Abstract
Recently, attention has been focused on comparing left ventricular (LV) endocardial (ENDO) with epicardial (EPI) pacing for cardiac resynchronization therapy. However, the effects of ENDO and EPI lead placement at multiple sites have not been studied in failing hearts. We hypothesized that differences in the improvement of ventricular function due to ENDO vs. EPI pacing in dyssynchronous (DYSS) heart failure may depend on the position of the LV lead in relation to the original activation pattern. In six nonfailing and six failing dogs, electrical DYSS was created by atrioventricular sequential pacing of the right ventricular apex. ENDO was compared with EPI biventricular pacing at five LV sites. In failing hearts, increases in the maximum rate of LV pressure change (dP/dt; r = 0.64), ejection fraction (r = 0.49), and minimum dP/dt (r = 0.51), relative to DYSS, were positively correlated (P < 0.01) with activation time at the LV pacing site during ENDO but not EPI pacing. ENDO pacing at sites with longer activation delays led to greater improvements in hemodynamic parameters and was associated with an overall reduction in electrical DYSS compared with EPI pacing (P < 0.05). These findings were qualitatively similar for nonfailing hearts. Improvement in hemodynamic function increased with activation time at the LV pacing site during ENDO but not EPI pacing. At the anterolateral wall, end-systolic transmural function was greater with local ENDO compared with EPI pacing. ENDO pacing and intrinsic activation delay may have important implications for management of DYSS heart failure.
Keywords: electrical dyssynchrony, tachycardia-induced heart failure, hemodynamic function, transmural mechanics
systolic heart failure (HF) is a growing health concern for aging populations in the United States and other developed countries (24). Approximately 30% of HF patients are burdened with electrical dyssynchrony (14), which further compounds their condition and can result in detrimental ventricular remodeling. Cardiac resynchronization therapy (CRT) has been shown to reverse many of these changes; however, 30% of CRT patients fail to improve clinically (26), suggesting a need for better pacing strategies.
Maximizing hemodynamic benefit by varying stimulus timing and left ventricular (LV) lead placement (5, 8) appears to be the best approach towards improving CRT efficacy. Traditionally, the LV lead is positioned at the epicardium of the lateral wall, a region of delayed electrical activation, in patients with a left bundle-branch block (LBBB). Kass et al. (20) showed improved LV systolic function during LV pacing of the delayed lateral wall in patients with LBBB, whereas right ventricular (RV) septal pacing resulted in minimal improvement, suggesting that baseline activation sequence is a major determinant of LV systolic function. Additionally, in failing canine hearts with LBBB, optimal LV lead placement, defined as pacing sites that resulted in ≥90% of maximum improvement in the maximum rate of LV pressure change (dP/dtmax), was found to occupy a substantial area of the lateral wall and was associated with greater reductions in mechanical dyssynchrony (17).
LV endocardial pacing has been proposed as a potential approach for further improving ventricular function with CRT. In a subset of HF patients, endocardial CRT led to more uniform intraventricular synchrony and improved LV filling compared with epicardial CRT (16). van Deursen et al. (35) showed in nonfailing canine hearts that endocardial CRT significantly reduced the transmural dispersion of repolarization time and increased LV contractility and stroke work to a greater extent, compared with epicardial pacing. Derval et al. (13) demonstrated in nonischemic HF patients that the change in contractility during LV pacing at the best endocardial site was far superior to that achieved by pacing at the usual epicardial LV site near the coronary sinus. Direct comparison at a single LV site using paired endocardial and epicardial electrodes, however, showed no significant differences between endocardial and epicardial LV pacing. Additionally, there was no significant difference between endocardial and epicardial LV pacing, performed at a single LV site, in patients with ischemic cardiomyopathy (32). The discrepancies between these studies suggest that further investigation into the differences between endocardial and epicardial biventricular pacing (BVP) in the failing heart is needed using a larger number of paired LV pacing sites. Furthermore, while previous studies have suggested that pacing of the LV lateral wall should result in the greatest improvements, the relationship between the baseline extent of electrical activation delay at the LV pacing site and hemodynamic improvement during CRT is not well understood.
Therefore, we sought to investigate the hypothesis that the transmural location of the LV pacing lead and its position in relation to the baseline activation pattern are important determinants of ventricular function during BVP in nonfailing and failing hearts with pacing-induced electrical dyssynchrony.
MATERIALS AND METHODS
All animal studies were performed according to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Additionally, surgical protocols were submitted to and approved by the Animal Subjects Committee of the University of California, San Diego, which is accredited by the American Association for Accreditation of Laboratory Animal Care.
Surgical preparation.
Acute pacing studies were performed in six nonfailing, adult mongrel dogs (18–23 kg) and in six chronic infarcted tachycardia-paced HF dogs (19–21 kg). Animals were induced at the terminal study with intravenous propofol (4–6 mg/kg), intubated, and mechanically ventilated with a mixture of isoflurane (2%) and medical-grade oxygen (2 l/min) to achieve a surgical plane of anesthesia. For failing animals, to maintain a sustainable hemodynamic state, inotropic support and supplemental analgesia were provided with dopamine (3–10 ug·kg−1·min−1 iv) and buprenorphine (0.02–0.05 mg/kg sc), respectively. After a medial sternotomy and left thoracotomy at the fifth intercostal junction, the heart was positioned in a pericardial cradle. Intramyocardial markers were implanted to study three-dimensional transmural mechanics in the anterolateral LV wall, as described previously (38). Briefly, gold or lead markers (2 mm) were sewn to the apex and the bifurcation of the left main coronary artery at the anterior base. A custom platform was secured to the anterolateral wall, between diagonal branches of the left anterior descending artery, and three columns of 4–6 gold or lead markers (1–1.2 mm) were implanted. The platform was removed, and gold or lead markers (1.7 mm) were sewn at the epicardial surface of the entrance of each transmural column. Bipolar pacing wires were sewn to the left atrial appendage for atrial pacing. Custom-made bipolar plunge electrodes (10) were positioned at five LV sites (Fig. 1), with each site consisting of an endocardial and epicardial electrode pair. Two of these sites were located in clinically relevant regions of the lateral and posterolateral wall near the coronary sinus. An additional plunge electrode was positioned at the endocardium of the right ventricular apex (RVA). These bipolar electrodes were also used for recording electrical events when not used for ventricular pacing. Electrical dyssynchrony was created by atrioventricular (AV) sequential pacing of the left atrial appendage and the RVA at a fixed AV delay of 40 ms. LV cavity pressure and rate of change were monitored with a high-fidelity micromanometer (Konigsburg Instruments, Pasadena, CA) placed in the lumen via puncture of the LV apex and closure with a purse-string suture. The micromanometer was matched to a statically calibrated fluid-filled pressure gauge, which was zeroed at a level approximate to the center of the heart. Aortic flow was obtained with a Doppler flow probe (model no. T208; Transonic Systems, Ithaca, NY). LV volumes were measured with a conductance catheter (Webster Laboratories, Baldwin Park, CA) positioned in the LV cavity via the left carotid artery. Arterial and aortic pressures were monitored with fluid-filled gauges positioned in the right femoral artery and left subclavian artery, respectively. Lead II electrocardiogram was recorded, along with local electrograms from nonpaced LV electrodes.
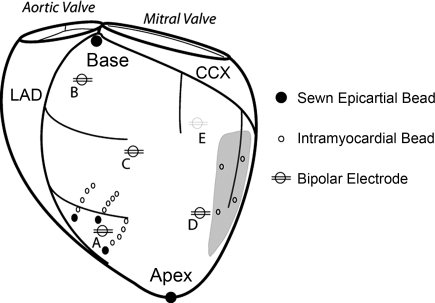
Fig. 1.
Experimental setup in nonfailing and failing hearts. Bipolar pacing electrodes were positioned on the left ventricle [i.e., in endocardial (ENDO) and epicardial (EPI) lead pairs] at the following locations: anterior apex (A), anterior base (B), anterolateral equator (C), lateral equator (D), and posterior coronary sinus (E; grey line). Note, in the nonfailing group the anterolateral equator location was replaced by a posterolateral equator location. Three columns of 4–6 intramyocardial markers (1–1.2 mm) were plunged into the anterolateral left ventricle and surface markers (1.7 mm) were sewn to the epicardial surface above each column. In the failing hearts, left ventricular scars (grey region) were present on the posterolateral wall and their locations were tracked with 3–4 gold markers (1.2–1.4 mm) implanted in the midwall. Left circumflex coronary (CCX) and left anterior descending (LAD) arteries are shown (black).
Tachycardia-induced HF animals.
We implemented a previously established canine model of tachycardia-induced HF and myocardial infarction (9), (19). In anesthetized dogs, arterial pressure was monitored by placing a small catheter, attached to a fluid-filled pressure transducer, into a side branch of the right femoral artery. Bipolar epicardial leads (CAPSURE EPI; model no. 4968; Medtronic, Minneapolis, MN) were sewn onto the right ventricular apical epicardium and routed to a programmable pacemaker capable of rates >180 beats/min (INSYNC III; model no. 8042; Medtronic). An implantable pressure gauge (model no. TA11PA-C40; Data Sciences International, St. Paul, MN) was inserted into the left atrial appendage to noninvasively monitor left atrial pressure once the chest had been closed. The left circumflex coronary artery was dissected, a marginal branch of the circumflex was permanently ligated, and the distal ends were embolized with multiple injections of microspheres (100–300 um; Biospheres Medical, Rockland, MA). LV scars were allowed to heal for 5 wk, and high rate ventricular pacing (220–250 beats/min) was performed for 5 wk. High rate pacing was discontinued for 24 h, and animals were then prepared for the terminal experimental study as described above. Cessation of high rate pacing 24 h before the terminal study was done primarily to ensure lower intrinsic sinus rates at the terminal study. Left atrial pressure was recorded at the onset, throughout the duration, and after 24 h of cessation of high rate pacing. Only animals with mean left atrial pressure values of three to four times the value recorded at the onset of high rate pacing, and displaying clinical signs of HF, were entered into the terminal experimental study.
Pacing protocol.
Atrial, RVA, and BVP were performed via stimulation of bipolar electrodes with square-wave pulse generators (model no. SD9; Grass Instruments, Quincy, MA). BVP was achieved via dual stimulation of the RVA electrode and one of the LV electrodes. In nonfailing hearts, LV pacing sites were anterior base, anterior apex, lateral equator, posterolateral equator, and posterior near the coronary sinus. In failing hearts, LV pacing sites were anterior base, anterior apex, anterolateral equator, lateral equator, and posterior near the coronary sinus (Fig. 1). All electrodes in the failing hearts were positioned ≥1 cm from the infarcted region. For each endocardial and epicardial pair, measurements during dyssynchrony were recorded to account for possible hemodynamic changes in baseline function over time. During pacing, hemodynamic variables were allowed to reach steady state before acquisition during expiration (∼2 min). Maximum and end-diastolic LV pressure, rate of LV pressure change (dP/dt), stroke volume, and ejection fraction were determined off-line using custom software in MATLAB 7.10 (Mathworks, Natick, MA). Pacing parameters were held constant at a frequency >20% of intrinsic rate, voltage >10% threshold value, and AV delay of 40 ms. During BVP, interventricular delay was held constant at 0 ms.
At the end of the study, animals were euthanized by an overdose of pentobarbital (100 mg/kg iv). Hearts were isolated in situ, perfused with cardioplegia, and perfusion fixed at zero LV pressure with buffered glutaraldehyde (5%).
Defining early, mid, and late LV activation sites.
During dyssynchrony, local LV activation times from all sites were measured, defined as the time from the onset of ventricular stimulus to the time of the maximum negative derivative of the electrode voltage (31). Hemodynamic improvement, expressed as percent change from dyssynchrony, and activation time at the LV pacing site were collected at all sites for nonfailing and failing hearts, respectively, and linear correlation analysis was performed. LV pacing sites were then categorized as early, mid, and late activation sites. Early activated (EARLY) sites were defined as having a transmural mean ≤ global mean − 1 SD; mid-activated (MID) sites defined as global mean − 1 SD < transmural mean < global mean + 1 SD; and late-activated (LATE) regions as having a transmural mean ≥ global mean + 1 SD. Epicardial activation was determined in greater detail for the failing animal group via a 128 unipolar electrode array sock positioned over the heart.
Three-dimensional coordinate reconstruction of implanted myocardial markers.
Radiopaque gold or lead markers (1–2 mm) were imaged with a biplane cineradiography system, and digital images from the two X-ray views were acquired at high temporal resolution (125 frames/s). Two-dimensional marker coordinates from each view were then corrected for spherical distortion (2) and reconstructed into three-dimensional coordinates (25) in a cardiac coordinate system define by five reference markers (27).
Histological measurement of mean fiber angle orientation.
To minimize the distortion effects of dehydration and shrinkage associated with paraffin embedding, histological measurements were obtained in freshly perfusion-fixed heart tissue. A transmural block from the anterolateral left ventricle, near the location of the implanted markers, was carefully excised with block edges cut parallel to the locally defined longitudinal, circumferential, and radial directions. Mean fiber angle measurements were then made at 1-mm transmural increments from epicardium to endocardium using a dissecting light microscope at low power (2). Mean fiber angle distributions in each animal were then corrected for geometric changes and variations in chamber pressure during fixation using the method of Takayama et al. (34). Briefly, three-dimensional strains were computed that mapped the deformation of the fixed ex vivo reference configuration to the in vivo end-diastolic configuration and used to calculate fiber angles in the deformed in vivo state.
Three-dimensional transmural fiber mechanics.
Nonhomogenous, Lagrangian strains were computed during atrial and local endocardial and epicardial activation at the anterolateral left ventricle. The reference frames were end diastole (i.e., notch of the micromanometer LV pressure) and the time of ventricular stimulus (i.e., time obtained from limb lead II ECG) for atrial and BVP, respectively. The deformed configuration was end systole (i.e., determined from aortic flow signal) for all pacing runs. Six independent cardiac strain components at end systole were computed at three transmural depths: subepicardium (25% wall depth), midwall (50% wall depth), and subendocardium (75% wall depth). At each wall depth, mean fiber angle measurements were used to rotate strains defined in a cardiac material coordinates (circumferential, longitudinal, and radial) into fiber material coordinates (fiber, cross-fiber, and radial) as described previously (11).
Histological measurement of volume, transmural extent, and wall thickness of LV scars.
Perfusion-fixed failing hearts with LV scars were skewered along a defined LV long axis (33), and cavity geometry was maintained with vinyl polysiloxane (3M ESPE Express, St. Paul, MN). With the impression material intact, the hearts were mounted in a custom Plexiglas cutting rig that allowed for serial short-axis sectioning in a defined plane perpendicular to the LV long axis. Multiple short axis slices (8–10) were sectioned at 5-mm increments. Digital images of apical and basal surfaces of each section were then recorded and imported to Image J (NIH). Scar tissue was identified grossly without the use of histological staining or light microscopy. For each slice, scar volume was defined as the average scar area from the apex and base surfaces multiplied by the slice thickness. Total scar volume was defined as percentage of total LV volume. Additionally, the transmural extent of the scar was qualitatively scored as follows: 1 (scar limited to either endocardial or epicardial surfaces), 2 (scar extends from surface to mid-wall), and 3 (scar extends from endocardial to epicardial surface). Transmural scar scores were then weighted by the slice thickness and averaged over all slices with scar present. Finally, LV wall thickness measurements were obtained from each slice and averaged at three anatomical locations: anterior (nonscar region), lateral (nonscar scar), and posterior (scar region).
Statistical analysis.
All measurements are reported as means ± SE, unless noted elsewhere, for absolute values and percentages. Statistical analysis was performed in SigmaPlot 11 (SyStat Software, San Jose, CA). For comparison of mean values between two groups, a two-tailed Student's t-test was used. Linear correlations between percent changes in hemodynamic parameters and activation time at the pacing site were performed in MATLAB, and t-statistics were calculated to test the null hypothesis of no correlation. Effects of transmural location and LV pacing site on changes in hemodynamic parameters were assessed with a two-way repeated-measures ANOVA. Post hoc comparisons between groups were performed with a Tukey's test. Significance was accepted for P < 0.05.
RESULTS
Animal characteristics and atrial paced function.
In failing hearts, mean left atrial pressure increased (P < 0.05) from 4.3 ± 1.7 to 24.0 ± 1.6 mmHg after 5 wk of high rate pacing (220–240 beats/min). After cessation of high rate pacing for 24 h, mean left atrial pressure decreased to 22.2 ± 1.1 mmHg (P = NS, compared with 24.0 ± 1.6 mmHg). End-diastolic in vivo wall thickness (i.e., estimated from myocardial markers) during atrial pacing was significantly decreased (P < 0.05) in the failing (9.3 ± 0.7 mm) compared with nonfailing (12.0 ± 1.0 mm) animals. Chamber dilatation was evident by significant increases in LV end-diastolic pressure (open-chest preparation) and unloaded LV volume-to-heart weight ratio (arrested and fixed at 0 mmHg) compared with the nonfailing hearts (Table 1). Maximum dP/dt, stroke volume, and ejection fraction were all significantly reduced in the failing compared with nonfailing hearts (Table 1). Three of nine animals enrolled in the failing protocol died prior to the conclusion of the terminal study. One was due to pacemaker failure and ventricular fibrillation, one due to ventricular fibrillation within an hour postmyocardial infarction, and one occurred during surgical preparation at the terminal study.
Table 1.
Animal characteristics and atrial paced function
| Body Weight, kg | LVV0, ml | LVV0/HW, ml/g | RR, ms | LVEDP, mmHg | dP/dtmax, mmHg/s | Stroke Volume, ml | Ejection Fraction, % | |
|---|---|---|---|---|---|---|---|---|
| Nonfailing | 25 ± 4 | 24 ± 3 | 0.15 ± 0.01 | 550 ± 9 | 8.5 ± 2.4 | 3,902 ± 632 | 28.2 ± 3.5 | 54.5 ± 4.5 |
| Failing | 19 ± 1* | 43 ± 5† | 0.27 ± 0.01† | 472 ± 19† | 15.8 ± 0.8† | 1,972 ± 309† | 9.3 ± 1.5† | 22.7 ± 3.7† |
Values are means ± SE; n = 5 (nonfailing) and 6 (failing). LVV0, left ventricular (LV) unloaded volume; HW, heart weight; RR, R-R interval; LVEDP, LV end-diastolic pressure; dP/dtmax, maximum rate of LV pressure change.
P < 0.01, failing vs. nonfailing;
P < 0.05, failing vs. nonfailing (comparisons between groups were performed with a Student's t-test).
Measured values of size, transmural extent, and wall thickness of LV scars.
Transmural scarring was observed in all animals, with the scar extending from the mid-basal plane towards the apex and located between the anterior and posterior papillary muscles. On average, scar volume (i.e., measured as %LV volume) was 12.8 ± 0.9% and ranged from 10.8 to 15.7%. Qualitative assessment of the transmural extent of the scar revealed marked myocardial damage across the wall. On average scars received a transmural score of 2.2 ± 0.1 (i.e., 3 representing a fully transmural scar), indicating that a majority of the scar volume extended throughout the wall thickness. Average wall thickness was 11.6 ± 0.7, 11.8 ± 0.4, and 7.4 ± 0.5 mm for the anterior, lateral, and posterior walls, respectively. Posterior (i.e., scar region) wall thickness was significantly lower than at the anterior (P < 0.001) and lateral (P < 0.0001) walls, indicating marked geometric remodeling due to myocardial infarction.
LV activation during dyssynchrony.
Electrical dyssynchrony (DYSS) was increased during DYSS for both nonfailing (114.0 ± 7.0 vs. 47.6 ± 5.0 ms; P < 0.001) and failing hearts (124 ± 9.0 vs. 54.4 ± 4.0 ms; P < 0.001) compared with atrial-paced values. In nonfailing hearts, mean transmural activation times for EARLY, MID, and LATE sites were 51.2 ± 8.0, 72.2 ± 10.5, and 87.6 ± 5.7 ms, respectively. In failing hearts, mean transmural activation times for EARLY, MID, and LATE were 54.4 ± 14.8, 80.5 ± 6.0, and 93.9 ± 5.4 ms, respectively. In the failing animal group, epicardial activation mapping revealed that the latest depolarized regions within the electrode array were activated at 106.0 ± 3.1 ms (referenced to ventricular stimulus), whereas the longest delay recorded from the epicardial bipolar pacing electrodes was 99.0 ± 3.0 ms. Qualitatively, in all six failing animals there was at least one LV epicardial lead near (<1 cm) or within the latest activated region measured within 128 electrode array.
Hemodynamic function for grouped endocardial and epicardial LV sites during BVP.
BVP at all LV sites were pooled into endocardial (ENDO) and epicardial (EPI) groups, respectively, to compare differences in hemodynamic parameters. In nonfailing hearts (Table 2), QRS duration was significantly reduced (P < 0.001) during ENDO (−31.7 ± 2.0%, relative to DYSS) compared with EPI (−12.9 ± 2.0%). Improvements in dP/dtmax (28.5 ± 3.7 vs. 16.6 ± 2.7%), stroke volume (21.2 ± 2.3 vs. 6.8 ± 2.7%), and stroke work (29.8 ± 4.7 vs. 16.6 ± 4.9%) were all significantly greater (P < 0.05) for ENDO than EPI. In failing hearts (Table 3), reduction in QRS width (−30.2 ± 1.7 vs. −14.3 ± 3.6%) was greater for ENDO than EPI (P < 0.001). Systolic parameters dP/dtmax (21.9 ± 3.1 vs. 11.0 ± 2.1%), stroke volume (21.1 ± 3.0 vs. 6.3 ± 2.6%), and ejection fraction (21.9 ± 2.9 vs. 7.1 ± 3.1%) were all greater for ENDO than EPI (P < 0.05). Additionally, dP/dtmin was greatest for ENDO (30.0 ± 4.9 vs. 13.3 ± 3.3%) compared with EPI (P < 0.05). Collectively, ENDO reduced electrical DYSS and improved hemodynamic function to a greater extent than EPI both in nonfailing and failing hearts.
Table 2.
Hemodynamic results during pacing in nonfailing hearts
| RR, ms | QRSd, ms | LVEDP, mmHg | LVPmax, mmHg | dP/dtmax, mmHg/s | dP/dtmin, mmHg/s | Stroke Volume, ml | Ejection Fraction, % | |
|---|---|---|---|---|---|---|---|---|
| DYSS | 531 ± 15 | 114 ± 7 | 7.0 ± 1.8 | 103 ± 10 | 2,487 ± 398 | −1,296 ± 214 | 19.2 ± 1.9 | 35.1 ± 2.7 |
| ENDO | 531 ± 5 | 78 ± 4* | 7.0 ± 0.6 | 115 ± 2† | 3,164 ± 103* | −1,596 ± 50† | 23.3 ± 0.3* | 42.4 ± 0.7* |
| EPI | 529 ± 5 | 98 ± 4 | 6.7 ± 0.4 | 112 ± 3 | 2,923 ± 70 | −1,424 ± 59 | 20.8 ± 0.7 | 37.6 ± 0.7 |
Values are means ± SE; n = 6. QRSd, QRS duration; LVPmax, LV maximum systolic pressure; dP/dtmin, minimum rate of LV pressure change.
P < 0.01, endocardial (ENDO) vs. epicardial (EPI);
P < 0.05, ENDO vs. EPI (comparisons performed with a two-way repeated measures ANOVA and Tukey's post hoc tests).
Table 3.
Hemodynamic results during pacing in failing hearts
| RR, ms | QRSd, ms | LVEDP, mmHg | LVPmax, mmHg | dP/dtmax, mmHg/s | dP/dtmin, mmHg/s | Stroke Volume, ml | Ejection Fraction, % | |
|---|---|---|---|---|---|---|---|---|
| DYSS | 471 ± 18 | 124 ± 9 | 13.4 ± 0.9 | 77.3 ± 4.3 | 1,062 ± 129 | −923 ± 70 | 6.1 ± 1.1 | 13.8 ± 2.3 |
| ENDO | 469 ± 17 | 86 ± 9* | 13.1 ± 1.0 | 83.9 ± 3.9 | 1,327 ± 147† | −1,179 ± 98† | 7.5 ± 1.1† | 17.2 ± 3.1† |
| EPI | 471 ± 17 | 101 ± 10 | 13.2 ± 4.2 | 81.7 ± 3.7 | 1,246 ± 152 | −1,078 ± 95 | 6.7 ± 1.0 | 15.7 ± 3.0 |
Values are means ± SE; n = 6.
P < 0.01, ENDO vs. EPI;
P < 0.05, ENDO vs. EPI (comparisons performed with a two-way repeated measures ANOVA and Tukey's post hoc tests).
Correlation between hemodynamic improvement and activation time at LV pacing site.
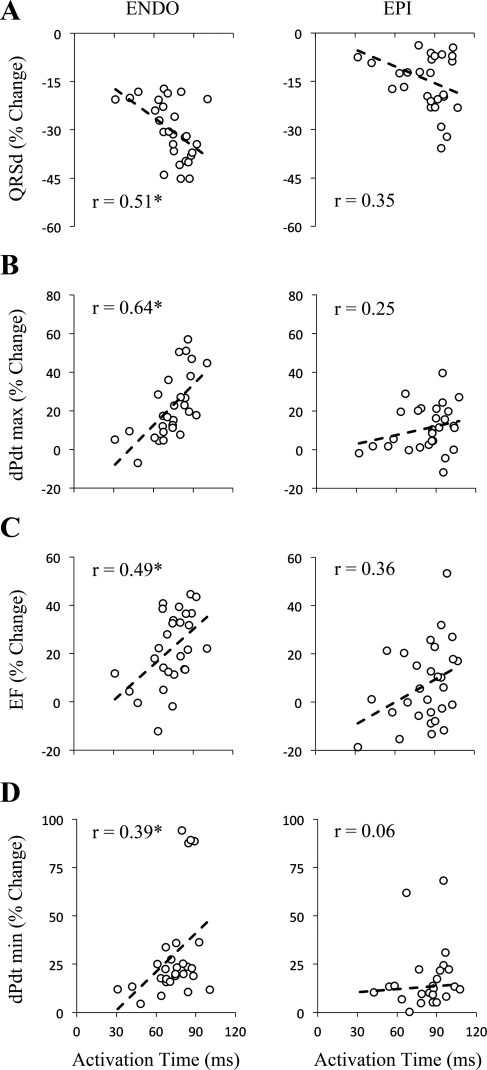
The following parameters: QRS duration (Fig. 2A), maximum dP/dt (Fig. 2B), ejection fraction (Fig. 2C), and minimum dP/dt (Fig. 2D) were all significantly correlated (P < 0.05) with activation time at the LV pacing site during ENDO (Fig. 2, left) but not EPI (Fig. 2, right) in failing hearts. During ENDO, correlation r values were 0.51, 0.64, 0.49, and 0.39 for QRS duration, maximum dP/dt, ejection fraction, and minimum dP/dt, respectively. During EPI the corresponding r values were 0.35, 0.25, 0.36, and 0.06, respectively. Qualitatively, these findings were similar in the nonfailing hearts (not shown); hemodynamic parameters were significantly correlated with activation time during ENDO but not EPI. These results indicate that activation time at the LV pacing site is a significant determinant of hemodynamic improvement during ENDO, but not EPI, BVP.
Fig. 2.
Grouped data during biventricular pacing at ENDO and 29 EPI sites in failing hearts. Activation time during dyssynchronous (DYSS) was used to correlate improvement (percent change) of hemodynamic parameters during ENDO and EPI. Percent changes in QRS duration (QRSd; A), maximum dP/dt (dPdt max; B), ejection fraction (EF; C), and minimum dP/dt (dPdt min; D) were all significantly correlated with activation time (P < 0.05) during ENDO (left) but not EPI (right). r, correlation r value. *P < 0.05, significant slope.
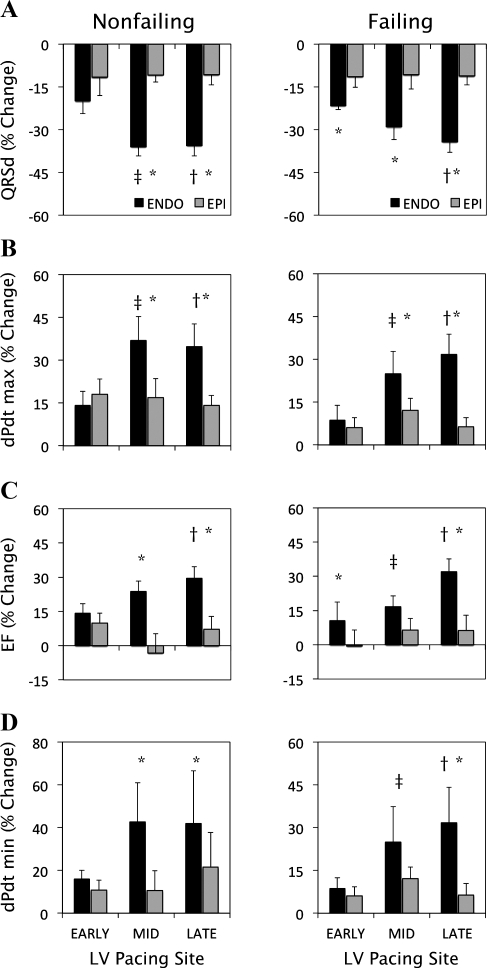
Figure 2 suggests that the differences between ENDO and EPI on ventricular function were greater at longer activation times. To investigate these changes, we compared the improvement in ventricular function during BVP at the EARLY, MID, and LATE activation sites. We also tested for interactions between main factors (i.e., transmural lead location and pacing site), which are presented in Fig. 3. In failing hearts, the reduction in QRS duration was greater (P < 0.05) during ENDO than EPI at all three sites (Fig. 3A, right). During ENDO, a greater reduction (P < 0.05) occurred while pacing at LATE compared with EARLY sites, whereas no differences were present between EARLY, MID, and LATE sites during EPI. Maximum dP/dt (Fig. 3B, right), stroke volume (Fig. 3C, right), and minimum dP/dt (Fig. 3D, right) were greater at LATE compared with EARLY sites during ENDO but not EPI. At LATE sites, all three hemodynamic parameters were significantly greater for ENDO than EPI. These findings were similar in the nonfailing hearts (Fig. 3, A–D, left). In both animal groups, there were significant interactions (P < 0.05) between transmural lead location and pacing site on levels of QRS duration and ventricular function. The results from Fig. 3 suggest that ENDO, compared with EPI, led to a lesser degree of electrical DYSS and greater pump function when pacing at LV sites with longer electrical activation delays.
Fig. 3.
Percent change from DYSS in QRS duration (A), maximum dP/dt (B), EF (C), and minimum dP/dt (D) during ENDO and EPI biventricular pacing (BVP) at various pacing sites of LV activation (EARLY, MID, and LATE) during ENDO and EPI BVP. Responses are shown for both nonfailing hearts (left) and failing hearts (right). *P < 0.05, ENDO vs. EPI. †P < 0.05, ENDO, LATE vs. EARLY. ‡P < 0.05, ENDO, MID vs. EARLY.
Transmural fiber angles and three-dimensional fiber strains at the anterolateral left ventricle.
In nonfailing hearts, mean fiber angles were −29.2 ± 3.0, 11.7 ± 4.9, and 52.6 ± 8.3° for the subepicardium, midwall, and subendocardium wall depths, respectively. In failing hearts, mean fiber angles were −7.5 ± 3.1, 34.8 ± 3.5, and 73.5 ± 5.5° for subepicardium, midwall, and subendocardium wall depths, respectively. Significant differences (P < 0.05) in mean fiber angle at all three wall depths between failing and nonfailing hearts were observed. Fiber strain components at end systole during atrial and local ENDO and EPI pacing at the anterolateral left ventricle are summarized in Table 4. In failing hearts, fiber shortening and wall thickening strain at the midwall were significantly greater (P < 0.05) during ENDO compared with EPI. At the subendocardium, cross-fiber shortening, wall thickening, and fiber-cross-fiber shear were all greater (P < 0.05) during ENDO compared with EPI. In nonfailing hearts, similar reductions in midwall fiber shortening and wall thickening strain, and subendocardial cross-fiber shortening and wall thickening, were observed for EPI compared with ENDO. Additionally, subepicardial fiber-cross-fiber shear was reduced with EPI compared with ENDO. Finally, fiber and cross-fiber shortening, wall thickening strain, and fiber-cross-fiber shear were all reduced (P < 0.05) at the subendocardium during atrial pacing in failing compared with nonfailing animals. Overall, these findings suggest that local endocardial pacing at the anterolateral left ventricle resulted in greater regional function than local epicardial pacing in all animals despite differences in atrial function between the nonfailing and failing groups. All authors agreed to the content of the data presented in this article.
Table 4.
End-systolic fiber strains at the anterolateral left ventricle during atrial and local biventricular activation in failing and nonfailing hearts
| Failing Hearts (n = 6) |
Nonfailing Hearts (n = 5) |
|||||
|---|---|---|---|---|---|---|
| Strain/Depth | ATRIAL | ENDO | EPI | ATRIAL | ENDO | EPI |
| Eff | ||||||
| Sub-epi | −0.05 ± 0.02 | −0.04 ± 0.01 | −0.01 ± 0.02 | −0.09 ± 0.02 | −0.06 ± 0.02 | −0.07 ± 0.02 |
| Midwall | −0.10 ± 0.02§ | −0.07 ± 0.02* | 0.01 ± 0.03‡ | −0.17 ± 0.02 | −0.16 ± 0.01* | −0.11 ± 0.02‡ |
| Sub-endo | −0.14 ± 0.02§ | −0.07 ± 0.03† | −0.07 ± 0.03‡ | −0.19 ± 0.02 | −0.18 ± 0.01 | −0.16 ± 0.02 |
| Ecc | ||||||
| Sub-epi | −0.06 ± 0.01 | −0.05 ± 0.02 | −0.03 ± 0.02 | −0.05 ± 0.01 | −0.01 ± 0.03 | −0.003 ± 0.03 |
| Midwall | −0.08 ± 0.02 | −0.07 ± 0.02 | −0.05 ± 0.03 | −0.08 ± 0.03 | −0.06 ± 0.06 | −0.04 ± 0.07 |
| Sub-endo | −0.08 ± 0.01§ | −0.08 ± 0.02* | 0.003 ± 0.03‡ | −0.20 ± 0.04 | −0.16 ± 0.07* | −0.07 ± 0.06‡ |
| Err | ||||||
| Sub-epi | 0.14 ± 0.03 | 0.07 ± 0.02 | 0.05 ± 0.02‡ | 0.15 ± 0.03 | 0.17 ± 0.06 | 0.16 ± 0.04 |
| Midwall | 0.27 ± 0.04 | 0.14 ± 0.03*† | 0.08 ± 0.04‡ | 0.37 ± 0.05 | 0.35 ± 0.06* | 0.29 ± 0.04 |
| Sub-endo | 0.42 ± 0.06§ | 0.20 ± 0.07*† | 0.14 ± 0.06‡ | 0.62 ± 0.08 | 0.55 ± 0.09* | 0.41 ± 0.07‡ |
| Efc | ||||||
| Sub-epi | −0.004 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | −0.02 ± 0.01 | −0.05 ± 0.02* | −0.006 ± 0.03 |
| Midwall | 0.01 ± 0.02 | −0.001 ± 0.01 | −0.011 ± 0.01 | 0.02 ± 0.01 | 0.004 ± 0.02 | 0.01 ± 0.01 |
| Sub-endo | 0.001 ± 0.01§ | 0.04 ± 0.05* | −0.03 ± 0.02‡ | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.07 ± 0.03 |
| Ecr | ||||||
| Sub-epi | 0.02 ± 0.02 | 0.02 ± 0.02 | −0.02 ± 0.02 | 0.02 ± 0.01 | −0.05 ± 0.02 | −0.03 ± 0.05 |
| Midwall | −0.02 ± 0.03 | −0.05 ± 0.02 | −0.05 ± 0.01 | 0.03 ± 0.02 | −0.07 ± 0.09 | −0.07 ± 0.08 |
| Sub-endo | −0.07 ± 0.06 | −0.07 ± 0.06 | −0.08 ± 0.04 | 0.001 ± 0.04 | −0.01 ± 0.09* | −0.07 ± 0.09 |
| Efr | ||||||
| Sub-epi | 0.05 ± 0.02 | 0.03 ± 0.02 | 0.02 ± 0.02 | 0.03 ± 0.01 | 0.07 ± 0.03* | 0.05 ± 0.02 |
| Midwall | 0.07 ± 0.04 | 0.04 ± 0.05 | 0.02 ± 0.03 | 0.07 ± 0.03 | 0.04 ± 0.04 | 0.03 ± 0.04 |
| Sub-endo | 0.07 ± 0.03 | 0.001 ± 0.08 | −0.02 ± 0.02‡ | 0.09 ± 0.05 | −0.004 ± 0.09*† | −0.04 ± 0.08‡ |
Values are means ± SE for failing (n = 6) and nonfailing (n = 5) hearts. ATRIAL, left atrial pacing; ENDO, biventricular pacing at local endocardial anterolateral surface; EPI, biventricular pacing at local epicardial anterolateral surface; Sub-epi, subepicardial wall depth (i.e., 25%); Sub-endo, subendocardial wall depth (i.e., 75%); Eff, fiber normal strain; Ecc, cross-fiber normal strain; Err, radial normal strain; Efc, fiber-cross-fiber shear strain; Ecr, cross-fiber-radial shear strain; Efr, fiber-radial shear strain.
P < 0.05, ENDO vs. EPI;
P < 0.05, ENDO vs. ATRIAL;
P < 0.05, EPI vs. ATRIAL;
P < 0.05, failing ATRIAL vs. nonfailing ATRIAL (comparisons performed with a two-way repeated-measures ANOVA and Tukey's post hoc tests).
DISCUSSION
This study demonstrates that improvements in LV pump function and electrical synchrony during endocardial BVP increases with activation time at the LV pacing site in dyssynchronously failing canine hearts. We also found that the amount of hemodynamic improvement during epicardial BVP did not significantly correlate with activation time and was typically less than that achieved during endocardial BVP. Additionally, BVP at regions with longer activation times resulted in greater electrical synchrony and hemodynamic function with endocardial compared with epicardial pacing. Regional fiber strain analysis revealed greater function at midwall and subendocardial wall depths during local endocardial compared with epicardial BVP in all animals. Finally, our findings on hemodynamic improvement with pacing were consistent between nonfailing and infarcted failing hearts, suggesting that endocardial BVP at LV sites with longer activation delays may provide a systematic approach towards improving pump function in dyssynchronously failing hearts.
Role of LV endocardial and epicardial activation on hemodynamic function.
In the normal heart during sinus rhythm, electrical depolarization at the endocardium precedes that on the epicardium; consequently, mechanical activation (e.g., time to 10% peak shortening) occurs earlier at the endocardium than the epicardium (3). With dyssynchrony, slow myocardial conduction results in depressed LV contractility (7). Pacing at the LV epicardium reverses transmural mechanical activation (3), leads to nonuniform shortening (10, 39), and impairs transmural function (38) compared with a normal activation sequence. Overall, mechanical dyssynchrony gives rise to reduced systolic performance (4, 29), since presystolic shortening and systolic stretching of early activated regions result in negative work that is not fully compensated by the increased work done by late activated regions (21). The imbalance in mechanical work distribution is thought to result in long-term myocardial remodeling in regions of early and late electrical activation (36), leading to further deteriorations in ventricular function. In the present study, endocardial BVP led to faster total ventricular depolarization than epicardial BVP, which may serve to reduce mechanical dyssynchrony. Previous studies (15) have measured faster conduction velocities at the endocardium compared with the epicardium. We used limb lead II ECG recordings to measure capture latency (i.e., interval between stimulus and the onset of depolarization) and found that epicardial stimulation significantly (P < 0.05) prolonged capture latency compared with endocardial stimulation in nonfailing (23.4 ± 0.9 vs. 33.1 ± 1.7 ms) and failing hearts (23.7 ± 0.7 vs. 29.5 ± 0.7 ms). We suspect that differences in capture latency may be due to layer-dependent differences in conductivity between the endocardial and epicardial surfaces, since stimulation threshold values were similar between all pacing sites. Faster conduction with endocardial pacing could potentially reduce the amount of mechanical dyssynchrony, resulting in more uniform work distribution and improved pump function. This potential mechanism could explain the findings from the present study in nonfailing and failing hearts, and those presented by van Deursen et al. (35) in nonfailing hearts, that endocardial, compared with epicardial, BVP results in greater hemodynamic improvement. Mechanical dyssynchrony, as indicated by temporal and spatial nonuniformity in fiber relengthening, may also play a role in impaired LV filling (18); therefore, a reduction in mechanical dyssynchrony during endocardial BVP could potentially improve diastolic filling to a greater extent than achieved with epicardial BVP.
Influence of intrinsic activation delay at the LV pacing site on hemodynamic function.
Multiple studies (5, 7, 12, 16, 23) have investigated the dependence of pacing site in determining hemodynamic function. Kass et al. (20) showed that the basal severity of electrical dyssynchrony and sequence of activation influences LV systolic function, whereas others (13) have shown that improvement in LV contractility is distributed uniformly across LV endocardial pacing sites. We hypothesized that the improvement in ventricular function depends on the intrinsic amount of activation delay at the LV pacing site. We found positive correlations (P < 0.05) between activation time at the pacing site and percent change in maximum dP/dt (r = 0.64), ejection fraction (r = 0.49), and minimum dP/dt (r = 0.39) during endocardial BVP. However, no significant correlations existed in these parameters with epicardial BVP. While epicardial pacing at sites with longer activation times showed trends for improvement (Fig. 2, A–C) in QRS duration, maximum dP/dt, and ejection fraction, we suspect that increased inter- and intra-animal variance in improvement resulted in nonsignificant findings.
Hemodynamic differences between endocardial and epicardial BVP varied with the length of activation delay at the LV pacing site (i.e., early, mid, and late). Specifically, maximum dP/dt, ejection fraction, and minimum dP/dt were all improved to a greater extent during endocardial, compared with epicardial, BVP at regions with longer activation delays. At early activated regions, commonly near the posterior coronary sinus, there were no significant differences in hemodynamic improvement between endocardial and epicardial BVP. This result may be in agreement with studies by Derval et al. (13), who found no difference between endocardial and epicardial LV pacing at the posterolateral wall near the coronary sinus; however, baseline electrical activation times were not reported and therefore make comparisons with our study difficult. In contrast to our findings, Derval et al. (13) found that LV pacing at the latest mechanically activated region rarely resulted in optimal hemodynamic function. The differences in the findings between their study (13) and ours may be explained by the following: 1) endocardial and epicardial comparisons were performed at only one paired site in Derval et al. (13), whereas we compared differences across a larger LV area at five paired sites; and 2) we used measures of local electrical depolarization to define sites of early, mid, and late activation, whereas Derval et al. (13) used echo-derived mechanical activation to identify the latest activated site.
In the current study, we did not observe negative hemodynamic responses during endocardial BVP in the infarcted failing hearts, which has been reported to occur clinically during epicardial BVP in ischemic hearts (6). We suspect there are a wide variety of issues that give rise to this observation, mainly location of the LV pacing electrode and electromechanical properties of the scar. We found that all endocardial and epicardial leads were positioned in nonscar tissue in the infarcted failing hearts, which could contribute to our findings. A recent study (30) in canine LBBB hearts with LV infarcts suggested that, “the best site [for hemodynamic improvement] does not coincide with the region of latest activation, but can be recognized as the site providing the most profound reduction in QRS duration.” However, previous studies showed that the greatest improvement in function did not necessarily correspond with the largest reduction in QRS duration (20) but was correlated with a marked reduction in mechanical dyssynchrony (17, 22). Furthermore, a closer examination of the study by Rademakers et al. (30) revealed that only in the presence of anterior scarring did pacing in latest activated site not yield maximal hemodynamic improvement. In the nonscarred and posterior scarred hearts, dP/dtmax was greater for late compared with early activated regions, based on the isochronal activation maps presented for representative hearts. We found that improvements in mechanical function and electrical synchrony during endocardial BVP strongly depended on the baseline activation time at the LV pacing site, whereas improvements during epicardial BVP did not. This finding could potentially explain the findings from Rademakers et al. (30), since only epicardial BVP was performed. The significant correlation observed in our data between percent change in QRS duration and activation time at endocardial pacing sites makes it difficult to separate the predictive benefits of the two (i.e., change in QRS duration and baseline activation time at the pacing site). Nevertheless, our findings indicate that improved hemodynamic function can be achieved through endocardial, but not epicardial, BVP at sites of later activation times at baseline.
Transmural fiber strains during local activation at the anterolateral left ventricle.
We compared end-systolic fiber strain components at three transmural depths (subepicardium, midwall, and subendocardium) during atrial and local endocardial and epicardial activation at the anterolateral left ventricle (Table 4). Previous studies have published end-systolic regional and transmural mechanics in healthy hearts at the anterolateral left ventricle during atrial (2, 38) and local epicardial pacing (3, 4, 37). We have extended the findings of these studies by providing regional fiber strain data during local endocardial pacing in the nonfailing and failing hearts. During atrial activation, failing hearts showed significant (P < 0.05) depression in fiber shortening, wall thickening, and fiber-cross-fiber shear predominantly at the subendocardium compared with nonfailing hearts. Our findings in nonfailing animals were in agreement with Ashikaga et al. (3), specifically, local epicardial pacing reduced fiber shortening at the midwall and cross-fiber shortening and wall thickening at the subendocardium compared with atrial (P < 0.05). In all animals, local endocardial activation resulted in greater fiber shortening, cross-fiber shortening, and wall thickening at midwall and subendocardial depths compared with local epicardial pacing (P < 0.05). Fiber-cross-fiber shear strain was significantly lowered at the subendocardium and subepicardium during local epicardial compared with endocardial pacing (P < 0.05) in failing and nonfailing hearts, respectively. Overall, the data provide evidence that local endocardial pacing results in greater transmural function than local epicardial pacing in both nonfailing and failing hearts.
Limitations.
The findings presented in this study represent acute changes in electrical and hemodynamic function during endocardial and epicardial BVP in nonfailing and failing canine hearts, which may differ from long-term responses. RVA pacing was used to induce electrical dyssynchrony, which may vary from actual structural abnormalities that may exist with LBBB. However, activation time delays measured during RVA were similar to reported values in canine LBBB (35), and this model has been used previously to mimic LV dyssynchrony (17). We chose to use RVA as our control instead of atrial pacing to better represent the conditions of electrical dyssynchrony. Comparing hemodynamic values during atrial and RVA pacing (Tables 1–3) reveals marked depression in function during RVA compared with atrial pacing. During BVP, AV delay was maintained at 40 ms to ensure ventricular capture; however, the short AV delay may have negative effects on LV filling. In a subset of nonfailing animals, AV delay was varied from 0 −120 ms and had a larger effect on functional improvement during epicardial compared with endocardial BVP.
Canine experimental models were used to mimic electrical dyssynchrony in failing human hearts; however, variations in disease severity, regions of conduction block, presence of ischemic heart disease with scar, coronary venous anatomy, and chronic presence of LBBB all serve to complicate human disease. Clinical studies with sufficient sample size are needed for further clarification and extrapolation of the results from this study. A well-established canine model of tachycardia-induced HF (1, 9, 28) was implemented in our failing group. We discontinued high rate pacing 24 h before the terminal study to allow for animals to return to a lower intrinsic sinus rate. During this period, mean left atrial pressure decreased slightly to 22.2 ± 1.1 mmHg (P = NS); however, hemodynamic parameters (Table 1) and transmural strain components (Table 4) were markedly depressed in failing compared with nonfailing animals during atrial pacing. Additionally, we acknowledge that controlling scar size can be problematic due to the large amount of collateral supply in the canine heart. Our measures of scar size (12.8 ± 0.9%) indicate that little variability was present in our experimental model, which we attribute to our approach of permanent vessel ligation followed by microsphere embolization of distal branches to minimize reperfusion via the collateral supply. Furthermore, we were unable to find significant correlations between scar size and hemodynamic improvement during endocardial BVP. Finally, there was a significant difference in stimulation rate between the nonfailing and failing groups (Table 1). It was difficult to ensure similar rates across animals groups, especially since the failing animals had higher intrinsic rates due to high rate pacing for 5 wk. Therefore, we used a consistent approach in determining pacing parameters. Specifically, stimulation rate was set at 10% above the intrinsic rate and delivered voltage of 20% above the stimulation threshold.
Conclusions.
Improvement in LV pump function and electrical synchrony during endocardial BVP increased with activation time at the LV pacing site, whereas improvement was markedly lower, compared with endocardial, and did not significantly differ across multiple LV sites during epicardial pacing. At the anterolateral wall, transmural function at end systole was largely improved with local endocardial compared with epicardial pacing. Additionally, endocardial BVP led to greater hemodynamic function when pacing at sites with increasing values of activation delay compared with epicardial pacing. Overall, these findings were consistent between nonfailing and failing hearts, despite significant differences in baseline function, and suggest that endocardial BVP may have potential advantages in optimizing hemodynamic improvement when positioning the LV lead in regions of increased activation delay.
GRANTS
Additional support for this study was provided by National Heart, Lung, and Blood Institute Grants 5P01-HL-46345 (to A. D. McCulloch) and 1R01-HL-96544 (to A. D. McCulloch).
DISCLOSURES
Primary funding for this study was provided by the University of California Discovery Grant No. ITL06-10159 (to A. D. McCulloch), which was cosponsored by Medtronic, who provided implantable electrodes, programmable pacemakers, surgical and interrogatory accessories, and technical support. Dr. Lawrence Mulligan is a Senior Principal Scientist in the Therapy Delivery Systems/Leads Research division at Medtronic.
ACKNOWLEDGMENTS
We are grateful to Drs. Irina Elrott and Bruce Ito for technical support with experimental preparation, surgical assistance, and postoperation animal care. We also thank Dr. Morton Printz for supplying us with the implantable left atrial pressure transducers and telemetric monitoring system.
REFERENCES
- 1. Armstrong P, Stopps T, Ford S, de Bold A. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation 74: 1075–1084, 1986 [DOI] [PubMed] [Google Scholar]
- 2. Ashikaga H, Criscione JC, Omens JH, Covell JW, Ingels NB., Jr Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol 286: H640–H647, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashikaga H, Omens JH, Ingels NB, Jr, Covell JW. Transmural mechanics at left ventricular epicardial pacing site. Am J Physiol Heart Circ Physiol 286: H2401–H2407, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badke FR, Boinay P, Covell JW. Effects of ventricular pacing on regional left ventricular performance in the dog. Am J Physiol Heart Circ Physiol 238: H858–H867, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, Boschat J, Benditt DG, Lurie KG. Evaluation of different ventricular pacing sites in patients with severe heart failure : results of an acute hemodynamic study. Circulation 96: 3273–3277, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bleeker GB, Kaandorp TAM, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 113: 969–976, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Burkhoff D, Oikawa RY, Sagawa K. Influence of pacing site on canine left ventricular contraction. Am J Physiol Heart Circ Physiol 251: H428–H435, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Butter C, Auricchio A, Stellbrink C, Fleck E, Ding J, Yu Y, Huvelle E, Spinelli J. Effect of Resynchronization Therapy Stimulation Site on the Systolic Function of Heart Failure Patients. Circulation 104: 3026–3029, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Coleman HN, Taylor RR, Pool PE, Whipple GH, Covell JW, Ross J, Braunwald E. Congestive heart failure following chronic tachycardia. Am Heart J 81: 790–798, 1971 [DOI] [PubMed] [Google Scholar]
- 10. Coppola BA, Covell JW, McCulloch AD, Omens JH. Asynchrony of ventricular activation affects magnitude and timing of fiber stretch in late-activated regions of the canine heart. Am J Physiol Heart Circ Physiol 293: H754–H761, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costa KD, May-Newman K, Farr D, O, äôDell WG, McCulloch AD, Omens JH. Three-dimensional residual strain in midanterior canine left ventricle. Am J Physiol Heart Circ Physiol 273: H1968–H1976, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dekker ALAJ, Phelps B, Dijkman B, van Der Nagel T, van Der Veen FH, Geskes GG, Maessen JG. Epicardial left ventricular lead placement for cardiac resynchronization therapy: Optimal pace site selection with pressure-volume loops. J Thorac Cardiovasc Surg 127: 1641–1647, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Derval N, Steendijk P, Gula LJ, Deplagne A, Laborderie J, Sacher F, Knecht S, Wright M, Nault I, Ploux S, Ritter P, Bordachar P, Lafitte S, Reant P, Klein GJ, Narayan SM, Garrigue S, Hocini M, Haissagueere M, Clementy J, Jais P. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol 55: 566–575, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Farwell D, Patel NR, Hall A, Ralph S, Sulke AN. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J 21: 1246–1250, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Frazier D, Krassowska W, Chen P, Wolf P, Danieley N, Smith W, Ideker R. Transmural activations and stimulus potentials in three-dimensional anisotropic canine myocardium. Circ Res 63: 135–146, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Garrigue S, Jais P, Espil G, Labeque J, Hocini M, Shah M, Haissaguerre M, Clementy J. Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol 88: 858–862, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Helm RH, Byrne M, Helm PA, Daya SK, Osman NF, Tunin R, Halperin HR, Berger RD, Kass DA, Lardo AC. Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation 115: 953–961, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Howard EJ, Coppola BA, Kerckhoffs RC, Omens JH, McCulloch AD, Covell JW. Abstract 21317: spatial heterogeneity of fiber relengthening underlies impaired lv filling in the dyssynchronous left ventricle. Circulation 122: A21317, 2010 [Google Scholar]
- 19. Issa ZF, Rosenberger J, Groh WJ, Miller JM, Zipes DP. Ischemic ventricular arrhythmias during heart failure: a canine model to replicate clinical events. Heart Rhythm 2: 979–983, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 99: 1567–1573, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Kerckhoffs RCP, Faris OP, Bovendeerd PHM, Prinzen FW, Smits K, McVeigh ER, Arts T. Electromechanics of paced left ventricle simulated by straightforward mathematical model: comparison with experiments. Am J Physiol Heart Circ Physiol 289: H1889–H1897, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation 106: 1760–1763, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lister JW, Klotz DH, Jomain SL, Stuckey JH, Hoffman BF. Effect of pacemaker site on cardiac output and ventricular activation in dogs with complete heart block. Am J Cardiol 14: 494–503, 1964 [DOI] [PubMed] [Google Scholar]
- 24. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 25. MacKay SA, Potel MJ, Rubin JM. Graphics methods for tracking three-dimensional heart wall motion. Comput Biomed Res 15: 455–473, 1982 [DOI] [PubMed] [Google Scholar]
- 26. McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction. JAMA 297: 2502–2514, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Meier GD, Ziskin MC, Santamore WP, Bove AA. Kinematics of the beating heart. IEEE Trans Biomed Eng 27: 319–329, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Packer DL, Bardy GH, Worley SJ, Smith MS, Cobb FR, Coleman RE, Gallagher JJ, German LD. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol 57: 563–570, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Prinzen FW, Augustijn CH, Allessie MA, Arts T, Delhass T, Reneman RS. The time sequence of electrical and mechanical activation during spontaneous beating and ectopic stimulation. Eur Heart J 13: 535–543, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Rademakers LM, van Kerckhoven R, van Deursen CJM, Strik M, van Hunnik A, Kuiper M, Lampert A, Klersy C, Leyva F, Auricchio A, Maessen JG, Prinzen FW. Myocardial infarction does not preclude electrical and hemodynamic benefits of cardiac resynchronization therapy in dyssynchronous canine hearts. Circ Arrhythm Electrophysiol 3: 361–368, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Spach MS, King TD, Barr RC, Boaz DE, Morrow MN, Herman-Giddens S. Electrical potential distribution surrounding the atria during depolarization and repolarization in the dog. Circ Res 24: 857–873, 1969 [DOI] [PubMed] [Google Scholar]
- 32. Spragg DD, Dong J, Fetics BJ, Helm R, Marine JE, Cheng A, Henrikson CA, Kass DA, Berger RD. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol 56: 774–781, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24: 339–347, 1969 [DOI] [PubMed] [Google Scholar]
- 34. Takayama Y, Costa KD, Covell JW. Contribution of laminar myofiber architecture to load-dependent changes in mechanics of LV myocardium. Am J Physiol Heart Circ Physiol 282: H1510–H1520, 2002 [DOI] [PubMed] [Google Scholar]
- 35. van Deursen C, van Geldorp IE, Rademakers LM, van Hunnik A, Kuiper M, Klersy C, Auricchio A, Prinzen FW. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol 2: 580–587, 2009 [DOI] [PubMed] [Google Scholar]
- 36. van Oosterhout MFM, Prinzen FW, Arts T, Schreuder JJ, Vanagt WYR, Cleutjens JPM, Reneman RS. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 98: 588–595, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Waldman LK, Covell JW. Effects of ventricular pacing on finite deformation in canine left ventricles. Am J Physiol Heart Circ Physiol 252: H1023–H1030, 1987 [DOI] [PubMed] [Google Scholar]
- 38. Waldman LK, Fung YC, Covell JW. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ Res 57: 152–163, 1985 [DOI] [PubMed] [Google Scholar]
- 39. Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol Heart Circ Physiol 276: H881–H891, 1999 [DOI] [PubMed] [Google Scholar]