the tissue level) and circulating factors (e.g., circulating adipokines and hormones) that were not examined in this study. Future studies should be directed at investigating additional mechanisms that could play a role in regulating in vivo functional differences in our model of HF/LV dysfunction, e.g., alterations in the responsiveness and regulation of β-adrenergic-mediated changes in contractile function.
In conclusion, we report that SAT diet feeding after the induction of mild-moderate HF/LV dysfunction can improve cellular contractility (cell shortening velocity); this change was associated with decreased MHC-β content. Furthermore, changes in isolated ventricular myocyte systolic and diastolic function correspond to improvements in in vivo myocardial contractile function and reductions in myocyte hypertrophy. These results suggest that the “cardioprotective” effect attributed to SAT feeding in HF may result from metabolic changes in the cardiac myocyte that lead to changes in myofilament function without affecting SR Ca2+ regulatory properties.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-081857 (to M. P. Chandler) and by American Heart Association Scientist Development Grant 0535361N (to M. P. Chandler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Cody Rutledge for the expert technical assistance during the initial stages of this project. The authors also thank Dr. Scott Howell (Department of Ophthalmology and The Visual Sciences Research Center) and Dr. Julian E. Stelzer (Department of Physiology and Biophysics, Case Western Reserve University) for the expert advice and technical assistance with the histological and myofilament assessments, respectively.
REFERENCES
- 1. Anand IS, Liu D, Chugh SS, Prahash AJC, Gupta S, John R, Popescu F, Chandrashekhar Y. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation 96: 3974–3984, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca2+ channel activity by a tyrosine kinase-independent mechanism. Mol Pharm 62: 554–565, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology 21: 380–387, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Berthiaume JM, Bray MS, McElfresh TA, Chen X, Azam SM, Young ME, Hoit BD, Chandler MP. Myocardial contractile response to physiological stress improves with high saturated fat feeding in heart failure. Am J Physiol Heart Circ Physiol 299: H410–H421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carnes CA, Janssen PML, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, Hamlin RL, Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem 282: 28063–28073, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Caton SJ, Bai YL, Burget L, Spangler LJ, Tschop MH, Bidlingmaier M. Low-carbohydrate high-fat diets: regulation of energy balance and body weight regain in rats. Obesity 17: 283–289, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Christopher BA, Huang HM, Berthiaume JM, McElfresh TA, Chen X, Croniger CM, Muzic RF, Chandler MP. Myocardial insulin resistance induced by high fat feeding in heart failure is associated with preserved contractile function. Am J Physiol Heart Circ Physiol 299: H1917–H1927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fauconnier J, Andersson DC, Zhang SJ, Lanner JT, Wibom R, Katz A, Bruton JD, Westerblad H. Effects of palmitate on Ca2+ handling in adult control and ob/ob cardiomyocytes. Diabetes 56: 1136–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Flagg TP, Cazorla O, Remedi MS, Haim TE, Tones MA, Bahinski A, Numann RE, Kovacs A, Schaffer JE, Nichols CG, Nerbonne JM. Ca2+-independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circ Res 104: 95–103, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Prahash AJC, Anand IS. Myocyte contractile function is intact in the post-infarct remodeled rat heart despite molecular alterations. Cardiovasc Res 48: 77–88, 2000 [DOI] [PubMed] [Google Scholar]
- 11. He JQ, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Hefti MA, Harder BA, Eppenberger HM, Schaub MC. Signaling pathways in cardiac myocyte hypertrophy. J Mol Cell Cardiol 29: 2873–2892, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Herron TJ, McDonald KS. Small amounts of α-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 90: 1150–1152, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Holt E, Tønessen T, Lunde PK, Semb SO, Wasserstrom JA, Sejersted OM, Christensen G. Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. J Mol Cell Cardiol 30: 1581–1593, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Howarth FC, Qureshi MA, Gbewonyo AJ, Tariq S, Adeghate E. The progressive effects of a fat enriched diet on ventricular myocyte contraction and intracellular Ca2+ in the C57BL/6J mouse. Mol Cell Biochem 273: 87–95, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hu FB. Diet and cardiovascular disease prevention: the need for a paradigm shift. J Am Coll Cardiol 50: 22–24, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci USA 89: 6452–6456, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: e391–e479, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115: 547–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, Jeor SS, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 102: 2284–2299, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, Solaro RJ, Shah AM. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 19: 1137–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 21. LeWinter M. Functional consequences of sarcomeric protein abnormalities in failing myocardium. Heart Fail Rev 10: 249–257, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med 12: 134–138, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010 [DOI] [PubMed] [Google Scholar]
- 24. McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase (p110α) pathway. J Biol Chem 279: 4782–4793, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Mercadier J, Lompre A, Wisnewsky C, Samuel J, Bercovici J, Swynghedauw B, Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res 49: 525–532, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Mitov MI, Holbrook AM, Campbell KS. Myocardial short-range force responses increase with age in F344 rats. J Mol Cell Cardiol 46: 39–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, Chandler MP, Hoit BD. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol 287: H2049–H2053, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Mørk HK, Sjaastad I, Sejersted OM, Louch WE. Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in postinfarction mice. Am J Physiol Heart Circ Physiol 296: H1069–H1079, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive Dahl salt-sensitive rat. Clin Expl Pharm Physiol 32: 825–831, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 48: 1116–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Palmer B. Thick filament proteins and performance in human heart failure. Heart Fail Rev 10: 187–197, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl ttransferase i activity in dogs with heart failure. J Card Fail 4: 121–126, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Ren J, Wold LE. Measurement of cardiac mechanical function in isolated ventricular myocytes from rats and mice by computerized video-based imaging. Biol Proc Online 3: 43–53, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res 79: 331–340, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipid 14: 281–287, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 98: 969–976, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Sharma N, Okere IC, Duda MK, Chess DJ, O'Shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res 73: 257–268, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sjaastad I, Bokenes J, Swift F, Wasserstrom JA, Sejersted OM. Normal contractions triggered by ICa,L in ventricular myocytes from rats with postinfarction CHF. Am J Physiol Heart Circ Physiol 283: H1225–H1236, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Solaro RJ, Kobayashi T. Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem 286: 9935–9940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66: 710–771, 1986 [DOI] [PubMed] [Google Scholar]
- 41. Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program. Ann NY Acad Sci 1188: 191–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taegtmeyer H, Stanley WC. Too much or not enough of a good thing? cardiac glucolipotoxicity versus lipoprotection. J Mol Cell Cardiol 50: 2–5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann NY Acad Sci 1047: 208–218, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the β (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol 278: H412–H419, 2000 [DOI] [PubMed] [Google Scholar]
- 45. van der Meer P, Lipsic E, Henning RH, Boddeus K, van der Velden J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol 46: 125–133, 2005 [DOI] [PubMed] [Google Scholar]
- 46. VanBuren P, Harris DE, Alpert NR, Warshaw DM. Cardiac V1 And V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res 77: 439–444, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149–151, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio 1: 251–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yue P, Long CS, Austin R, Chang KC, Simpson PC, Massie BM. Post-infarction heart failure in the rat is associated with distinct alterations in cardiac myocyte molecular phenotype. J Mol Cell Cardiol 30: 1615–1630, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Zhang XQ, Moore RL, Tillotson DL, Cheung JY. Calcium currents in postinfarction rat cardiac myocytes. Am J Physiol Cell Physiol 269: C1464–C1473, 1995 [DOI] [PubMed] [Google Scholar]