Abstract
Stromal cell-derived factor-1α (SDF-1) has been reported to mediate cardioprotection through the mobilization of stem cells into injured tissue and an increase in local angiogenesis after myocardial infarction. However, little is known regarding whether SDF-1 induces acute protection following global myocardial ischemia/reperfusion (I/R) injury and if so, by what molecular mechanism. SDF-1 binding to its cognate receptor CXCR4 has been shown to activate STAT3 in a variety of cells. STAT3 is a cardioprotective factor and may mediate SDF-1/CXCR4-induced acute protection. We hypothesized that SDF-1 would improve myocardial function through CXCR4-increased STAT3 activation following acute I/R. Isolated mouse hearts were subjected to 25-min global ischemia/40-min reperfusion and divided into groups of 1) vehicle; 2) SDF-1; 3) AMD3100, a CXCR4 inhibitor; 4) SDF-1 + AMD3100; 5) Stattic, a STAT3 inhibitor; 6) SDF-1 + Stattic; 7) cardiomyocyte-restricted ablation of STAT3 (STAT3KO); 8) STAT3KO + SDF-1; 9) Ly294002, an inhibitor of the Akt pathway; and 10) SDF-1 + Ly294002. Reagents were infused into hearts within 5 min before ischemia. SDF-1 administration significantly improved postischemic myocardial functional recovery in a dose-dependent manner. Additionally, pretreatment with SDF-1 reduced cardiac apoptotic signaling and increased myocardial STAT3 activation following acute I/R. Inhibition of the SDF-1 receptor CXCR4 neutralized these protective effects by SDF-1 in hearts subjected to I/R. Notably, inhibition of the STAT3 pathway or use of STAT3KO hearts abolished SDF-1-induced acute protection following myocardial I/R. Our results represent the first evidence that the SDF-1/CXCR4 axis upregualtes myocardial STAT3 activation and, thereby, mediates acute cardioprotection in response to global I/R.
Keywords: CXCL12, signal transducer and activator of transcription 3, cardioprotection
myocardial ischemia/reperfusion (I/R) injury occurs during coronary artery disease and cardiac surgery. Despite advances in understanding of the cellular and molecular mechanisms that modulate the severity of myocardial I/R, the treatment of ischemic heart disease remains a challenge. It is well established that I/R injury induces myocardial inflammation with resultant overexpression of inflammatory cytokines and chemokines (8, 23, 25). Among these, stromal cell-derived factor 1α (SDF-1), a constitutively expressed chemokine, has recently been receiving much interest in its role in the treatment of ischemic diseases. SDF-1 expression is increased in the heart instantly after myocardial infarction (MI; Refs. 3, 17, 21). Enhanced SDF-1 after myocardial ischemia results in migration of stem cells into the injured heart and thus leads to increased vascular density, enhanced tissue regeneration, and improved cardiac function (7, 35). In addition, administration of SDF-1 promotes myocyte survival in the heart 72 h after MI (21). However, it is unknown whether SDF-1 is able to mediate rapid and direct cardioprotection following global myocardial I/R, and if so, by what molecular mechanism.
The majority of SDF-1-induced biological effects are initiated by binding to its cognate receptor CXCR4, which is present in cardiac myocytes (17, 21). CXCR4 is a G-protein-coupled receptor with 7-transmembrane domains, and SDF-1 functions as the unique ligand for it (24). The SDF-1/CXCR4 axis has been reported to play a critical role in guiding the movement of stem cells to target tissues or organs (3, 7, 35). In addition, SDF-1/CXCR4 signaling is able to modulate cell proliferation and survival. By binding to CXCR4, SDF-1 has been reported to activate a series of downstream signaling pathways. SDF-1/CXCR4-triggered signal transducer and activator of transcription 3 (STAT3) activation is one of critical pathways that has been shown to facilitate cell growth, inhibit apoptosis, and mediate migration of mesenchymal stem cells (6, 10, 20). Of note, it is well documented that STAT3 activation plays a cardioprotective role following myocardial I/R injury. However, up to date, no study has investigated the relationship among SDF-1, CXCR4, and STAT3 in the heart, and no information exists regarding the role of STAT3 in SDF-1-mediated cardioprotection. On the other hand, although SDF-1 has been shown to upregulate myocardial Akt activation, and thus to mediate protection after MI, it is still unknown whether SDF-1-activated Akt signaling plays a role in the heart subjected to acute global I/R.
Therefore, in this study, to investigate the direct effect of SDF-1 on acute cardioprotection and to elucidate whether STAT3 plays a role in SDF-1-modulated cardiac function following I/R, we utilized an isolated heart perfusion system (Langendorff) to remove any potential confounding effects derived from systemic actions on the heart. We aimed to identify that 1) delivery of SDF-1 directly into the isolated heart before ischemia would improve myocardial functional recovery and upregulate cardiac STAT3 activation following acute I/R through CXCR4; and 2) blockade of STAT3 activation or cardiac-restricted ablation of STAT3 (STAT3KO) would abolish SDF-1/CXCR4-induced acute cardioprotection in the heart subjected to I/R, but inhibition of Akt pathway would not.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice (8- to 10-wk-old; Jackson Laboratories, Bar Harbor, ME) as wild-type group (WT) and with cardiac-restricted deletion of STAT3 (STAT3KO) were fed a standard diet and acclimated in a quiet quarantine room for ≥10 days before experiment. All animal studies conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996). The protocols were reviewed and approved by the Indiana Animal Care and Use Committee of Indiana University.
Isolated heart perfusion system (Langendorff).
Langendorff I/R experiments were performed in isolated mouse hearts as described previously (26–28). Briefly, hearts were rapidly excised via median sternotomy and placed in 4°C KH solution. The aorta was rapidly cannulated and the heart was perfused in the isovolumetric Langendorff mode (70 mmHg) and paced at 400 beats/min except during ischemia. A water-filled latex balloon was passed into the left ventricle. End diastolic pressure was adjusted to a level between 8 and 15 mmHg. The left ventricular developed pressure (LVDP) and the maximum positive and negative values of first derivative of left ventricular pressure (±dP/dt) were continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments, Milford, MA). Immediately at the end of reperfusion, hearts were snap frozen with liquid nitrogen and stored at −80°C.
Experiment groups.
All isolated mouse hearts were subjected to the same I/R protocol: 15-min equilibration followed by 25-min global ischemia (37°C) and 40-min reperfusion. Mouse hearts were randomly divided into (n = 4–7/group) the following: 1) WT-vehicle; 2) WT-SDF-1 (25 ng/ml); 3) WT-AMD3100 (5 μg/ml), a specific inhibitor of CXCR4; 4) WT-AMD3100 + SDF-1; 5) WT-Stattic, an inhibitor of STAT3 activation (20 μM); 6) WT-SDF-1 + Stattic; 7) STAT3KO-vehicle; 8) STAT3KO-SDF-1; 9) WT-LY294002, an inhibitor of Akt pathway (2.5 μM); and 10) WT-SDF-1 + LY294002. SDF-1 was infused into the isolated mouse hearts through a port above the aortic root (intracoronary delivery) over a continuous 3-min period before ischemia. All other infusions (AMD3100, Stattic and LY294002) were given over a continuous 5-min period before ischemia (2 min before SDF-1 infusion; Fig. 1A). A dose-response curve was created using doses of 5, 15, and 25 ng/ml of SDF-1 following global myocardial I/R ; 25 ng/ml of SDF-1 were found to provide the most optimal results and was utilized for experiments. The doses of other drugs were chosen based on previous literature that illustrated the effective doses of each drug (11, 17).
Fig. 1.
A: schematic illustrates experimental protocol performed in isolated mouse hearts. B: stromal cell-derived factor-1α (SDF-1) improves postischemic myocardial functional recovery in a dose-dependent manner. Myocardial function at the end of reperfusion is represented as % of equilibration (Eq). Results are means ± SE. *P < 0.05, ***P < 0.001 vs. group of 0 ng/ml of SDF-1 (vehicle). #P < 0.05, ##P < 0.01 vs. group of 5 ng/ml of SDF-1. C: changes of left ventricular developed pressure (LVDP) and maximum positive and negative values of first derivative of left ventricular pressure (±dP/dt) in groups of vehicle (0 ng/ml of SDF-1) and SDF-1-treated hearts (25 ng/ml) following acute global ischemia/reperfusion (I/R) injury, represented as %Eq. Results are means ± SE; n = 7/group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle at corresponding time point.
Western blot.
Western blot analysis was performed to measure activation of STAT3, Akt, and ERK1/2, as well as apoptosis-related proteins caspase-3, caspase-8, Bcl-2, Bax, and Bcl-XL. Heart tissue was homogenized in cold RIPA buffer (Sigma, Saint Louis, MO) and was centrifuged at 12,000 rpm for 10 min. The protein extracts (15 μg/lane) were subjected to electrophoresis on a 4–12% bis-Tris protein gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. After blocking, the membranes were incubated with the following primary antibodies: Akt, phosphor-Akt, STAT3, phosphor-STAT3 (Tyr705), ERK1/2, p-ERK1/2, Bax, Bcl-XL (1:1,000 dilution, Cell Signaling Technology, Beverly, MA), caspase-3, caspase-8, Bcl-2 (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), and GAPDH (1:5,000 dilution, Biodesign International, Saco, ME). Membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG secondary antibody (Pierce, Rockford, IL), and detection was performed using supersignal west pico stable peroxide solution (Pierce). Films were scanned and band density was analyzed using TotalLab software (Nonlinear USA, Durham NC).
Lactate dehydrogenase activity.
Lactate dehydrogenase (LDH) assay was performed in coronary effluents collected at equilibration time and at 5 min of reperfusion according to manufacturer instructions, in duplicate, using a commercially available cytotoxicity detection kit (Roche Applied Science, Indianapolis, IN).
Presentation of data and statistical analysis.
All reported values are means ± SE. Data was compared using two-way ANOVA with post hoc Tukey test or Student's t-test. A probability value of <0.05 was considered statistically significant.
RESULTS
Role of SDF-1 in the improvement of myocardial function following acute I/R.
Twenty-five minutes of ischemia followed by 40-min reperfusion significantly depressed myocardial function by ∼60–70% in isolated mouse hearts. Intracoronary delivery of SDF-1 before ischemia improved postischemic myocardial function in a dose-dependent manner (Fig. 1B), with the most optimal results at 25 ng/ml. Therefore, a dose of 25 ng/ml was chosen to investigate acute effect of SDF-1 on cardiac function following global I/R in this study. We did not utilize the higher doses of SDF-1 than 25 ng/ml because the current dose was optimal to identify the mechanisms of action of SDF-1 in global myocardial I/R injury here. We observed significantly improved cardiac contractility and relaxation as early as 20 min into reperfusion in SDF-1-pretreated hearts compared with the vehicle control group (Fig. 1C), and the SDF-1-mediated preservation of myocardial function remained until the end of the experiment.
Effect of SDF-1 on myocardial damage following I/R.
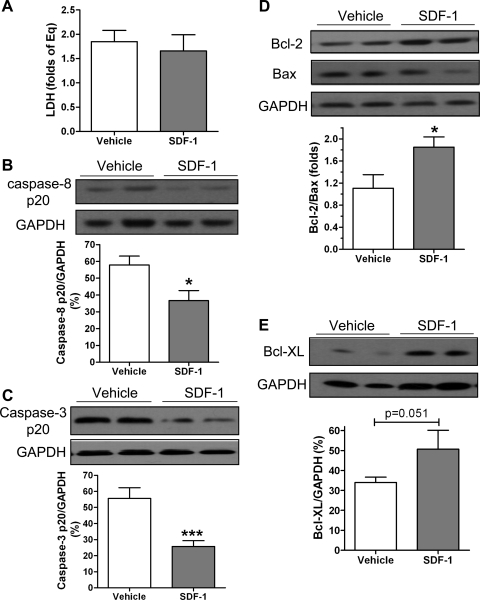
To examine the effect of SDF-1 on myocardial injury, we measured LDH levels in coronary effluents collected at equilibration time and at 5-min reperfusion after 25-min ischemia. Elevated LDH release usually indicates the damage of cell membrane integrity and is one of the most widely used indicators for cell viability and tissue injury. We observed that I/R increased LDH release in both vehicle and SDF-1 groups. However, SDF-1 pretreatment did not decrease LDH levels in coronary effluents (Fig. 2A).
Fig. 2.
Myocardial damage is determined by lactate dehydrogenase (LDH) assay and detection of apoptotic-related proteins between vehicle and SDF-1 (25 ng/ml)-treated hearts subjected to I/R. A: LDH release in coronary effluents collected at 5 min of reperfusion after ischemia, represented as folds of equilibration. B: cleaved caspase-8 p20 by Western blot. C: cleaved caspase-3 p20. D: antiapoptotic protein Bcl-2 and its proapoptotic partner Bax. E: antiapoptotic protein Bcl-XL. B–E: top: representative blots (2 lanes/group); bottom: bar graph of densitometry data represented as %GAPDH. Results are means ± SE; n = 5–7/group. *P < 0.05, ***P < 0.001 vs. vehicle.
To investigate whether SDF-1 suppresses apoptosis following acute myocardial I/R, we measured the proapoptotic proteins caspase-8, caspase-3, and Bax and the antiapoptotic proteins Bcl-2 and Bcl-XL. Our previous study (29, 30) indicated that I/R increased myocardial activity of caspase-8 and caspase-3. In this study, pretreatment with SDF-1 significantly reduced cleaved caspase-8 by ∼30% and decreased caspase-3 p20 by >50% in the hearts subjected to I/R (Fig. 2, B and C). In contrast, upregulated antiapoptotic signaling was noticed in SDF-1-pretreated hearts as exhibited by significantly increased Bcl-2-to-Bax ratios (Fig. 2D) and a trend of elevated expression of Bcl-XL (Fig. 2E). These data suggest that SDF-1 is able to directly suppress apoptotic signaling in the heart subjected to acute I/R.
SDF-1 mediated acute protection through its receptor CXCR4 following myocardial I/R.
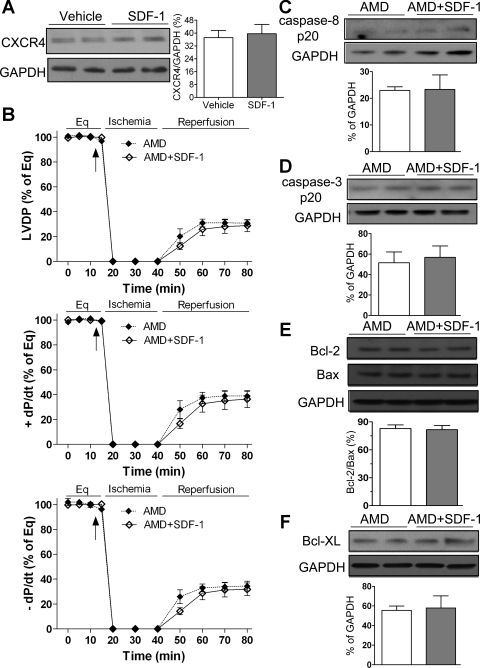
SDF-1 initiates its biological effects primarily through binding to its cognate receptor CXCR4. To elucidate whether SDF-1-induced acute cardioprotection is mediated through CXCR4 following I/R injury, we utilized a specific blocker of CXCR4, AMD3100, in this study. We first observed that myocardial CXCR4 expression was not increased by administration of SDF-1 in the heart subjected to I/R (Fig. 3A). Using AMD3100 abolished SDF-1-improved myocardial function as exhibited by similar levels of postischemic LVDP and ±dP/dt between AMD3100 + SDF-1 group and its counterpart (Fig. 3B). In addition, pretreatment with SDF-1 neither attenuated myocardial levels of cleaved caspase-8 and caspase-3 nor increased ratio of Bcl-2 to Bax or expression of Bcl-XL in AMD3100 group after I/R (Fig. 3, C–F), suggesting that SDF-1-induced acute protection is mediated through its receptor CXCR4 in the heart following global I/R.
Fig. 3.
Role of CXCR4 in SDF-1-induced acute protection following I/R. A: myocardial expression of CXCR4 between vehicle and SDF-1-treated group. Top: representative blots of CXCR4 and GAPDH (2 lanes/group). Bottom: bar graph of densitometry data represented as %GAPDH. B: blockade of CXCR4 by AMD3100 (AMD) neutralizes SDF-1-improved myocardial functional recovery following I/R. Results are means ± SE; n = 5/group. C–F: administration of SDF-1 did not affect apoptotic-related proteins caspase-8 p20 (C); caspase-3 p20 (D); Bcl-2, Bax, and Bcl-2-to-Bax ratios (E); or Bcl-XL (F) in AMD3100-treated hearts after I/R compared with control group. C–F: top: representative blots (2 lanes/group); bottom: densitometry data (%GAPDH). Results are means ± SE; n = 4–5/group.
Effect of SDF-1 on myocardial activation of STAT3, Akt, and ERK1/2 after I/R.
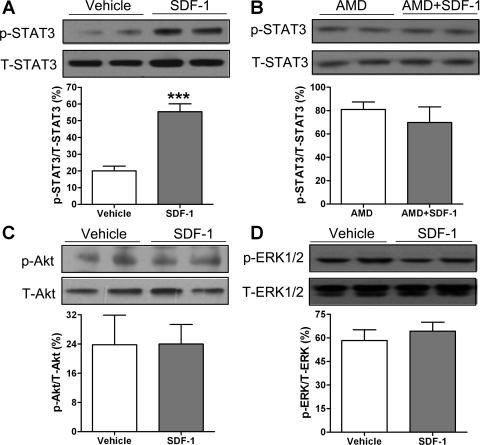
It has been well-established that the signaling pathways Akt, ERK, and STAT3 are involved in protecting cells against injury and promoting cell survival. In addition, SDF-1 binding to its receptor CXCR4 has been reported to initiate activation of STAT3, Akt, and ERK in a variety of cells. In the current study, we observed that infusion of SDF-1 before ischemia significantly upregulated myocardial p-STAT3 (an active form) levels following I/R, whereas the level of total STAT3 was not affected by SDF-1, leading to increased myocardial activation of STAT3 by >2.5 times compared with the vehicle group (Fig. 4A). In addition, blockade of the SDF-1 receptor by using AMD3100 neutralized SDF-1-increased STAT3 activation in the heart subjected to I/R (Fig. 4B). Unexpectedly, SDF-1 treatment did not upregulate the phosphorylated levels of Akt or ERK in hearts following acute I/R (Fig. 4, C and D).
Fig. 4.
Myocardial activation of signal transducer and activator of transcription 3 (STAT3; A), Akt (C), and ERK (D) after SDF-1 treatment in the hearts subjected to acute I/R. B, Inhibition of CXCR4 in SDF-1-activated STAT3. A–D: top: representative blots (2 lanes/group) of phosphor- (p-) and total-protein kinase (T-); bottom: bar graph of densitometry data (%total-protein kinase). Results are means ± SE; n = 4–7/group. ***P < 0.001 vs. vehicle.
Role of STAT3 in SDF-1-mediated acute cardioprotection following I/R.
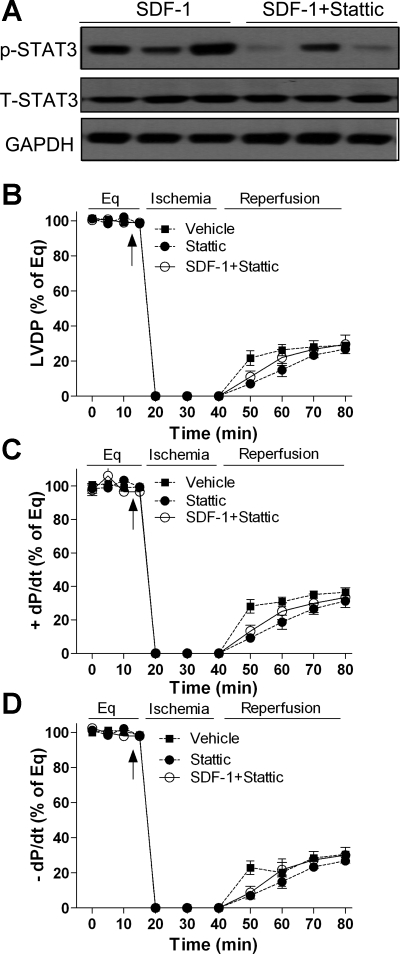
To identify the effect of STAT3 signaling on SDF-1-protected myocardial function in response to acute I/R, we blocked the myocardial STAT3 pathway by infusing the STAT3 inhibitor Stattic before SDF-1 treatment in the isolated hearts. Infusion of Stattic significantly reduced SDF-1-increased p-STAT3 level in the hearts but did not affect myocardial total STAT3 (Fig. 5A), leading to decreased STAT3 activation. As a result, using Stattic markedly impaired recovery of LVDP and ±dP/dt in the SDF-1 + Stattic group compared with the SDF-1-treated group and abolished SDF-1 protection of myocardial function to levels comparable to those seen in the vehicle control group following I/R (Fig. 5, B−D). Stattic alone did not affect postischemic myocardial functional recovery compared with the vehicle group.
Fig. 5.
Blockade of myocardial STAT3 activation in SDF-1-improved myocardial function following I/R. A: myocardial levels of phosphor-STAT3 (p-STAT3) and total-STAT3 (T-STAT3) are analyzed by Western blot after administration of STAT3 inhibitor Stattic in response to acute I/R. B−D: inhibition of STAT3 activation abolishes SDF-1-improved myocardial function following I/R. Results are means ± SE; n = 5–7/group.
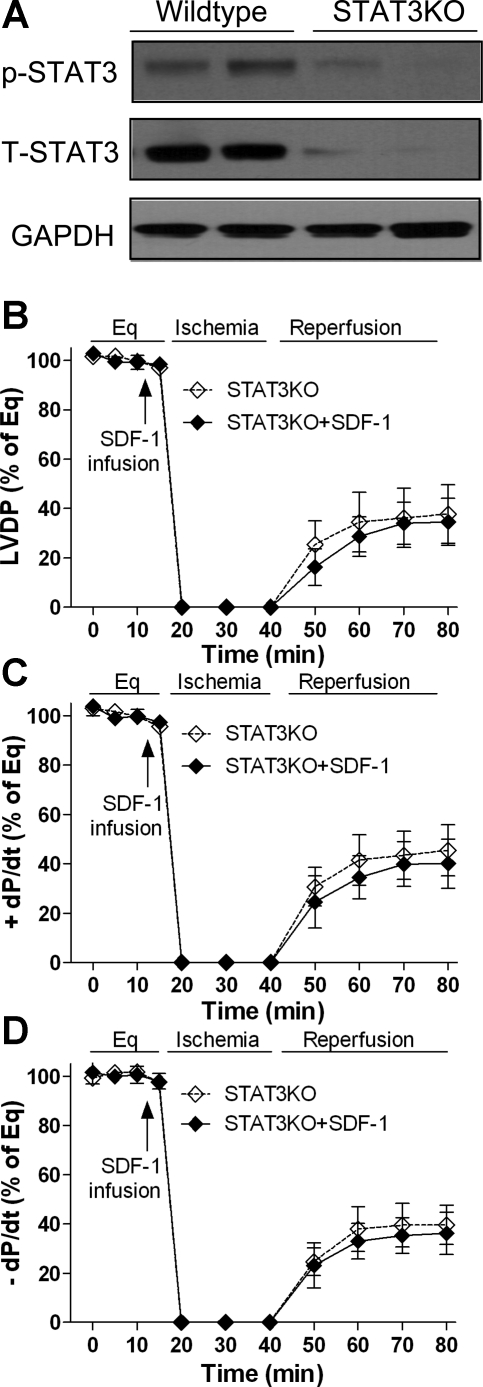
To further verify the role of STAT3 in myocardial SDF-1 signaling, we utilized mice with cardiomyocyte-restricted STAT3KO. Cardiac-restricted deletion of STAT3 resulted in significantly reduced p-STAT3 and total STAT3 in the heart (Fig. 6A). Administration of SDF-1 before ischemia did not improve myocardial functional recovery in STAT3KO mouse hearts compared with their untreated counterparts following I/R (Fig. 6, B−D).
Fig. 6.
Cardiac-restricted ablation of STAT3 in SDF-1-preserved cardiac functional recovery following I/R. A: Western blot assay indicates myocardial levels of p-STAT3 and T-STAT3 between wild-type and STAT3KO mice. B−D: changes of LVDP and ±dP/dt in the STAT3KO hearts following I/R with or without SDF-1 treatment. Results are means ± SE; n = 5–6/group.
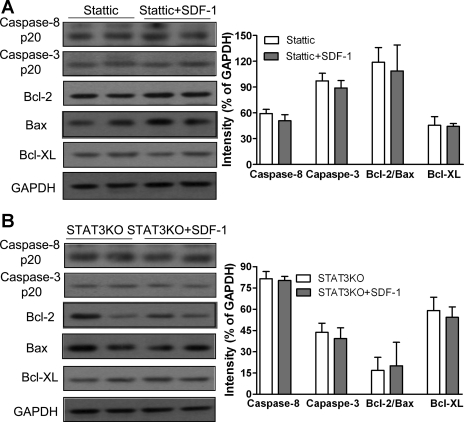
Consistent with the results of myocardial function, we observed that pretreatment of SDF-1 neither reduced activity of caspase-8 and caspase-3 nor increased ratio of Bcl-2 to Bax or expression of Bcl-XL in Stattic-treated hearts (Fig. 7A) or STAT3KO hearts (Fig. 7B) compared with their untreated counterparts.
Fig. 7.
Disruption of STAT3 pathway on SDF-1-suppressed apoptotic-signaling following acute global I/R injury. SDF-1 treatment neither reduces proapoptotic proteins caspase-8 and caspase-3 nor upregulates antiapoptotic signaling Bcl-2-to-Bax and Bcl-XL in STAT3 inhibitor (Stattic)-treated hearts (A) or cardiac-restricted STAT3KO mouse hearts (B) compared with their counterparts after I/R. Results are means ± SE; n = 5–6/group.
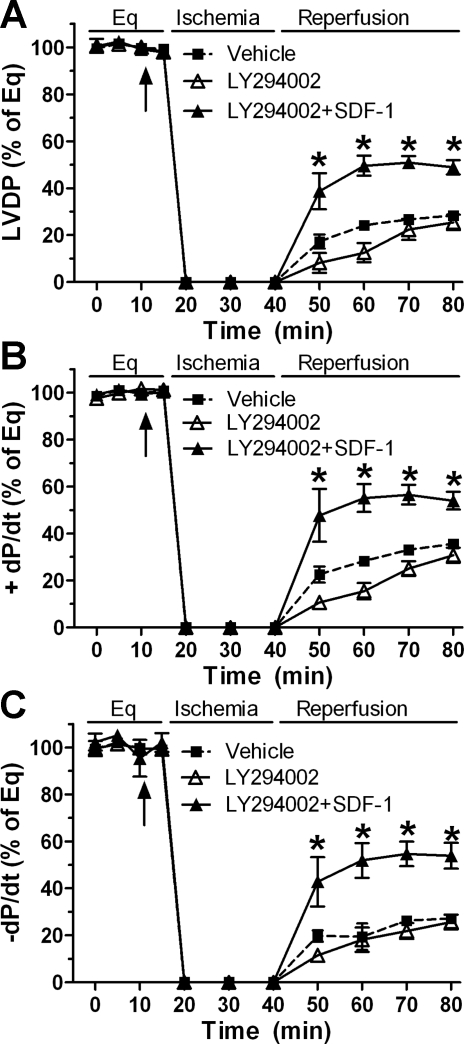
To further obviate the effect of Akt signaling in SDF-1-mediated cardioprotection, we utilized LY294002, an inhibitor of the Akt pathway. In line with our previous results in which pretreatment with SDF-1 did not affect myocardial Akt activation following global I/R, blockade of Akt signaling by using LY294002 did not attenuate SDF-1-improved cardiac function (Fig. 8), suggesting that SDF-1-induced acute protection is not mediated through the Akt pathway during global I/R injury.
Fig. 8.
Akt pathway in SDF-1-improved myocardial function following I/R. Inhibition of the Akt signaling by LY294002 does not affect SDF-1-protected postischemic LVDP (A), +dP/dt (B), and −dP/dt (C). Results are means ± SE; n = 5–6/group. *P < 0.05 vs. vehicle or LY294002 alone at corresponding time point.
DISCUSSION
In the present study, an in vitro perfused heart system was utilized to determine the role of SDF-1 in modulating acute cardioprotection following I/R, which excludes the effect of SDF-1 on mobilization and recruitment of stem cells into the sites of injury. In addition, this model is directly related to clinical cardiac surgery, which leads to obligatory global myocardial I/R injury. Herein, we demonstrated that intracoronary delivery of SDF-1 before ischemia significantly improved myocardial function and reduced apoptotic signaling following I/R through CXCR4. Interestingly, SDF-1 treatment also upregulated myocardial STAT3 activation but not Akt or ERK. Inhibition of STAT3 activation or cardiomyocyte-restricted STAT3 ablation neutralized SDF-1-mediated cardioprotection in the heart subjected to acute I/R.
SDF-1, also called CXCL12, is an important member of the CXC group of chemokines and has been widely explored in the hematopoietic system. SDF-1 and its cognate receptor CXCR4 are critical for development processes including cardiac development, lymphopoiesis, and central nervous system development. Lack of either SDF-1 or CXCR4 in mice has resulted in fetal lethality with multiple developmental defects (24, 37). Of note, a major function of the SDF-1/CXCR4 axis is chemoattraction during stem cell homing. In particular, bone marrow-derived stem cells and endothelial progenitor cells are able to migrate along an SDF-1 gradient into ischemic tissue through their expressed CXCR4 (3, 7, 35). In addition to its role in stem cell migration, development, and organogenesis, the SDF-1/CXCR4 axis also mediates other specific effects that are likely distinct to each tissue/cell. SDF-1/CXCR4 has been shown to improve proliferation of endothelial progenitor cells and their differentiation into vascular cords (33). Additionally, the anti-inflammatory or plaque-stabilizing effect of SDF-1/CXCR4 has been observed in atherosclerosis (34). Furthermore, in the central nervous system, SDF-1/CXCR4 is able to modulate neurotransmission through direct control of neuron excitability (12). However, it is unclear what rapid effect SDF-1/CXCR4 exerts in the heart following acute I/R.
Cardiac levels of SDF-1 are increased immediately after MI (3, 17, 21). Although accumulating evidence has clearly indicated that the SDF-1/CXCR4 axis is able to mediate cardioprotection through recruiting stem cells, promoting angiogenesis, increasing vascular density, and advancing myocardial structure (3, 7, 14), the elevation of SDF-1 after MI is transient and insufficient to attract stem cells homing to the injured site for cardiac repair and angiogenesis (1). Therefore, increasing SDF-1 by delivery of the SDF-1 protein and gene after MI has been shown to be promising in the treatment of ischemic heart disease (3, 7, 14). It is evident that intracardiac injection of SDF-1 reduced myocardial damage and improved cardiac function within 72 h after MI (21). However, it remains unknown regarding the effect of SDF-1/CXCR4 on acute protection of myocardial function early after ischemia. Our current study, by using the Langendorff I/R model, addressed that pretreatment with SDF-1 improved left ventricular contractility and compliance following global I/R and provides direct evidence of SDF-1-protection of cardiac function in response to myocardial ischemia. In addition, AMD3100, a specific inhibitor of CXCR4, abolished SDF-1-improved myocardial function following acute I/R, suggesting that SDF-1 binding to CXCR4 mediates this rapid protection in the heart subjected to myocardial I/R.
Cell death is a key factor leading to myocardial damage and cardiac dysfunction after I/R injury. SDF-1 has been shown to promote cell survival and exert an antiapoptotic effect in a variety of cells. Both SDF-1-treated myeloid progenitor cells and SDF-1-expressing transgenic myeloid progenitors exhibit enhanced survival and decreased apoptosis (4, 5). SDF-1 also decreases serum deprivation-induced apoptosis in endothelial progenitor cells (36). In addition, SDF-1-overexpressing pancreatic β-cells demonstrate promoted survival with attenuated caspase-3 activity and increased Bcl-2 following glucose deprivation (32). Furthermore, SDF-1 has been reported to protect cardiomyocytes from death against ischemic and hypoxic injury (17, 21). In the present study, we demonstrated that infusion of SDF-1 before ischemia significantly reduced proapoptotic signaling in the ischemic heart as exhibited by decreased activity of caspase-8 and caspase-3 and increased antiapoptotic signaling as shown by elevated Bcl-2-to-Bax ratios. Conversely, blockade of CXCR4 neutralized SDF-1-alleviated apoptotic signaling in the hearts subjected to I/R. These findings suggested that the SDF-1/CXCR4 axis might mediate acute cardioprotection through attenuation of myocyte death following I/R. In addition, given that necrotic cell death plays an important role in causing myocardial dysfunction following acute I/R injury (9), we determined the effect of SDF-1 on necrosis by measurement LDH release in coronary effluents collected at 5 min of reperfusion after ischemia. Based on the time period here, most LDH release is from necrosis-induced cell damage (9). We observed that SDF-1 did not decrease LDH levels compared with vehicle group, implying that SDF-1-improved cardiac function is unlikely through reduction of necrotic cell death. Being consistent with our findings, more and more recent studies (13, 16, 31) have demonstrated that decreasing apoptosis and apoptotic signaling are able to protect I/R-induced cardiac dysfunction by using a Langendorff model.
It is well documented that binding of SDF-1 to CXCR4 activates a series of downstream protein kinases, such as Akt, ERK, and STAT3. Saxena et al. (21) have demonstrated that administration of SDF-1 activates the cell-survival factor Akt in the heart 72 h after in vivo coronary ligation and thus suppresses myocyte death after MI. In addition, Hu et al. (17) have reported rapid and transient activation of ERK and Akt in the myocyte culture as early as 5 min after adding SDF-1, but these increased levels returned to baseline 30 min after treatment. Surprisingly, in this study, delivery of SDF-1 did not increase myocardial activation of Akt or ERK in the heart subjected to acute I/R. In contrast, we observed dramatically upregulated STAT3 activation in SDF-1-treated hearts following I/R. These disparate findings are likely due to the following: 1) the use of different dose/concentration of SDF-1, and 2) the use of different experimental models. In our study, we utilized at least an eight-time lower dose of SDF-1 than previous studies did (17, 21). This low dose of SDF-1 might not be enough to activate myocardial Akt pathway. In addition, we employed an isolated heart perfusion system compared with the in vivo MI model or cardiomyocyte culture in previous studies (17, 21). In fact, to identify the role of Akt in SDF-1-preserved cardiac function, we used a specific inhibitor of the Akt pathway. We reported that blockade of Akt signaling did not affect SDF-1-improved myocardial function, implying that the Akt pathway is not involved in SDF-1-mediated acute protection following global I/R. However, it requires further investigation regarding the interaction among SDF-1/CXCR4-activated STAT3, Akt, and ERK pathways in the heart subjected to I/R. Of note, CXCR4 contains two domains involved in Jak2/STAT3 signaling and binding of SDF-1 to CXCR4 is able to trigger STAT3 activation (2). SDF-1-increased phosphorylation of STAT3 has been observed to facilitate growth and spread of small cell lung cancer (20). In addition, SDF-1 has been shown to protect chronic lymphocytic leukemia B cells from apoptosis through upregulated STAT3 activation (6). Furthermore, SDF-1/CXCR4 mediates migration of mesenchymal stem cells through activation of STAT3 (10). Herein, we expanded this observation of SDF-1/CXCR4-increased STAT3 activation to the organ of heart.
Studies from our group and others (15, 18, 27, 30) have demonstrated that STAT3 activation is increased in the ischemic heart and plays an important role in mediating cardioprotection in response to I/R injury. Inhibition of STAT3 signaling in the heart has been shown to increase myocardial injury following infarction (19). Results from restricted deficiency of STAT3 in cardiomyocytes also demonstrate the beneficial effect of STAT3 after MI (15). In addition, recent evidence has demonstrated that the STAT3 signaling is involved in mediating preconditioning- and postconditioning-induced protection in the heart subjected to I/R (11, 22). However, it is unclear whether increased cardiac STAT3 activation is responsible for SDF-1-mediated acute protection in the heart following I/R. In this study, we clearly demonstrated that disruption of the STAT3 pathway by using STAT3 inhibitor or cardiac-restricted STAT3KO abolished SDF-1-improved myocardial function and neutralized SDF-1-reduced apoptotic signaling following I/R. Therefore, our study represents the first evidence that the SDF-1/CXCR4 axis upregulates myocardial STAT3 activation and, thereby, mediates acute cardioprotection in response to I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute R00-HL-0876077 (to M. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110: 3300–3305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahr B, Denizot M, Robert-Hebmann V, Brelot A, Biard-Piechaczyk M. Identification of the cytoplasmic domains of CXCR4 involved in Jak2 and STAT3 phosphorylation. J Biol Chem 280: 6692–6700, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362: 697–703, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Broxmeyer HE, Cooper S, Kohli L, Hangoc G, Lee Y, Mantel C, Clapp DW, Kim CH. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol 170: 421–429, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, Hangoc G, Shaheen M, Li X, Clapp DW. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol 73: 630–638, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood 106: 1824–1830, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol 42: 792–803, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98: 699–710, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Freude B, Masters TN, Robicsek F, Fokin A, Kostin S, Zimmermann R, Ullmann C, Lorenz-Meyer S, Schaper J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol 32: 197–208, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27: 857–865, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol 295: H1649–H1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem 96: 1540–1550, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hadzimichalis NM, Baliga SS, Golfetti R, Jaques KM, Firestein BL, Merrill GF. Acetaminophen-mediated cardioprotection via inhibition of the mitochondrial permeability transition pore-induced apoptotic pathway. Am J Physiol Heart Circ Physiol 293: H3348–H3355, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation 109: 2454–2461, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 95: 187–195, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122: 2170–2182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation 116: 654–663, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104: 979–981, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, Yamauchi-Takihara K. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res 47: 797–805, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Pfeiffer M, Hartmann TN, Leick M, Catusse J, Schmitt-Graeff A, Burger M. Alternative implication of CXCR4 in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer 100: 1949–1956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation 117: 2224–2231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res 79: 127–133, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 104: I308–I303, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 288: E321–E326, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol 290: H2204–H2209, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Wang M, Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation 118: S38–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation 114: I282–289, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Wang M, Tsai BM, Kher A, Baker LB, Wairiuko GM, Meldrum DR. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol Heart Circ Physiol 288: H221–H226, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol 293: H2101–H2108, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122: 1308–1318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes 56: 2946–2957, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 28: 644–650, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res 102: 209–217, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 21: 3197–3207, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, Fu G. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis 201: 36–42, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599, 1998 [DOI] [PubMed] [Google Scholar]