Abstract
microRNA-210 (miR-210) is upregulated in hypoxia, but its function in cardiomyocytes and its regulation in response to hypoxia are not well characterized. The purpose of this study was to identify upstream regulators of miR-210, as well as to characterize miR-210's function in cardiomyocytes. We first showed miR-210 is upregulated through both hypoxia-inducible factor (HIF)-dependent and -independent pathways, since aryl hydrocarbon nuclear translocator (ARNT) knockout mouse embryonic fibroblasts (MEF), lacking intact HIF signaling, still displayed increased miR-210 levels in hypoxia. To determine the mechanism for HIF-independent regulation of miR-210, we focused on p53 and protein kinase B (Akt). Overexpression of p53 in wild-type MEFs induced miR-210, whereas p53 overexpression in ARNT knockout MEFs did not, suggesting p53 regulates miR-210 in a HIF-dependent mechanism. Akt inhibition reduced miR-210 induction by hypoxia, whereas Akt overexpression increased miR-210 levels in both wild-type and ARNT knockout MEFs, indicating Akt regulation of miR-210 is HIF-independent. We then studied the effects of miR-210 in cardiomyocytes. Overexpression of miR-210 reduced cell death in response to oxidative stress and reduced reactive oxygen species (ROS) production both at baseline and after treatment with antimycin A. Furthermore, downregulation of miR-210 increased ROS after hypoxia-reoxygenation. To determine a mechanism for the cytoprotective effects of miR-210, we focused on the predicted target, apoptosis-inducing factor, mitochondrion-associated 3 (AIFM3), known to induce cell death. Although miR-210 reduced AIFM3 levels, overexpression of AIFM3 in the presence of miR-210 overexpression did not reduce cellular viability either at baseline or after hydrogen peroxide treatment, suggesting AIFM3 does not mediate miR-210's cytoprotective effects. Furthermore, HIF-3α, a negative regulator of HIF signaling, is targeted by miR-210, but miR-210 does not modulate HIF activity. In conclusion, we demonstrate a novel role for p53 and Akt in regulating miR-210 and demonstrate that, in cardiomyocytes, miR-210 exerts cytoprotective effects, potentially by reducing mitochondrial ROS production.
Keywords: microribonucleic acid, reactive oxygen species, cell death, hypoxia
hypoxic stress drives numerous phenotypic changes in cardiomyocytes, including a shift in substrate utilization away from fatty acid metabolism (22), a decrease in contractility (6, 32), and increased sensitivity to acidosis-induced apoptosis (26). Many of these adaptations are driven by hypoxia-inducible factor-1α (HIF-1α), a transcription factor that is a master regulator of the cellular hypoxic response. However, other signaling pathways, including the p53 and protein kinase B (Akt) pathways, also play crucial roles in modulating cellular metabolic adaptation and survival in the setting of hypoxia (3, 19, 28).
microRNAs, small RNA molecules ∼22 nucleotides in length that downregulate gene expression by translational repression, are the major posttranscriptional mechanism by which gene expression is controlled and are predicted to target up to 30% of the mammalian genome (15). These molecules have been implicated in several processes of direct relevance to the heart, including cardiac development (11), cardiac hypertrophy (38), heart failure (35), and angiogenesis (13). Modulation of microRNA to treat ischemic heart disease is a field of great interest (17). Therefore better characterization of microRNAs in the heart at baseline and in response to various stressful stimuli is of biological and clinical importance.
microRNA-210 (miR-210), a direct transcriptional target of HIF-1α (21), has emerged as a critical element of the cellular hypoxia response in a broad variety of cell types ranging from cancer cell lines (27) to human umbilical vein endothelial cells (14). This microRNA has diverse functions, including modulating angiogenesis (14), stem cell survival (25), and hypoxia-induced cell cycle arrest (40). Recently, miR-210-based gene therapy using minigenes in mice has been shown to improve cardiac function in a model of myocardial infarction (20). However, our understanding of the regulation of miR-210, or of the effects of miR-210 in cardiomyocytes, remains limited.
The purpose of this study was to examine mechanisms of miR-210 induction aside from HIF-1α and to evaluate the effects of miR-210 on cell death and reactive oxygen species (ROS) production in cardiomyocytes. We first demonstrate that miR-210 is regulated by p53 and Akt. We further show that miR-210 overexpression in cardiomyocytes has protective effects in response to oxidant stress, reduces mitochondrial ROS production, and decreases mitochondrial mass. Knock down of miR-210 in the setting of hypoxia increases mitochondrial ROS production and increases mitochondrial mass in H9c2 cells exposed to 0.5% oxygen.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney (HEK293) cells (ATTC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (CellGro, Manassas, VA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA) and 5 mg/ml of sodium pyruvate. H9c2 cells (ATTC) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS. Wild-type (WT) mouse embryonic fibroblasts (MEFs) as well as aryl hydrocarbon nuclear translocator (ARNT) knockout (KO), p53 KO, and Akt 1/Akt 2 double-KO MEFs (hereafter referred to as Akt KO MEFs for simplicity) were cultured in modified Eagle's medium (CellGro) supplemented with 10% FBS. p53 KO and Akt KO MEF cell lines were kind gifts from Dr. Nissim Hay (University of Illinois, Chicago, IL). ARNT KO MEFs were kindly provided by Dr. Celeste Simon (Abramson Family Cancer Research Institute, University of Pennsylvania School of Medicine). Neonatal rat cardiomyocytes (NRCM) were prepared from 1- to 2-day-old Sprague-Dawley rats as previously described (1). The animal protocol used to isolate NRCMs was submitted to and approved by the institutional review board at Northwestern University Feinberg School of Medicine.
Transfection.
HEK293 cells and MEFs were transfected using Lipofectamine reagent (Invitrogen), and assays were conducted 48 h later. All plasmid transfections were done using Lipofectamine in conjunction with Plus Reagent (Invitrogen). For p53 and Akt transfection experiments, MEFs were plated on six-well plates, and 4 μg of the appropriate plasmid along with 0.4 μg enhanced green fluorescent protein (GFP) plasmid (Stratagene) were cotransfected into the cells. Two micrograms of pSUPER and pSUPER-miR-210 plasmids, or 2 μg of pCMV-SPORT and pCMV-AIFM3 plasmids, were transfected into HEK293 cells plated on six-well plates for cell death assays. For miR-210 knock down experiments, scrambled control and anti-miR-210 AntimiR miRNA inhibitors were purchased from Ambion (Austin, TX) and transfected into NRCM with Transmessenger transfection reagent from Qiagen (Valencia, CA).
microRNA arrays.
NRCM were plated in six-well plates at a density of 1.2 million cells per well. Seventy two hours after primary isolation, cells were cultured in either hypoxia or normoxia for a further 48 h. Total RNA was then extracted from cells using RNA-Stat (Teltest, Friendswood, TX). After RNA quality was assessed using the Agilent bioanalyzer system, samples were shipped to Exiqon (Vedbaek, Denmark), where hybridizations were performed in-house. At the time the experiment was performed, the Exiqon platform was capable of detecting 280 of the 287 known rat microRNAs. The following two-color hybridizations using Cy3 and Cy5 labeling were performed: 1.5% O2 vs. normoxia (n = 3 independent biological samples in each group) and 0.5% O2 vs. normoxia (n = 3 independent biological samples in each group). To minimize the effects of dye bias, the raw microarray data were then normalized in-house by Exiqon using M vs. A plots in which the log2 intensity ratio between the two labeled samples is plotted against the log2 mean intensity of the two labeled samples. The locally weighted scatterplot smoothing regression algorithm was then applied to the data to generate normalized log2 Cy3-to-Cy5 ratios (4). To generate uncorrected P values for the fold change data from the microarray, one-sample t-testing was performed (36). Correction for multiple comparisons was done using the Bonferroni step-down method (24). All microarray data have been submitted to the Gene Expression Omnibus at the National Center for Biotechnology Information (accession no. GSE24954).
Quantitative real-time PCR.
Taqman microRNA assays (Applied Biosystems) were performed to quantify miR-210 levels. RNA species-specific cDNA templates were generated according to manufacturer protocols for both miR-210 and U6, a small RNA that served as a normalization control. Quantitative RT-PCR was then performed on an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems), and miR-210 expression relative to U6 control was calculated using the 2−ΔΔCt method. The reagents for the assay, including primers for miR-210 and U6 control, were purchased from Applied Biosystems.
Cell death.
Cell death assays were conducted using the trypan blue assay and propidium iodide staining followed by flow cytometry. After treatment with varying doses of hydrogen peroxide, cells were gently collected after treatment with 0.25% trypsin and resuspended in PBS. For the trypan blue assay, an aliquot of cells was then mixed with trypan blue for a final concentration of 0.2% trypan blue. After a 5-min room temperature incubation, cells were loaded on a hemocytometer, and the fraction of dead cells was calculated as cells staining blue over total cell count. For propidium iodide staining, a 200-μl aliquot of cell suspension was stained with propidium iodide at a final concentration of 5 μg/ml. After a 15-min room temperature incubation, the fraction of propidium iodide-positive cells was detected using flow cytometry as described below.
Measurement of mitochondrial membrane potential.
After being washed, NRCM or H9c2 cells were stained with 100 nM tetramethylrhodamine esther (TMRE) in fresh media. Next the cells were gently trypsinized with 0.25% Trypsin, centrifuged at 250 g for 5 min, and resuspended in 500 μl of PBS with 1% BSA. Flow cytometry experiments were then performed as described below.
Flow cytometry.
Flow cytometry experiments were conducted using a BD LSR II Flow Cytometer (BD, Franklin Lakes, NJ) after incubating cells with either nonyl acridine orange (NAO) at 25 nM for mitochondrial biogenesis experiments or propidium iodide as described above. Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
ROS studies.
NRCM were plated in a six-well format. For overexpression experiments, 48 h after plating, cells were transfected with a multiplicity of infection of 10 GFP or miR-210 adenovirus. Ninety six hours after plating, cells were treated with antimycin A, a complex III inhibitor, at a final concentration of 100 μM for 30 min before being stained with Mitosox (5 μM) and Hoescht (10 μM) nuclear stain from Invitrogen according to the manufacturer instructions. Cells were then imaged with a Zeiss fluorescent microscope, and a total of 15 high-power fields were captured in three channels (red for Mitosox, green for GFP, and blue for Hoescht). These cells were then analyzed in ImageJ (National Institutes of Health, Bethesda, MD) by measuring average red fluorescence in regions with green fluorescence (successfully infected with adenovirus) and without blue fluorescence (to mask out nonspecific nuclear staining by Mitosox). For knock down experiments, 48 h after plating, cells were transfected with scrambled control and anti-miR-210 miRNA inhibitors. The following day after a change to fresh media, cells were exposed to hypoxia for 48 h, followed by 1 h of reoxygenation. The remainder of the analysis was performed as described above, with the exception that the entire microscope field was measured for red fluorescence instead of masking with the green channel for transfected cells.
Hypoxia.
All hypoxia experiments were conducted in a hypoxia glove box (Coy, Grass Lake, MI).
Plasmids and adenovirus construction.
The HIF3α 3′-untranslated region (UTR) construct was created by cloning the HIF-3α 3′-UTR into pMIR-REPORT (Promega, Madison, WI) using the following primers: HIF-3α forward primer: GGACGCGTGCCGGCTCCTCTCCCCATCTGC and HIF-3α reverse primer: CCTCACCTTGGTAGGCACCAGAGTTTAAACCC. The 3′-UTR of AIFM3 was cloned into the pMIR-Report vector between the SpeI and SacI restriction sites using the following primers: AIFM3 forward primer: ACTAGTGCTCACATGCAGTAGACTT and AIFM3 reverse primer: GAGCTCGATCTGCAAATTTGTGCGT. The miR-210 sensor construct used as a positive control was created by inserting two perfect matches to miR-210 into the pMIR-report plasmid between the SpeI and HindIII restriction sites by first ligating together the following two synthesized oligonucleotides: sensor top strand: CTAGTTCAGCCGCTGTCACACGCACAGGGATCCTCAGCCGCTGTCACACGCACAGA, sensor bottom strand: AGCTTCTGTGCGTGTGACAGCGGCTGAGGATCCCTGTGCGTGTGACAGCGGCTGAA. All oligonucleotides were purchased from Integrated DNA Technologies. The 3× HRE-luciferase construct was a gift from Dr. Navdeep Chandel. The pSUPER-miR-210 construct was a gift from Dr. Fabio Martelli. The p53 and p53 dominant-negative constructs are from Clontech (Mountain View, CA). The Akt constitutively active plasmid was obtained from Addgene (Addgene plasmid 16244) (41). miR-210 adenovirus and GFP adenovirus were generated using the AdEasy adenovirus system (Invitrogen). The miR-210 adenovirus construct expresses both miR-210 and GFP from separate promoters.
Luciferase assays.
Luciferase assays were performed in HEK293 cells after cotransfection with pRL-TK Renilla plasmid for normalization (Promega). Luminescence was measured using a Berthold Lumat LB 9507 luminometer with the Dual-Glo luciferase assay kit (Promega).
Western blot.
HEK293 cells after 48 h treatment with either GFP or miR-210 adenovirus were lysed using RIPA buffer (Pierce, Rockford, IL) with added protease inhibitor cocktail (G-Biosciences, Maryland Heights, MO). Twenty five micrograms of total protein were loaded on a 4–12% SDS-PAGE gradient gel (Invitrogen), transferred to a nitrocellulose membrane, incubated with rabbit anti-AIFM3 antibody (ProSci, Poway, CA) at 1:500 dilution, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Enhanced chemiluminesence was then detected (Pierce). The membrane was then stripped, and anti-actin antibody (Santa Cruz Biotechnology) was used as a loading control.
Statistical methods.
Data are reported as means ± SE. Statistical analysis was performed using Microsoft Excel Software (Microsoft, Redmond, WA). Statistical methods for the microarray analysis are discussed under microRNA arrays. For all other experiments, significance threshold was set at P = 0.05, and Student t-test was used to assess statistical significance.
RESULTS
miR-210 is upregulated in cardiomyocytes.
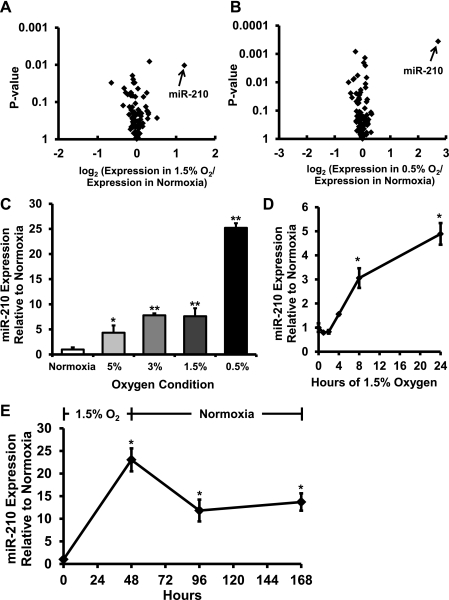
To screen for microRNAs regulated by hypoxia, we performed microRNA microarrays comparing NRCM exposed to normoxia vs. either 1.5 or 0.5% O2 for 48 h. miR-210 was the most strongly upregulated microRNA in both the 1.5 and 0.5% O2 conditions and, after correction for multiple comparisons, was the only significantly upregulated microRNA (Fig. 1, A and B). These observations were then confirmed by quantitative real-time PCR, which demonstrated that miR-210 is upregulated by hypoxia in a dose-responsive manner, increasing 7.6-fold in the 1.5% O2 condition and 25.2-fold in the 0.5% O2 condition. An intermediate level of upregulation was seen in the 3 and 5% O2 conditions, but no significant difference in the amount of miR-210 induction was seen between these two conditions (Fig. 1C). In NRCM cultured in 1.5% O2, miR-210 levels are increased significantly within 8 h (Fig. 1D). Furthermore, miR-210 levels remained elevated for up to 120 h in NRCM returned to normoxia after being cultured in 1.5% O2 for 48 h (Fig. 1E).
Fig. 1.
microRNA-210 (miR-210) is upregulated in hypoxic cardiomyocytes. A: volcano plot of microRNA expression level and uncorrected P value in the 1.5% O2 for 48 h condition. B: volcano plot of microRNA expression level and uncorrected P value in the 0.5% O2 for 48 h condition. miR-210 is the most strongly induced microRNA in both conditions and, after correction for multiple comparisons, is the only significantly upregulated microRNA (corrected P = 0.04 in the 0.5% O2 condition); n = 3 independent biological samples in each group. C: RT-PCR data confirm that hypoxia upregulates miR-210 in an O2 dose-responsive manner (*P < 0.05 and **P < 0.01). D: miR-210 is induced in neonatal rat cardiomyocytes (NRCM) as soon as 8 h after hypoxia exposure (*P < 0.05 compared with time 0). E: miR-210 levels remain elevated in NRCM up to 120 h after withdrawal of cells from hypoxia (*P < 0.05 compared with time 0). Data are presented as means ± SE; n = 3 in each group.
p53 upregulates miR-210.
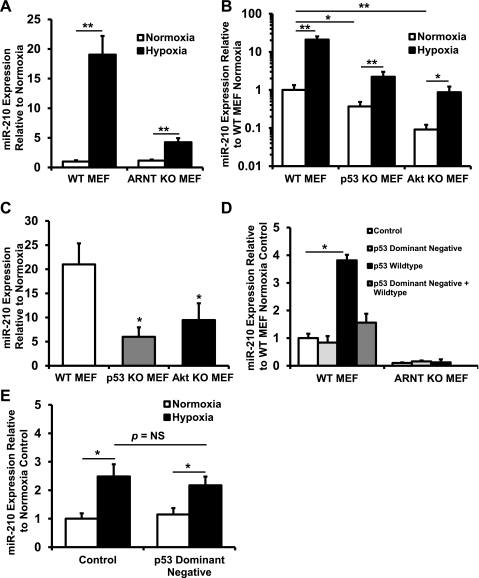
HIF-mediated upregulation of miR-210 has been described (7, 21). Because a number of additional pathways and transcription factors are activated in response to hypoxia, we asked whether other factors might also regulate miR-210. Both HIF-1α and HIF-2α need to dimerize with ARNT to exert their biological effects, and a deletion of ARNT results in complete inactivation of the HIF pathway. We therefore used ARNT KO MEFs to test whether miR-210 is regulated in a HIF-independent pathway. In WT MEFs, after 48 h of hypoxia (1.5% O2), miR-210 levels increased 19.0-fold compared with 3.7-fold in ARNT KO MEFs (Fig. 2A). Next we assessed the role of the p53 and Akt pathways in miR-210 regulation. Compared with WT MEFs, both at baseline and in response to hypoxia, p53 and Akt KO MEFs express lower levels of miR-210 (Fig. 2B). Moreover, the fold increase in miR-210 levels with hypoxia in both p53 and Akt KO MEFs was reduced (Fig. 2C). To confirm that p53 overexpression upregulates miR-210, we transfected WT and ARNT KO MEFs with p53 WT, dominant-negative, and control plasmids. The p53 dominant-negative plasmid did not upregulate miR-210 in either the ARNT KO MEFs or the WT MEFs. However, transfection with p53 WT plasmid led to a 3.8-fold increase in miR-210 levels in the WT MEFs but not in the ARNT KO MEFs (Fig. 2D). To test whether p53 is involved in HIF-independent hypoxic induction of miR-210, we transfected p53 dominant-negative plasmid into ARNT KO MEFs and showed that inhibition of p53 did not blunt the hypoxia-induced increase in miR-210 (Fig. 2E). Taken together, these results suggest that p53 regulates miR-210 and that this regulation is likely dependent on HIF.
Fig. 2.
miR-210 is regulated through hypoxia-inducible factor (HIF)-dependent and -independent pathways. A: hypoxia (1.5% O2) upregulates miR-210 both in wild-type (WT) and aryl hydrocarbon nuclear translocator (ARNT) knockout (KO) mouse embryonic fibroblasts (MEFs) lacking HIF signaling, a known inducer of miR-210 (**P < 0.01 compared with normoxia, n = 6). The degree of miR-210 induction, however, is lower in ARNT KO MEFs. B: p53 KO MEFs and Akt KO MEFs have lower levels of miR-210 expression both at baseline and in response to hypoxia (*P < 0.05 and **P <0.01 compared with WT MEF; n = 6 in all groups). C: both p53 KO MEFs and Akt KO MEFs have reduced fold increase of miR-210 (*P < 0.05 compared with WT MEF, n = 6 for each group). D: p53 plasmid, but not p53 dominant-negative plasmid, transfected into WT MEFs induces miR-210 expression (*P < 0.05 compared with pCMV control plasmid transfection, n = 3). Transfection of dominant-negative plasmid with WT plasmid abrogates induction of miR-210 by WT p53. However, in ARNT KO MEF, p53 overexpression does not increase miR-210 levels. E: p53 dominant-negative plasmid transfection into ARNT KO MEFs does not reduce induction of miR-210 by hypoxia (*P < 0.05 compared with normoxia, n = 3). NS, not significant. Data are presented as means ± SE.
Akt upregulates miR-210.
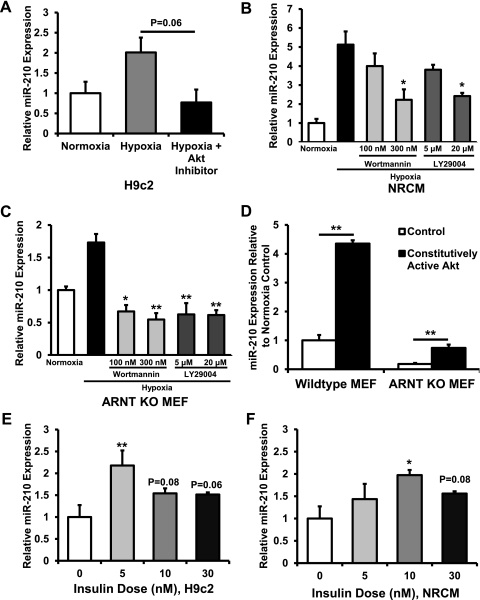
Initial studies demonstrated reduced miR-210 expression in Akt KO MEFs both at baseline and in response to 1.5% O2 (Fig. 2, B and C). To confirm Akt regulation of miR-210, H9c2 cardiomyoblasts were exposed to normoxia, hypoxia, and hypoxia plus 5 μM Calbiochem Akt Inhibitor IV, followed by measurement of miR-210 levels. Akt Inhibitor IV abolished the induction of miR-210 with hypoxia (Fig. 3A). In NRCM (Fig. 3B), high-dose wortmannin and LY-29004, two different inhibitors of phosphatidylinositol 3-kinase (PI 3-kinase), an upstream activator of Akt, also abolished the induction of miR-210 by hypoxia. In ARNT KO MEFs, both wortmannin and LY-29004 abolished the increase in miR-210 levels (Fig. 3C). Furthermore, transfection of constitutively active Akt plasmid into both WT and ARNT KO MEFs increased miR-210 expression (Fig. 3D). Finally, addition of insulin, a PI 3-kinase-dependent activator of Akt (8), to H9c2 and NRCM induced miR-210 expression (Fig. 3, E and F). Taken together, these results suggest that Akt increases miR-210 levels through a HIF-independent mechanism.
Fig. 3.
Akt upregulates miR-210. A: In H9c2 cells, there was a reduction in miR-210 response to hypoxia after treatment with Akt inhibitor (P = 0.06 compared with hypoxia alone). B: in NRCM, treatment with the phosphatidylinositol 3-kinase (PI 3-kinase) inhibitors wortmannin at 300 nM and LY-29004 at 20 μM prevented induction of miR-210 by hypoxia (*P < 0.05 compared with hypoxia alone). C: in ARNT KO MEFs, treatment with wortmannin and LY-29004 prevented induction of miR-210 by hypoxia (*P < 0.05 and **P < 0.01 compared with hypoxia alone). D: constitutively active Akt plasmid transfection in both WT MEFs and ARNT KO MEFs induces miR-210 expression (**P < 0.01 compared with control transfection). E: in H9c2 cells, insulin treatment increased miR-210 levels (**P < 0.01 compared with no insulin treatment, n = 3 for each group). F: findings in NRCM were similar (*P < 0.05 compared with no insulin treatment, n = 3 for each group). Data are presented as means ± SE; n = 3 for each group.
miR-210 overexpression protects against hydrogen peroxide-induced cell death.
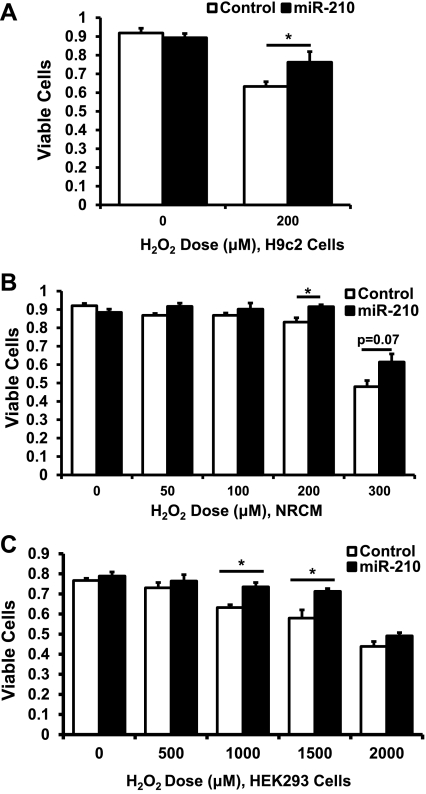
Because miR-210 is induced in cardiomyocytes in response to hypoxia and in response to the prosurvival pathway of Akt activation (30), we asked whether miR-210 overexpression could protect against cell death. In H9c2 cells and NRCM, miR-210-expressing adenovirus vs. GFP-expressing adenovirus control conferred a cytoprotective effect in response to oxidant stress by hydrogen peroxide (Fig. 4, A and B). Similar results were seen in HEK293 cells in which miR-210 overexpression was induced by plasmid transfection (Fig. 4C).
Fig. 4.
miR-210 overexpression exerts cytoprotective effects. A: H9c2 cells infected with miR-210-expressing adenovirus at a multiplicity of infection of 10 demonstrate increased viability after 200 μM hydrogen peroxide treatment for 1 h compared with green fluorescent protein (GFP) adenovirus control by trypan blue exclusion (*P < 0.05). B: results are similar in NRCM transfected with miR-210-expressing adenovirus at a multiplicity of infection of 10 (*P < 0.05). C: human embryonic kidney (HEK293) cells transfected with miR-210-expressing plasmid demonstrate increased viability at 1,000 and 1,500 μM hydrogen peroxide doses (*P < 0.05). Data are presented as means ± SE; n ≥ 3 in each group.
miR-210 targets apoptosis-inducing factor, mitochondrion-associated 3.
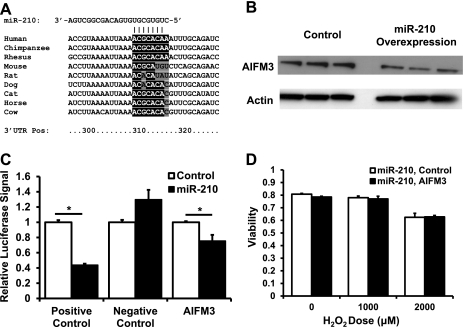
We next sought to identify novel targets mediating the cytoprotective effects of miR-210 by performing an in silico search using TargetScanS (16). Among the predicted targets was apoptosis-inducing factor, mitochondrion-associated 3 (AIFM3), a protein that has been reported to induce apoptosis (39). miR-210 is predicted to target human AIFM3; however, the predicted binding site of miR-210 on rat and mouse AIFM3 is not conserved, making it less likely that miR-210 regulation of AIFM3 is the mechanism for the cytoprotective effects of this microRNA in rat and mouse-derived cell lines (Fig. 5A). Nevertheless, because of its potential relevance to human disease, we elected to study this interaction further and performed these studies in the human-derived HEK293 cell line given the dearth of human cardiac cell lines. miR-210 overexpression led to a decrease in AIFM3 protein levels, suggesting that this protein is a target of miR-210 (Fig. 5B). We then cloned the AIFM3 3′-UTR into the pMIR-report luciferase construct and confirmed that AIFM3 is targeted by miR-210 (Fig. 5C). To test whether AIFM3 overexpression can abrogate the protective effects of miR-210, we overexpressed miR-210 in HEK293 cells along with either AIFM3 plasmid or control. Both at baseline and with hydrogen peroxide, no increase in cell death was seen with AIFM3 overexpression in the presence of miR-210 (Fig. 5D). Thus AIFM3 is a target of miR-210, but it does not modulate the cytoprotective effects of miR-210.
Fig. 5.
Apoptosis-inducing factor, mitochondrion-associated 3 (AIFM3) is targeted by miR-210 but does not mediate the cytoprotective effects of miR-210. A: alignment of the 3′-untranslated region (UTR) of AIFM3 from various species with the seed sequence of miR-210. Human AIFM3 is targeted by miR-210 but mouse and rat AIFM3 are not. Bases highlighted in black are seed sequence matches according to TargetScan S. Bases highlighted in gray are seed sequence mismatches. Note that, for the purposes of microRNA targeting, an adenine on the mRNA aligning with the first base pair of a microRNA is considered a seed match. Nos. along the bottom denote the base position along the 3′-UTR in human AIFM3. B: Western blot of HEK293 cells infected with miR-210 adenovirus vs. control adenovirus and probed with AIFM3 antibody. miR-210 overexpression reduces AIFM3 expression. C: luciferase assay with AIFM3 3′-UTR cloned into the 3′-position of the luciferase reporter gene demonstrates that plasmid-mediated miR-210 overexpression reduces luciferase activity, indicating that AIFM3 is a target of miR-210 (*P < 0.05). Positive control is a luciferase construct in which two perfect matches to the miR-210 sequence were cloned into the 3′-position of the luciferase reporter gene. Negative control is a luciferase construct in which nothing was cloned into the 3′-position of the luciferase reporter gene. D: AIFM3 overexpression in the presence of miR-210 overexpression does not reduce cellular viability either at baseline or in the presence of hydrogen peroxide. Data are presented as means ± SE; n ≥ 3 in each group.
miR-210 decreases mitochondrial ROS production and mitochondrial biogenesis.
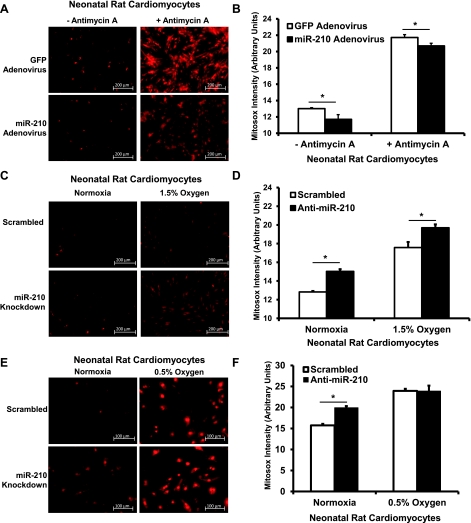
We next focused our studies on cellular ROS production and mitochondrial biogenesis. Hypoxia increases ROS levels through increased superoxide production at complex III of the mitochondrial respiratory chain (5, 18). Thus we hypothesized that, since miR-210 is upregulated in hypoxia, it may mediate a reduction in ROS levels, which may in turn mediate its cytoprotective effects. To test this, we exposed NRCM pretreated with miR-210 adenovirus vs. GFP adenovirus to antimycin A, a complex III inhibitor that induces mitochondrial superoxide production. Fluorescence microscopy was performed, and cytosolic fluorescence was analyzed. miR-210 adenovirus overexpression led to significant reductions in Mitosox fluorescence both in the baseline and antimycin A-treated conditions (Fig. 6, A and B). Furthermore, miR-210 knock down in NRCM both at baseline and in cells exposed to 1.5% O2 for 48 and 1 h of reoxygenation increased Mitosox fluorescence (Fig. 6, C and D). However, Mitosox fluorescence did not increase in cells treated with 0.5% O2 for 48 h followed by 1 h reoxygenation (Fig. 6, E and F).
Fig. 6.
miR-210 modulates superoxide production in NRCM. A: NRCM infected with miR-210-expressing adenovirus demonstrate decreased Mitosox positivity both at baseline and after 30 min of antimycin A treatment. The brightly stained nuclei were excluded from analysis by masking out the regions that exhibited Hoescht positivity. B: quantification of cytoplasmic Mitosox signal in NRCM demonstrates reduced Mitosox intensity in the miR-210-pretreated groups (*P < 0.05). C: anti-miR-210 increases Mitosox signal both at baseline and after 48 h of hypoxia at 1.5% O2 followed by 1 h of reoxygenation. D: quantification demonstrates increased Mitosox intensity in the anti-miR-210 groups (*P < 0.05). E: no difference in mitochondrial reactive oxygen species (ROS) was seen after 48 h of 0.5% O2 followed by 1 h reoxygenation. F: quantitation of data (*P < 0.05). Data are presented as means ± SE. For each experiment, n = 15 fields analyzed in each group.
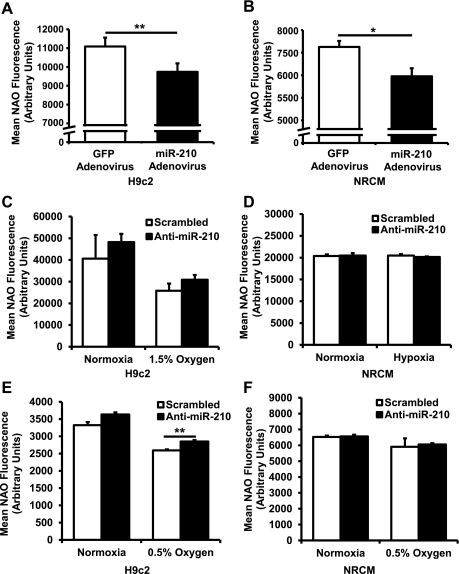
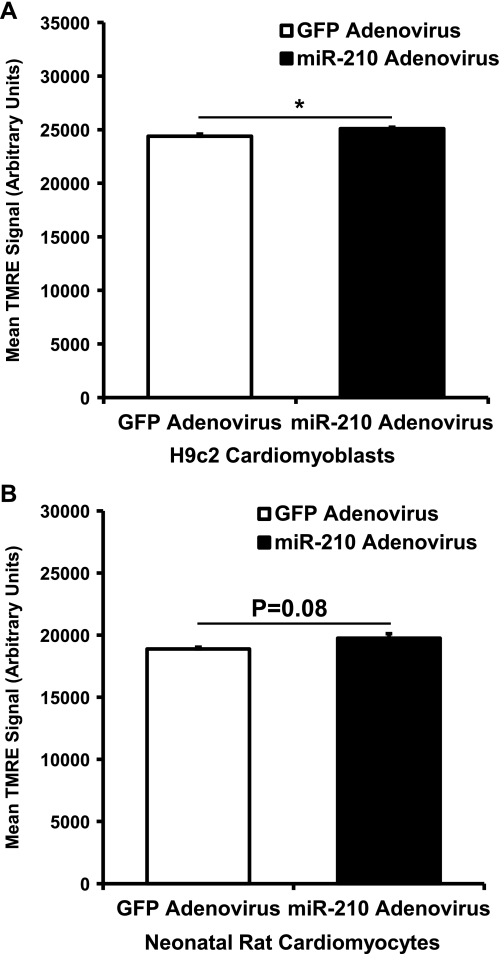
Flow cytometry on H9c2 cells stained with NAO, a mitochondria-binding dye and an indicator of mitochondrial levels, demonstrated a decrease in mitochondrial levels with adenovirus-mediated miR-210 overexpression compared with GFP control (Fig. 7A). Similar results were seen in NRCM (Fig. 7B). On the other hand, knock down of miR-210 did not increase mitochondrial mass in H9c2 cells or NRCM at baseline or after exposure to hypoxia, with the exception of a slight increase in H9c2 cells after 0.5% O2 (Fig. 7, C-F). Furthermore, miR-210 overexpression in H9c2 cells (Fig. 8A) and NRCM (Fig. 8B) had only minimal effects on mitochondrial membrane potential as assessed by staining with TMRE. This is relevant to interpretation of the NAO staining experiments, since loading of this dye is mitochondrial membrane potential-dependent (23).
Fig. 7.
miR-210 reduces mitochondrial biogenesis. A: miR-210 adenovirus overexpression in H9c2 cells reduces signal of nonyl acridine orange (NAO), a marker of mitochondrial mass (**P < 0.01, n = 6). B: miR-210 adenovirus overexpression in NRCM demonstrates similar results (*P < 0.05, n = 6). C: at baseline or after 48 h of 1.5% O2, anti-miR-210 does not increase mitochondrial mass in H9c2 cells (n = 3). D: results in NRCM were similar (n = 3). E: after 48 h of 0.5% O2, anti-miR-210 increases mitochondrial mass in H9c2 cells (**P < 0.01, n = 3). F: however, in NRCM treated with 0.5% O2 for 48 h, no change in mitochondrial mass was seen (n = 3). Data are presented as means ± SE.
Fig. 8.
Mitochondrial membrane potential in miR-210-treated cells. A: in NRCM treated with miR-210-expressing adenovirus, mitochondrial membrane potential as assessed by tetramethylrhodamine esther (TMRE) staining is slightly increased compared with GFP control (*P < 0.05, n = 3). B: in H9c2 treated with miR-210-expressing adenovirus, there was a trend toward a statistically significant increase in TMRE signal (n = 3), but the magnitude of change is very small.
HIF-3α is a target of miR-210, but miR-210 does not modulate HIF signaling.
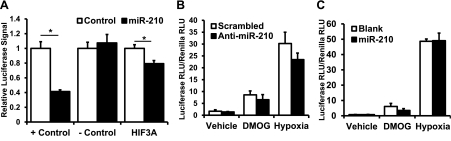
Our results thus far suggest that miR-210 exerts protective effects and is regulated by HIF-dependent and -independent pathways. Among the proposed general mechanisms of action for microRNAs is to act as a rheostat, fine-tuning the level of activation of a given signaling pathway (2). Because miR-210 is robustly induced by HIF, we asked whether miR-210 could also act upstream of HIF and modulate HIF signaling. Our interest in this question was increased when we noted that one of the potential targets of miR-210 is HIF-3α based on in silico analysis using TargetScanS. The function of HIF-3α remains incompletely understood; however, studies suggest that certain isoforms of HIF-3α can inhibit HIF-1α activity (29, 34). We hypothesized that miR-210 inhibition of HIF-3α might derepress HIF-1α activity, thereby enhancing HIF-1α signaling and potentially contributing to the cytoprotective effects of miR-210. To test this hypothesis, we first cloned the 3′-UTR of HIF-3α into the pMIR-Report construct, performed a luciferase assay, and confirmed that HIF-3α is targeted by miR-210 (Fig. 9A). We then transfected HEK293 cells with a luciferase construct containing three tandem HIF-responsive elements in the promoter (3× HRE-luciferase construct) that increases luciferase transcription in the presence of increased HIF. These cells were then exposed to one of three conditions: vehicle only; 1 mM dimethyloxaloylglcycine (DMOG), which functions as a hypoxia mimetic by inhibiting prolyl-4-hydroxylase; or 1.5% O2 for 24 h. As expected, DMOG and 1.5% O2 treatment resulted in significant increases in luciferase signal. However, knock down of miR-210, which we hypothesized would decrease overall HIF activity by increasing HIF-3α, resulted in no significant change in HRE-luciferase signal compared with scrambled control transfection (Fig. 9B). Similarly, plasmid-mediated miR-210 overexpression, which we hypothesized would increase HIF activity by decreasing HIF-3α, did not significantly alter HRE-luciferase signal in miR-210 transfection vs. blank control (Fig. 9C). These results suggest that, although HIF-3α is targeted by miR-210, in this experimental system, miR-210 does not regulate HIF activity.
Fig. 9.
miR-210 targets HIF-3α but does not modulate HIF signaling. A: luciferase assay demonstrates HIF-3α is a target of miR-210 (*P < 0.05, n = 6). B: anti-miR-210 in vehicle only, 1 mM dimethyloxaloylglcycine (DMOG), and hypoxic conditions does not change HRE-luciferase reporter construct activity (n = 3). C: similarly, miR-210 overexpression in vehicle only, 1 mM DMOG, and hypoxic conditions also does not change HRE-luciferase reporter construct activity (n = 3). Data are presented as means ± SE.
DISCUSSION
The purpose of this study was to examine mechanisms of miR-210 induction aside from HIF-1α and to evaluate the effects of miR-210 on cell death and ROS production in cardiomyocytes. First, we explored upstream mechanisms of miR-210 activation and found that p53 upregulates miR-210, most likely through a HIF-dependent mechanism, whereas Akt activation of miR-210 appears to be HIF-independent. Second, we found that miR-210 overexpression in cardiomyocytes exerts cytoprotective effects. Third, in cardiomyocytes, miR-210 overexpression reduces mitochondrial ROS, whereas miR-210 knock down increases mitochondrial ROS both at baseline and in the setting of hypoxia. Fourth, miR-210 overexpression decreases mitochondrial mass in cardiomyocytes, but miR-210 knock down only has limited effects on mitochondrial mass both at baseline and in hypoxia. Last, while AIFM3 and HIF-3α appear to be targets of miR-210, we could not demonstrate functional significance of these interactions in further studies on cell death with AIFM3 or modulation of HIF activity with HIF-3α.
The hypoxic induction of miR-210 has been studied previously, and the roles of HIF-1α (7, 12, 21, 27) and potentially HIF-2α (27, 40) have been demonstrated by several groups. We demonstrate that p53 can also upregulate miR-210 but that this is likely dependent upon intact baseline HIF signaling. Our results also suggest that the presence of p53 is necessary for full induction by hypoxia of miR-210 expression. We did not directly investigate the molecular mechanisms responsible for these observations, but at least two possibilities exist. First, p53 may regulate miR-210 at the posttranscriptional level through a mechanism reported by Suzuki et al. (33). They found that p53, through interaction with Dicer, is necessary for correct processing of some microRNAs from the pre-miRNA stem-loop stage to the fully active microRNA; however, whether miR-210 processing is p53-dependent was not checked. Second, p53 may regulate miR-210 at the transcriptional level by binding directly to the miR-210 promoter. Additional studies such as chromatin immunoprecipitation and evaluation of relative levels of pre-miR-210 and mature miR-210 in the presence and absence of p53 are needed to distinguish between these possibilities. Further studies are also necessary to determine what mediates the HIF-independent hypoxic regulation of miR-210 by Akt demonstrated by our data.
The effects of miR-210 on cell death appear to be cell type-dependent, since miR-210 has been found to be either cytoprotective or cytotoxic, depending on the cell type studied (9, 20, 25, 27, 31). Our results demonstrating protection against oxidant-induced stress in H9c2 and NRCM cells with miR-210 overexpression are consistent with the findings by Hu et al. (20) that gene therapy with miR-210 in mice reduces infarct size and that, in the mouse cardiomyocyte-derived HL-1 cell line, miR-210 overexpression reduces caspase 3/7 activity. miR-210 has also been found to be prosurvival in bone marrow-derived mesenchymal stem cells (25) and a breast cancer cell line (27), whereas proapoptotic effects have been seen in an esophageal cancer cell line (37), a lung cancer cell line (31), and human pulmonary artery epithelial cells (9). Interestingly, Favaro et al. (14a) found differing effects on apoptosis in MCF7 and HCT116 cells, depending on the experimental setup: in 0.1% O2, miR-210 knock down increased apoptosis, but, in normoxia, miR-210 overexpression increased apoptosis. The reasons for these differing effects are unclear but may be related to differences in stress stimulus, duration of treatment, or cell type. It is important also to emphasize that the magnitude of cytoprotection found in our studies was relatively modest, although similar to that found by other studies (20).
The transcriptional targets of miR-210 that may mediate its effects on cell death have also been investigated. In bone marrow-derived mesenchymal cells, reduction in caspase-8-associated protein-2 levels by miR-210 appears to explain the protective effects of miR-210 induction (25). Another study demonstrated that the transcript for protein tyrosine phosphatase 1B is a target of miR-210 and that its levels in the heart are decreased in the infarct border zones of mouse hearts treated with miR-210-expressing minigene (20). In this study, we investigated in the human-derived HEK293 cell line the potential protective role of AIFM3 inhibition by miR-210. This protein has been shown to be targeted to the inner mitochondrial membrane of mitochondria, and overexpression of this protein has been shown to induce apoptosis (39). However, we found that targeting of this protein by miR-210 does not mediate the cytoprotective effects of miR-210. Furthermore, because the target site for miR-210 in the 3′-UTR of AIFM3 is not conserved in rodents, this mechanism cannot explain the results in cell death studies obtained in rat-derived NRCM and H9c2 cells.
The effects of miR-210 on ROS seen in our studies are in some respects opposite to those found by the two other published reports on the effects of miR-210 on ROS generation. It is not clear what accounts for these differences in the effect of miR-210 modulation on ROS levels among these studies. However, the differences seen among studies regarding miR-210's role in both cytoprotection and ROS suggest that the effects of this molecule are dependent upon the specific cellular context. An alternative explanation may lie in the differing methods of ROS detection among the studies. Chan et al. (9) detected ROS using dichlorofluorescein diacetate, which is most sensitive to cytoplasmic hydrogen peroxide, whereas Mitosox used in our studies predominantly detects mitochondria-derived superoxide.
Although the molecular pathways that cause the decrease in ROS are not clear, it appears that miR-210-mediated regulation of iron-sulfur cluster scaffold homolog (ISCU) may be an important determinant of levels of electron transport chain constituents (9, 10, 14). Further studies in cardiomyocytes examining miR-210 regulation of ISCU and the role of miR-210 in regulating cytoplasmic ROS production may yield insights into the apparently differential effects of miR-210 on ROS. Finally, it is interesting that, after treatment with 0.5% O2, we saw no significant change in mitochondrial ROS with miR-210 knock down. It is possible that, under this degree of hypoxia, other mechanisms supervene and miR-210 no longer exerts effects on mitochondrial ROS production despite approximately threefold higher miR-210 levels at 0.5% O2 compared with 1.5% O2.
To assess whether miR-210 functions as a rheostat fine-tuning overall HIF activity, we evaluated whether miR-210 activation can feed back on HIF regulation by decreasing HIF-3α, a negative regulator of HIF. While we demonstrated HIF-3α to be a target of miR-210, our functional assays did not demonstrate a difference in HIF activity at baseline or in hypoxia with either miR-210 knock down or overexpression. It has been demonstrated in A549 lung epithelial cells that miR-210 can positively regulate HIF-1α (31), possibly through regulation of succinate dehydrogenase complex, subunit D (SDHD), a component of complex II of the electron transport chain. The differences between these results and those reported here may be explained by cell type or time course under which the studies were conducted. Furthermore, it is still possible that HIF-3α plays a role in miR-210-mediated positive feedback on HIF-1α activity in A549 cells, in addition to the proposed model involving SDHD.
In summary, our results indicate a novel role for p53 and Akt in regulating miR-210 expression. We also demonstrate a role for miR-210 in protection against oxidant stress, which is consistent with the work of others in cardiomyocytes but is divergent from other studies in cancer cell lines and human pulmonary artery epithelial cells. Furthermore, we demonstrate that miR-210 reduces mitochondrial ROS production and mitochondrial mass in cardiomyocytes. Some of our findings regarding ROS production are divergent from other groups' findings, further highlighting potential cell-specific effects of this hypoxia-inducible microRNA. Together, our findings implicate miR-210 as a microRNA with cytoprotective effects in cardiomyocytes and as a node integrating the p53, Akt, and HIF signaling pathways.
GRANTS
R. K. Mutharasan is supported by National Heart, Lung, and Blood Institute (NHLBI) Grant 5F32HL-095339 and by American Heart Association Grant 0825722G. H. Ardehali is supported by NHLBI Grants K08 HL-079387 and R01 HL-087149.
DISCLOSURES
None.
ACKNOWLEDGMENTS
We thank Dr. Paul Schumacker for use of the hypoxia chamber and critical comments; Dr. Navdeep Chandel for use of the hypoxia chamber, the MEF cell lines, and 3× HRE-luciferase construct; and Dr. Iasha Sznajder for use of the hypoxia chamber.
REFERENCES
- 1. Ardehali H, O'Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ Res 97: 740–742, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5: 396–400, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Beitner-Johnson D, Rust RT, Hsieh TC, Millhorn DE. Hypoxia activates Akt and induces phosphorylation of GSK-3 in PC12 cells. Cell Signal 13: 23–27, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Berger JA, Hautaniemi S, Jarvinen AK, Edgren H, Mitra SK, Astola J. Optimized LOWESS normalization parameter selection for DNA microarray data (Abstract). BMC Bioinformatics 5: 194, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Budinger GR, Chandel N, Shao ZH, Li CQ, Melmed A, Becker LB, Schumacker PT. Cellular energy utilization and supply during hypoxia in embryonic cardiac myocytes. Am J Physiol Lung Cell Mol Physiol 270: L44–L53, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14: 1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Ceci M, Ross J, Jr, Condorelli G. Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. J Mol Cell Cardiol 37: 905–912, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29: 4362–4368, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res 104: 724–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res 69: 1221–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daubman S. MicroRNAs in angiogenesis and vascular smooth muscle cell function. Circ Res 106: 423–425, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, Vojnovic B, Pires das Neves R, Glazer P, Iborra F, Ivan M, Ragoussis J, Harris AL. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One 5: e10345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res 3: 280–289, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun 331: 718–725, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122: S124–131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35: 856–867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem 276: 27605–27612, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson J, Duchen MR, Heales SJ. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: implications for assays of cardiolipin and mitochondrial mass. J Neurochem 82: 224–233, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Jung SH, Bang H, Young S. Sample size calculation for multiple testing in microarray data analysis. Biostatistics 6: 157–169, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 284: 33161–33168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA 99: 12825–12830, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 100: 2373–2379, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Maynard MA, Evans AJ, Shi W, Kim WY, Liu FF, Ohh M. Dominant-negative HIF-3 alpha 4 suppresses VHL-null renal cell carcinoma progression. Cell Cycle 6: 2810–2816, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto S, Murphy AN, Brown JH. Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerg Biomembr 41: 169–180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouyssegur J, Gounon P, Hofman P, Barbry P, Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 18: 465–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silverman HS, Wei S, Haigney MC, Ocampo CJ, Stern MD. Myocyte adaptation to chronic hypoxia and development of tolerance to subsequent acute severe hypoxia. Circ Res 80: 699–707, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature 460: 529–533, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka T, Wiesener M, Bernhardt W, Eckardt KU, Warnecke C. The human HIF (hypoxia-inducible factor)-3alpha gene is a HIF-1 target gene and may modulate hypoxic gene induction. Biochem J 424: 143–151, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Topkara VK, Mann DL. Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther 25: 171–182, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Tsai CA, Chen YJ, Chen JJ. Testing for differentially expressed genes with microarray data. Nucleic Acids Res 31: e52, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem 286: 420–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang N, Zhou Z, Liao X, Zhang T. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life 61: 566–571, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Xie Q, Lin T, Zhang Y, Zheng J, Bonanno JA. Molecular cloning and characterization of a human AIF-like gene with ability to induce apoptosis. J Biol Chem 280: 19673–19681, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle 8: 2756–2768, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 275: 8027–8031, 2000 [DOI] [PubMed] [Google Scholar]