Abstract
The aim of this investigation was to evaluate sex differences in baroreflex and heart rate variability (HRV) dysfunction and indexes of end-organ damage in the TG(mRen2)27 (Ren2) rat, a model of renin overexpression and tissue renin-angiotensin-aldosterone system overactivation. Blood pressure (via telemetric monitoring), blood pressure variability [BPV; SD of systolic blood pressure (SBP)], spontaneous baroreflex sensitivity, HRV [HRV Triangular Index (HRV-TI), standard deviation of the average NN interval (SDNN), low and high frequency power (LF and HF, respectively), and Poincaré plot analysis (SD1, SD2)], and cardiovascular function (pressure-volume loop analysis and proteinuria) were evaluated in male and female 10-wk-old Ren2 and Sprague Dawley rats. The severity of hypertension was greater in Ren2 males (R2-M) than in Ren2 females (R2-F). Increased BPV, suppression of baroreflex gain, decreased HRV, and associated end-organ damage manifested as cardiac dysfunction, myocardial remodeling, elevated proteinuria, and tissue oxidative stress were more pronounced in R2-M compared with R2-F. During the dark cycle, HRV-TI and SDNN were negatively correlated with SBP within R2-M and positively correlated within R2-F; within R2-M, these indexes were also negatively correlated with end-organ damage [left ventricular hypertrophy (LVH)]. Furthermore, within R2-M only, LVH was strongly correlated with indexes of HRV representing predominantly vagal (HF, SD1), but not sympathetic (LF, SD2), variability. These data demonstrated relative protection in females from autonomic dysfunction and end-organ damage associated with elevated blood pressure in the Ren2 model of hypertension.
Keywords: renin angiotensin system, autonomic nervous system
premenopausal women have a lower risk of hypertension and cardiovascular disease (CVD) than men; however, after menopause this apparent protection is lost (34). Likewise, the degree of blood pressure (BP) elevation and associated cardiovascular damage in rodent models of hypertension is less severe in young females than in males (32). Ovariectomy accelerates the development and the severity of hypertension in females in animal models, which suggests modulation of BP and cardiovascular injury by sex hormones and the relative protection of female sex.
The role of the renin-angiotensin-aldosterone system (RAAS) in the regulation of cardiovascular function is well established. Modulation of autonomic control by the brain RAAS is an important determinant of normal BP homeostasis, and maladaptations in this system are a major contributor to hypertension and CVD. Alterations in ANG II have been implicated in reduced baroreflex sensitivity, reduced heart rate variability (HRV), and autonomic dysfunction. Furthermore, sex hormones modulate brain and systemic RAAS and baroreflex control in female rats (13, 53). However, direct comparisons of males and females in models of RAAS overactivation are lacking, especially in regard to HRV, baroreflex control, and the associated cardiac complications.
The TG(mRen2)27 (Ren2) rat overexpresses the mouse renin transgene and is a model of tissue RAAS activation. Hypertensive male Ren2 rats develop progressive cardiovascular abnormalities including systolic and diastolic dysfunction, cardiac remodeling, glomerular filtration barrier injury, and proteinuria (17, 50, 51). Although circulating and renal ANG II levels are low (36), the male Ren2 exhibits elevated vascular, heart, and brain tissue ANG II levels and impaired baroreflex sensitivity (18, 37). However, although female Ren2 rats are less hypertensive than males, direct sex comparisons in the aforementioned indexes of end-organ damage have not been examined.
Hemodynamic variability, including blood pressure variability (BPV) and beat-to-beat HRV, is a strong and independent predictor of morbidity and mortality for both healthy individuals and populations at risk for CVD (1). Decreased HRV have been implicated as a predictor for cardiovascular dysfunction in conditions such as arrhythmia, heart failure, and cardiac hypertrophy (3, 9). Power spectral analysis of HRV has been validated as a noninvasive method of evaluating sympathetic and vagal variability (38, 48) and is utilized extensively as a valuable tool to investigate autonomic dysfunction in both humans and rodents. However, sex differences in HRV in the context of inappropriate RAAS activation and cardiac damage have not been studied. Therefore, we hypothesized that when compared with males, female sex would be associated with less autonomic dysfunction and related cardiovascular pathology in the Ren2 model of hypertension.
MATERIALS AND METHODS
Animal Care and Use
All animal procedures were approved in advance by the Harry S. Truman Veterans Memorial Hospital Subcommittee for Animal Safety and the Institutional Animal Care and Use Committee of the University of Missouri. Animals were cared for in accordance with National Institutes of Health guidelines. Five-wk-old male (M) and randomly cycling female (F) heterozygous transgenic Ren2 and Sprague-Dawley (SD) littermate rats (Wake Forest University) were housed under standard temperature and humidity laboratory conditions in which light and dark cycles were 12 h each.
Telemetric BP Monitoring
SD-M (n = 8), SD-F (n = 7), R2-M (n = 6), and R2-F (n = 8) rats 7 wk of age were anesthetized (2% isoflurane in a stream of air containing 40% O2) and instrumented with an abdominal aorta catheter attached to a radiotransmitter (Data Sciences International, St. Paul, MN) as previously described (16). After a 3-wk recovery, rats were monitored in 300-s bins every 15 min for 3 12-h light and 3 12-h dark cycles (sampling rate = 1,000 Hz). Parameters evaluated include systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), and spontaneous cage activity [counts of lateral movement per minute (cpm)]. Blood pressure variability (BPV) was calculated as the standard deviation of the SBP (SDSBP) measured during three light and three dark cycles.
Baroreflex Sensitivity
Telemetry data was analyzed post hoc for spontaneous baroreflex sensitivity (sBRS) using the sequence method (6, 48) with the aid of HemoLab software (http://www.haraldstauss.com/HemoLab/HemoLab.php). Analysis was performed on bins of 300 s collected every 30 min for three 12-h light and three 12-h dark cycles. Baroreflex sequences were identified as ramps of four or more consecutive beats in which both SBP and interpulse interval (PI) increased or decreased simultaneously, and sBRS was calculated via linear regression (R for inclusion = 0.8) as the slope (in ms/mmHg) of that relationship.
HRV
HRV analysis was performed on telemetry data according to published recommendations (1) using LabChart software (ADInstruments, Colorado Springs, CO). For each animal, four 300-s data bins from one light and one dark cycle were selected post hoc. A 90-s interval free from movement or behavior artifact was selected from each bin and low-pass filtered (45 Hz). Individual Poincaré plots and tachograms were examined as guides for eliminating ectopic beats (7). HRV was evaluated in the time and frequency domains and using Poincaré plot analysis.
Time domain.
The HRV Triangular Index (HRV-TI) was calculated as the integral of the density distribution [the number of all NN (normal RR) intervals] divided by the maximum of the density distribution (the number of NN intervals in the modal bin), and standard deviation of the average NN interval (SDNN) was calculated as the standard deviation of the NN interval (the square root of variance) (1).
Frequency domain.
Spectral analysis of the MAP and PI was performed using fast Fourier transformation. A Hann window (1024) was applied with 75% overlap and a bin size of 0.5 ms. Oscillatory components were separated into very low frequency (VLF; <0.05 Hz), low frequency (LF; 0.05–0.75 Hz), or high frequency (HF; 0.75–2.5 Hz) bands. The power of LF and HF HRV components were expressed in normalized units (nu) as a percentage of total power minus the VLF component (1). Efferent vagal parasympathetic activity is a major contributor to the HF component. Both sympathetic and vagal influences contribute to the LF component; thus the ratio of LF to HF (nu) is commonly utilized as a measure of sympathovagal balance (1).
Poincaré plot analysis.
To quantify the shape of the Poincaré plot, an ellipse was fitted to the distribution of points, and two standard descriptors (SD1 and SD2) were calculated (7). SD1 measures the dispersion of points perpendicular to the line of identity through the ellipse and is interpreted as a measure of short-term (vagal) variability, whereas SD2 measures the dispersion along the line of identity and is interpreted as a measure of long-term (sympathetic) variability (7, 14, 25, 27, 29).
Cardiac Catheterization and Cardiac Function Testing
Cardiac catheterization to examine left ventricle (LV) diastolic and systolic function was performed on anesthetized (above) 10-wk-old SD-M (n = 10), SD-F (n = 3), R2-M (n = 6), and R2-F (n = 7) rats using an Advantage PV System (Scisense, Ontario, CA) as described previously (17).
Histomorphometric Analysis
Animals were euthanized via exsanguination under isoflurane anesthesia (as above) approximately midway through the light cycle. The LV and septum (S) were dissected and the wet weight-to-body weight (BW) ratio was determined (LV + S/BW; n = 6–12 each group). LV cardiomyocyte cross-sectional area and myocardial peri-arterial fibrosis (Verhoff van Gieson stain quantification) were evaluated on paraformaldehyde-fixed paraffin-embedded mounted LV sections as described previously (n = 5 in each group) (51).
Tissue Oxidative Stress
As described previously, production of superoxide anions was measured via the lucigenin-enhanced chemiluminescence method on whole LV homogenates (51). NADPH oxidase activity was measured using spectrophotometric techniques on plasma membrane LV fractions. Reactive oxygen species (ROS) production and NADPH oxidase activity values were normalized to total protein and daily SD controls (n = 4–8 each group).
Proteinuria
Urine protein (colorimetric assay) and creatinine (Jaffe reaction assay) concentrations were analyzed on an automated clinical chemistry analyzer (Olympus America, Centerville, PA) using commercial assays as described previously (n = 5–8 each group) (50).
Statistical Analysis
Differences in outcomes across groups were determined using two-way ANOVA to examine effects of sex (male vs. female), strain (SD vs. Ren2), and the interaction. Differences between light and dark cycle SBP within males and females were determined using repeated measures ANOVA. Post hoc comparisons were made using Fisher's Protected Least Significant Differences. Relationships between HRV indexes and SBP or LV hypertrophy (LVH) were analyzed using Pearson's product-moment correlation coefficient and linear regression analysis; the strength of a relationship was described as weak (0.0 < r < 0.3), moderate (0.3 < r < 0.7), or strong (0.7 < r < 1.0). All differences were considered significant when P < 0.05 (Sigma Plot 12.0; Systat Software).
RESULTS
Telemetry Measures
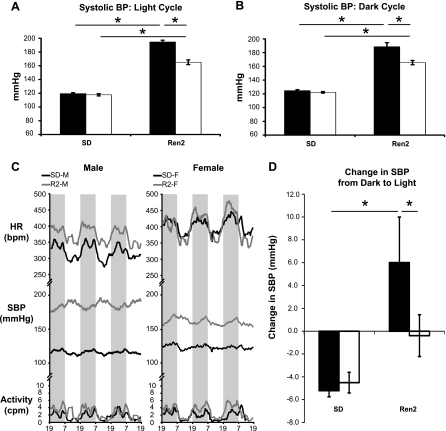
Both R2-M and R2-F demonstrated increases in SBP (Fig. 1, A and B), DBP, and MAP compared with their respective SD counterparts during both the light and dark cycles (Table 1). Although no differences in SBP, DBP, or MAP were observed between SD-M and SD-F, these values were lower in R2-F than in R2-M during both the light and dark cycles. When compared with SD-M, R2-M showed a 61% increase in 24-h MAP, whereas MAP in R2-F was only increased by 39% compared with SD-F. Furthermore, the variability of BP (SDSBP) was elevated in R2-M during the light cycle and tended to be elevated during the dark cycle (P = 0.051) compared with SD-M. During both time periods, SDSBP was also elevated in R2-F compared with SD-F but was less than R2-M. No HR differences were observed among the groups during the dark cycle, but during the light cycle HR was elevated in SD-F compared with SD-M and in SD-F compared with R2-F (Table 1). Spontaneous cage activity was increased in R2 compared with SD rats during the dark cycle, but no effect of sex was present (Table 1). Interestingly, R2-M displayed a reversed circadian BP profile, with higher pressures during the light (quiescent) cycle compared with the dark (active) cycle (Fig. 1, C and D). When compared with the dark period, SBP decreased in SD-M and SD-F, but increased in R2-M, during the light cycle (Fig. 1D). No effect of time of day was observed for R2-F.
Fig. 1.
Systolic blood pressure (SBP) values and cyclicity. A and B: SBP in male (black bars) and female (white bars) Sprague-Dawley (SD) and Ren2 rats. During both the light (A) and dark (B) cycles, SBP was elevated in Ren2 compared with their SD counterparts and in Ren2 males (R2-M) compared with Ren2 females (R2-F). C: representative tracings (moving average) of activity, SBP, and heart rate (HR) during 3 light (white) and 3 dark (shaded) cycles. R2-M displayed reversed circadian blood pressure (BP) profiles, with higher BP during the light cycle. Some R2-F displayed normal, some inverted, and some intermediate (phase-shifted; shown) circadian profiles. D: difference in mean SBP between dark and light in male (black bars) and female (white bars) SD and Ren2 rats. SBP decreased from the dark to light cycle in SD male (SD-M) and SD female (SD-F) but increased in R2-M. cpm, Counts per minute; bpm, beats per minute. *P < 0.05.
Table 1.
Telemetry data from 10-week-old male and female SD and Ren2 rats
| SD-M | SD-F | R2-M | R2-F | |
|---|---|---|---|---|
| n | 7 | 7 | 6 | 8 |
| Diastolic blood pressure, mmHg | ||||
| Light | 82.3 ± 1.20 | 80.5 ± 2.28 | 139.8 ± 2.15*§ | 116.1 ± 3.05† |
| Dark | 87.4 ± 1.41 | 85.1 ± 1.40 | 133.1 ± 4.38*§ | 114.1 ± 2.48† |
| Mean arterial pressure, mmHg | ||||
| Light | 98.8 ± 1.27 | 98.0 ± 2.01 | 166.8 ± 2.24*§ | 140.9 ± 3.25† |
| Dark | 104.0 ± 1.35 | 102.3 ± 1.24 | 160.2 ± 5.03*§ | 139.7 ± 2.81† |
| Heart rate, beats/min | ||||
| Light | 340.7 ± 11.80 | 380.4 ± 6.98‡ | 338.8 ± 7.12 | 347.2 ± 5.53† |
| Dark | 389.9 ± 14.6 | 424.8 ± 8.71 | 406.4 ± 6.95 | 408.8 ± 10.09 |
| Activity, cpm | ||||
| Light | 0.9 ± 0.18 | 1.18 ± 0.11 | 1.4 ± 0.38 | 1.7 ± 0.21 |
| Dark | 2.1 ± 0.30 | 2.5 ± 0.17 | 4.1 ± 0.76* | 4.8 ± 0.61† |
| SDSBP, mmHg | ||||
| Light | 1.17 ± 0.171 | 0.96 ± 0.043 | 1.81 ± 0.052*§ | 1.51 ± 0.106† |
| Dark | 1.04 ± 0.144 | 0.86 ± 0.079 | 1.41 ± 0.104§ | 1.29 ± 0.092† |
Values are means ± SD; n, sample size. Comparisons are across groups. cpm, Counts per minute; SDSBP, standard deviation of systolic blood pressure.
P < 0.05 Ren2 male (R2-M) vs. Sprague-Dawley male (SD-M);
P < 0.05 Ren2 female (R2-F) vs. Sprague-Dawley female (SD-F);
P < 0.05 SD-F vs. SD-M;
R2-M vs. R2-F.
sBRS (Sequence Method)
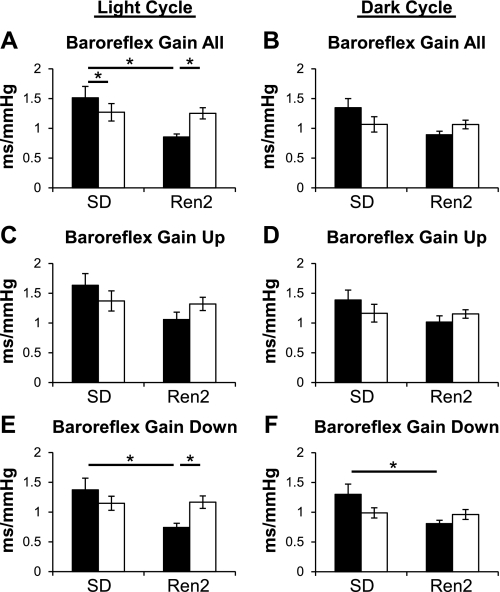
More baroreflex sequences were identified in females than males during both the light and dark cycles, but no effect of strain on number of baroreflex sequences was present (data not shown). When compared with SD-M, SD-F exhibited decreased overall baroreflex sensitivity (all baroreflex sequences; Fig. 2A) during the light cycle.
Fig. 2.
Baroreflex sensitivity. Baroreflex gain (in ms/mmHg) during the light (A, C, E) and dark (B, D, F) cycles in male (black bars) and female (white bars) SD and Ren2 rats. A and B: overall baroreflex gain (combination of up and down sequences) was decreased during the light cycle in R2-M vs. SD-M, but not in R2-F compared with SD-F. C and D: up sequence gain [SBP and interpulse interval (PI) increase] was not different during either light or dark cycles. E and F: down sequence gain (SBP and PI decrease) was less in R2-M than in SD-M during the light and dark cycles. Down sequence gain was not different in R2-F compared with SD-F during either the light or dark cycles. *P < 0.05.
Overall baroreflex sensitivity was diminished during the light cycle by 44% in R2-M compared with SD-M, whereas no differences were observed between R2-F and SD-F (Fig. 2A). R2-M sBRS gain was 32% lower than R2-F. During the dark cycle differences in overall sBRS were not significant, although there was a similar trend for decreased sBRS in R2 compared with SD rats (P = 0.059) and a trend for strain to have a greater effect in males than in females (interaction, P = 0.063; Fig. 2B).
Although strain effects approached significance (P = 0.062) during the light cycle, no differences in baroreflex bradycardia (up sBRS sequences) were observed during either the light or dark cycle (Fig. 2, C and D).
During both the light and dark cycles, baroreflex tachycardia (down sBRS sequences) was less in R2-M than in SD-M but was not different between R2-F and SD-F (Fig. 2, E and F). In addition, the gain of down sBRS sequences was less in R2-M than in R2-F during the light cycle (Fig. 2E).
HRV
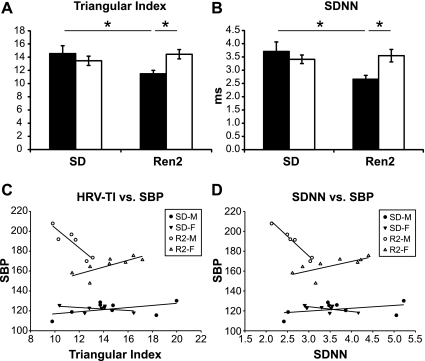
Time domain.
During the dark cycle, R2-M HRV was decreased compared with both SD-M and R2-F as measured by both HRV-TI and SDNN, whereas no differences were found between R2-F and SD-F (Fig. 3, A and B). No sex or strain effects were observed for either measure during the light cycle (data not shown). HRV-TI was negatively correlated with SBP in R2-M (r = −0.893; P = 0.0165) and positively correlated in R2-F (r = 0.752; P = 0.0313; Fig. 3C). Similarly, SDNN and SBP were negatively correlated in R2-M (r = −0.984; P < 0.001) and positively correlated R2-F (r = 0.707; P = 0.0500; Fig. 3D). In R2-M, HRV-TI and SDNN explained 79.7% and 96.8% of the variation in SBP, respectively (r2). In R2-F, 56.5% and 50.0% of the variation in SBP was explained by HRV-TI and SDNN.
Fig. 3.
HR variability (HRV) in the time domain during the dark cycle. A: HRV Triangular Index (HRV-TI) in male (black bars) and female (white bars) SD and Ren2 rats was diminished in R2-M but not R2-F compared with SD-M and SD-F, respectively. B: R2-M, but not R2-F, displayed diminished standard deviation of the average NN interval (SDNN) compared with SD-M and SD-F, respectively. C: scatter plot of HRV-TI vs. dark cycle SBP within SD-M, SD-F, R2-M, and R2-F. Lines represent the plot of the linear relationship (linear regression) within each group. The relationship between HRV-TI and SBP was significant in R2-M (negative slope) and R2-F (positive slope) but not SD-M or SD-F. D: scatter plot and linear regression of SDNN vs. dark cycle SBP within each group. SDNN was significantly correlated with SBP in R2-M (negative slope) and R2-F (positive slope). *P < 0.05.
Frequency domain.
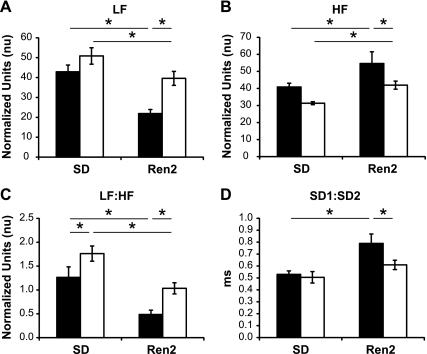
During the light cycle, SD and R2 females exhibited increased LF power (nu), decreased HF power (nu), and increased LF:HF (nu) compared with their male counterparts, but no strain effects were present (data not shown). During the dark cycle, LF (nu) was decreased in both R2-M and R2-F compared with SD-M and SD-F, respectively, but LF power was elevated in R2-F compared with R2-M (Fig. 4A). Conversely, both R2-M and R2-F demonstrated increased HF (nu) over SD-M and SD-F, respectively, whereas HF power was decreased in R2-F compared with R2-M (Fig. 4B). Finally, similar to the LF data, the ratio of LF to HF (nu) was decreased in R2-F compared with SD-F and in R2-M compared with both SD-M and R2-F (Fig. 4C). Raw values for total, VLF, LF, and HF power are reported in Table 4 as recommended (1) to describe in total the power distribution in spectral components. LF power raw values were correlated with overall sBRS gain during both the light (r = 0.657; P < 0.001) and dark (r = 0.365; P = 0.0258) cycles.
Fig. 4.
HRV by spectral analysis and Poincare plot analysis during the dark cycle. A: low frequency (LF), representing a combination of sympathetic and vagal variability with sympathetic predomination, in male (black bars) and female (white bars) SD and Ren2 rats. LF was decreased in R2-F compared with SD-F and in R2-M compared with SD-M and R2-F. B: high frequency (HF), representing vagal variability, was increased in R2-M and R2-F compared with SD-M and SD-F, respectively, and in R2-M compared with R2-F. C: LF:HF, representing the balance of sympatho-vagal activity, was decreased in R2-F compared with SD-F and in R2-M compared with both SD-M and R2-F. D: ratio of long-term vagal (SD1) to short-term sympathetic (SD2) variability was increased in R2-M compared with SD-M. R2-F were not different from SD-F but were decreased compared with R2-M. *P < 0.05.
Table 4.
HRV spectral analysis: raw values
| Total, ms2 |
VLF, ms2 |
LF, ms2 |
HF, ms2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | |
| SD-M | 12.0 ± 3.63 | 12.7 ± 2.41 | 5.0 ± 1.85 | 5.4 ± 1.16 | 2.8 ± 0.98 | 3.2 ± 0.64 | 3.8 ± 1.07 | 3.1 ± 0.81 |
| SD-F | 10.8 ± 2.93 | 11.1 ± 1.29 | 3.8 ± 1.30 | 4.5 ± 0.48 | 4.1 ± 1.09 | 3.5 ± 0.61 | 2.5 ± 0.63 | 2.0 ± 0.27 |
| R2-M | 8.0 ± 1.38 | 6.9 ± 0.97 | 2.5 ± 0.62 | 2.0 ± 0.41 | 1.7 ± 0.41 | 1.0 ± 0.13 | 3.7 ± 0.59 | 2.9 ± 0.92 |
| R2-F | 16.5 ± 3.52 | 12.8 ± 1.80 | 7.3 ± 3.17 | 4.2 ± 0.86 | 4.0 ± 0.50 | 3.3 ± 0.62 | 4.5 ± 0.79 | 3.4 ± 0.55 |
Values are means ± SD; n = 7 sample size for total and very low frequency (VLF) groups, 6 sample size for low frequency (LF), and 8 sample size for high frequency (HF).
Poincaré plot analysis.
Poincaré plot analysis revealed sex but not strain effects in the ratio of short-term to long-term variability during the light cycle, with SD1:SD2 decreased in SD-F compared with SD-M (data not shown). Consistent with a decreased LF:HF (nu), during the dark cycle, the SD1-to-SD2 ratio was increased in R2-M compared with both SD-M and R2-F, whereas no changes were observed between SD-F and R2-F (Fig. 4D).
Abnormalities in End-Organ Structure and Function
Body weight and proteinuria.
Female SD and R2 rats weighed less than their respective male counterparts; in addition, R2-M weighed less than SD-M. Total urine protein-to-urine creatinine ratio was elevated in R2-M relative to R2-F and SD controls (Table 2).
Table 2.
Age, body weight, ventricular weights, and proteinuria in male and female SD and Ren2 rats
| Parameter | P Value | SD-M | SD-F | R2-M | R2-F |
|---|---|---|---|---|---|
| n | 12 | 10 | 6 | 12 | |
| Age, weeks | |||||
| Strain | 0.792 | 10.6 ± 0.2 | 10.98 ± 0.1‡ | 10.6 ± 0.2 | 10.8 ± 0.1 |
| Sex | 0.037 | ||||
| Interaction | 0.522 | ||||
| Body weight, g | |||||
| Strain | 0.003 | 378 ± 10 | 214 ± 6‡ | 312 ± 19*§ | 221 ± 3 |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| LV + S weight, mg | |||||
| Strain | 0.001 | 777 ± 21 | 509 ± 18‡ | 1,044 ± 66*§ | 636 ± 20† |
| Sex | 0.001 | ||||
| Interaction | 0.001 | ||||
| RV w, mg | |||||
| Strain | 0.918 | 235 ± 14 | 155 ± 7‡ | 234 ± 17§ | 154 ± 5 |
| Sex | 0.001 | ||||
| Interaction | 0.989 | ||||
| LV + S/body weight, mg/g | |||||
| Strain | 0.001 | 2.07 ± 0.05 | 2.46 ± 0.06‡ | 3.34 ± 0.09*§ | 2.87 ± 0.07† |
| Sex | 0.301 | ||||
| Interaction | 0.001 | ||||
| RV/body weight, mg/g | |||||
| Strain | 0.629 | 0.62 ± 0.03 | 0.76 ± 0.04 | 0.75 ± 0.04*§ | 0.70 ± 0.02† |
| Sex | 0.979 | ||||
| Interaction | 0.001 | ||||
| Proteinuria, mg/mg | |||||
| Strain | 0.001 | 2.3 ± 0.36 | 0.9 ± 0.12 | 6.0 ± 0.63*§ | 1.4 ± 0.31 |
| Sex | 0.001 | ||||
| Interaction | 0.003 |
Values are means ± SD; n, samples sizes. LV, left ventricle; RV, right ventricle; S, septum.
P < 0.05 R2-M vs. SD-M;
P < 0.05 R2-F vs. SD-F;
P < 0.05 SD-F vs. SD-M;
R2-M vs. R2-F.
Pressure-volume (PV) loop analysis of LV function.
LOAD-INDEPENDENT INDEXES.
With the exception of Tau (τ), the time constant of isovolumic relaxation, these indexes were generated following occlusion of the inferior vena cava (IVC) to vary preload.
SYSTOLIC INDEXES.
Both end-systolic elastance (Ees), i.e., the slope of the end systolic PV relationship (ESPVR), and the maximum systolic elastance (Emax) tended to be elevated in females compared with males (Table 3). No differences were found between the rat strains or sexes in the time from end diastole to Emax. Preload recruitable stroke work (PRSW), an index that incorporates both systolic and diastolic properties, was elevated in Ren2 compared with SD, particularly between R2-M and SD-M (Table 3).
Table 3.
Load-independent systolic and diastolic indexes in a subset of 11-week-old male and female SD and Ren2 rats obtained by PV loop analysis during preload reduction
| Parameter | P Value | SD-M | SD-F | R2-M | R2-F |
|---|---|---|---|---|---|
| n | 9 | 3 | 6 | 7 | |
| Systolic indexes | |||||
| Ees, slope of ESPVR, mmHg/μl | |||||
| Strain | 0.209 | 1.32 ± 0.23 | 1.79 ± 0.11 | 1.59 ± 0.20 | 2.22 ± 0.39 |
| Sex | 0.056 | ||||
| Interaction | 0.776 | ||||
| Emax, mmHg/μl | |||||
| Strain | 0.603 | 1.03 ± 0.24 | 1.29 ± 0.13 | 0.93 ± 0.14 | 1.65 ± 0.33 |
| Sex | 0.052 | ||||
| Interaction | 0.345 | ||||
| Time from end diastole to Emax, ms | |||||
| Strain | 0.470 | 90 ± 7 | 96 ± 8 | 86 ± 2 | 94 ± 13 |
| Sex | 0.119 | ||||
| Interaction | 0.782 | ||||
| PRSW, mmHg | |||||
| Strain | 0.006 | 73.2 ± 7.2 | 83 ± 10 | 106 ± 8.0* | 103.6 ± 16 |
| Sex | 0.662 | ||||
| Interaction | 0.501 | ||||
| Diastolic indexes | |||||
| Slope of EDPVR, mmHg/μl | |||||
| Strain | 0.005 | 0.0034 ± 0.0004 | 0.0048 ± 0.0002 | 0.0100 ± 0.0020* | 0.0075 ± 0.0020 |
| Sex | 0.725 | ||||
| Interaction | 0.198 | ||||
| τ, Glantz, ms | |||||
| Strain | 0.003 | 14.0 ± 0.4 | 13.7 ± 1.9 | 18.0 ± 1.4* | 17.3 ± 2.7† |
| Sex | 0.480 | ||||
| Interaction | 0.897 |
Values are means ± SD; n, samples sizes. Ees, end-systolic elastance; Emax, maximum systolic elastance; ESPVR and EDPVR, end-systolic and end-diastolic pressure-volume (PV) relationship, respectively; PRSW, preload recruitable stroke work.
P < 0.05 R2-M vs. SD-M;
P < 0.05 R2-F vs. SD-F;
P < 0.05 SD-F vs. SD-M;
R2-M vs. R2-F.
DIASTOLIC INDEXES.
To evaluate LV wall compliance we calculated the LV chamber stiffness constant [the slope of the end-diastolic PV relationship (EDPVR) during variable preload conditions]. EDPVR slope was elevated in Ren2 compared with SD, and this difference was more pronounced in males. τ was elevated in both male and female Ren2 rats compared with their SD counterparts.
PV AREA (PVA) ANALYSIS OF LV ENERGETICS.
Analysis of PVA normalized to LV + S weight detected no significant strain, treatment, or interaction effects, suggesting that myocardial oxygen consumption per unit weight did not vary among treatment groups (Supplemental Table S2; P > 0.05).
Myocardial remodeling.
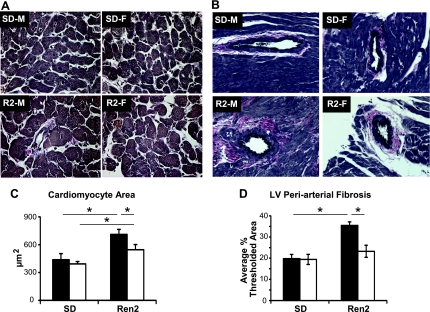
LV and S wet weight normalized to body weight (LV + S/BW; Table 2), LV cardiomyocyte cross-sectional area (Fig. 5, A and C), and LV peri-arterial fibrosis (Fig. 5, B and D) were elevated in R2-M compared with SD-M and in R2-F compared with SD-F, but were reduced in R2-F compared with R2-M.
Fig. 5.
Left ventricular (LV) remodeling. A: representative images of LV cardiomyocyte cross-sections. Scale bar equals 50 μm. B: representative images of LV Verhoff van Gieson stain (VVG) staining for collagen. Scale bar equals 50 μm. C: quantification of LV cardiomyocyte cross-sectional area in male (black bars) and female (white bars) SD and Ren2 rats. Cardiomyocyte area was increased in both R2-M and R2-F compared with SD-M (438.0 ± 65.2) and SD-F, respectively; R2-M was elevated over R2-F. D: quantification of LV fibrosis revealed increased VVG staining in R2-M compared with SD-M and in R2-F compared with SD-F, but not in R2-M compared with R2-F. *P < 0.05.
Oxidative stress.
NADPH OXIDASE ACTIVITY.
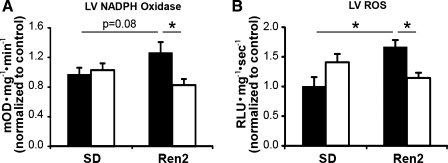
R2-F displayed less LV NADPH oxidase activity compared with R2-M (Fig. 6, A and C). In R2-M, LV NADPH oxidase activity tended to be higher than in SD-M (P = 0.08) and was elevated compared with R2-F.
Fig. 6.
End organ oxidative stress. A: LV NADPH oxidase activity in R2-M was elevated compared with R2-F and tended to be elevated compared with SD-M. R2-F was not higher than SD-F. B: LV reactive oxygen species (ROS) production was elevated in R2-M compared with both SD-M and R2-F but not in R2-F compared with SD-F. RLU, relative light units. *P < 0.05.
SUPEROXIDE LEVELS.
Superoxide production was elevated in the LV of R2-M compared with both SD-M and R2-F. No differences were observed between R2-F and SD-F (Fig. 6, B and D).
Correlation of HRV and End-Organ Damage
The relationships between HRV indexes and LVH (LV + S/BW) during the dark cycle are presented in Table 5. When all groups were included in the analysis, HRV-TI and SDNN were moderately negatively correlated with LVH, as were indexes of HRV characterized by sympathetic predominance, including LF (nu), LF:HF (nu), and SD2. SD1:SD2, an index representing the short-term (vagal) to long-term (sympathetic) ratio, was moderately positively correlated with LVH (Table 5).
Table 5.
Correlation coefficients (r) representing the strength of the linear relationships between indexes of HRV and left ventricular hypertrophy (LV + S/body weight) during the dark cycle in all animals and within each group
| Parameter | All | SD-M | SD-F | R2-M | R2-F |
|---|---|---|---|---|---|
| n | 29 | 8 | 7 | 6 | 8 |
| HRV-TI | −0.38*† | −0.76*‡ | −0.48 | −0.87*‡ | 0.25 |
| SDNN | −0.41*† | −0.66 | −0.55 | −0.93*‡ | 0.50 |
| LF, nu | −0.61*† | −0.28 | −0.33 | 0.65 | −0.16 |
| HF, nu | 0.36 | 0.06 | 0.46 | −0.89*‡ | −0.30 |
| LF:HF, nu | −0.59*† | −0.23 | −0.28 | 0.78 | 0.13 |
| SD1 | 0.11 | −0.62 | 0.22 | −0.94*‡ | 0.41 |
| SD2 | −0.50*† | −0.64 | −0.61 | −0.47 | 0.46 |
| SD1:SD2 | 0.47*† | 0.19 | 0.51 | −0.87*‡ | 0.18 |
Values are means ± SD; n, samples sizes. For all HF, P = 0.058. HRV-TI, heart rate variability triangular index; SDNN, standard deviation of the average NN interval; SD, standard descriptors.
P < 0.05;
moderate significant correlations;
strong significant correlations.
When groups were analyzed individually, no significant correlations between HRV indexes and LVH were found within SD-F or R2-F. In SD-M, HRV-TI was negatively correlated with LVH. In R2-M, HRV-TI and SDNN were strongly negatively correlated with LVH. Strong negative correlations were also found between LVH and HF (nu), SD1, and SD1:SD2 in R2-M. No correlations were found between LVH and LF:HF or measures of HRV that predominantly represent sympathetic variability (LF [nu], SD2; Table 5).
DISCUSSION
To our knowledge, these data are the first to demonstrate a role of sex in autonomic regulation of RAAS-associated hypertension and cardiovascular target organ injury in the Ren2 rat. Male Ren2 rats developed increased BPV, reduced HRV, and, in agreement with previous reports, baroreflex impairment (18), hypertension, and end-organ damage manifested as cardiac dysfunction (17), myocardial remodeling (51), elevated proteinuria (50), and heart tissue oxidative stress (50, 51). These effects had not been examined previously in female Ren2 rats and were found to be attenuated compared with male Ren2 rats. Our observations extend previous work demonstrating sex differences in the development of hypertension and end organ damage in humans and preclinical models of pressure overload, in which ovarian hormones confer relative female protection (32).
A clinical feature consistent with CVD onset is the loss of nocturnal dipping wherein the normal nighttime (quiescent period) drop in BP disappears; it is noteworthy that this abnormality is associated with LVH and proteinuria and predicts future cardiovascular events (19, 47). Our finding of cardiac hypertrophy and increases in proteinuria concomitant with inverted circadian BP rhythm in R2-M is a pattern similar to that observed in human hypertensive non-dippers. It is noteworthy that in R2-M the increase in BP during the light cycle (quiesecent period) was concomitant with decreased baroreflex gain. In R2-F, baroreflex gain was not compromised in the light cycle and no changes were observed in SBP from the dark to light cycle. Thus it is possible that preserved baroreflex gain in R2-F partially compensates for the pathological processes that contribute to an inverted circadian rhythm in R2-M. Previous reports in male Ren2 and ANG II-infused rats relate alterations in normal circadian patterns to changes in brain RAAS (11), alpha-adrenergic activity, and/or clock gene expression (28), although plasma concentrations of RAAS components (ANG I and ANG II) appear to have no significant circadian rhythm in the Ren2 (45). Our data support an additional role for sex and baroreflex function in nocturnal circadian BP regulation in a setting of RAAS activation.
We report differences in sBRS between normotensive SD male and female rats during the light cycle, with females exhibiting lower baroreflex gain, which is in accordance with some (2, 21), but not all (16, 26), previous studies. It is possible these different findings reflect variation in cycling status, as it is known that baroreflex function fluctuates during the human menstrual and rat estrus cycle (24, 35). Although our rats were randomly cycling, telemetry and baroreflex measures were averaged over the course of three consecutive nights and days and thus included the majority of cycle days. It is well-documented that ANG II acts via central angiotensin type 1 receptors (AT1R) to modulate sympathetic outflow and baroreflex function. Circulating ANG II acts via AT1R on neurons within circumventricular organs, which project directly or indirectly to brain regions that integrate baroreflex function, specifically the nucleus tractus solitarii, rostral ventral lateral medulla, and paraventricular nucleus (8, 22). In addition, local endogenous RAAS in these same regions also participates in baroreflex modulation (40). Our data showing decreased sBRS in R2-M are consistent with a previous study demonstrating baroreflex impairment in older male Ren2 (18). Our novel observation that female Ren2 rats were protected from baroreflex dysfunction suggests a protective mechanism against overactive brain RAAS, since previous studies have demonstrated increases in brain ANG II and ANG 1–7 in female Ren2 rats (46). In particular, the preservation of baroreflex function in R2-F in our study is in accordance with a previous report (52) in which ANG II-infusion blunted baroreflex bradycardia in male, but not female, rats.
Our experiments revealed sex differences in HRV in the Ren2. Analysis in the time domain (Triangular Index and SDNN) revealed that R2-M had decreased HRV and thus a decreased responsiveness to BP alterations, evidenced by increased BPV (SDSBP). HRV in R2-F was not reduced, demonstrating female Ren2 rats maintain some ability to compensate for BP fluctuations. It is well-accepted that sex hormones impact both peripheral and brain RAAS. In the Lew.Tg(mRen2) hypertensive rat, a congenic strain derived from the Ren2, plasma concentrations of ANG I and ANG II are highest in males, whereas plasma ANG 1–7 levels are elevated in females (39). Furthermore, severity of ANG II-induced hypertension is sex-specific despite identical dosing paradigms between males and females, and it has been suggested the relative protection of females is due to the central nervous system effects of estrogen on components of the brain RAAS and inhibition of ANG II-induced increases in ROS production (49, 52, 53). In particular, central estrogen modulates autonomic tone via central estrogen receptor (ER) activation and could account for the relative preservation of HRV in R2-F. The ability to more normally regulate BP fluctuations and maintain baroreflex function may, in part, explain the blunted hypertension in female Ren2 rats. That HRV and SBP were only correlated in the Ren2 but not SD rats highlights the strong influence of HRV on BP at higher pressures. Furthermore, the positive direction of the relationship in R2-F indicates that individual females with reduced HRV are still protected from BP elevations.
In regard to HRV frequency domain analyses, based on previous models of established hypertension, we might have expected an increase in sympatho-vagal balance, since elevated BP is often associated with elevated sympathetic activity. Indeed, one report (23) has demonstrated elevated LF HRV and LF-to-HF ratio in male Ren2 rats 20–30 wk of age using similar spectral analysis methods. In contrast, our 10-wk-old R2-M exhibited increased HF and decreased LF and LF-to-HF ratio, consistent with previous findings of reduced plasma catecholamines in the Ren2 (45). At 10 wk of age, the progression of hypertension is just beginning to plateau (17), and many compensatory mechanisms might be triggered during this early stage of the disease. It is important to recognize autonomic variability measured by HRV spectral analysis reflects modulation and not underlying autonomic tone (1). Indeed, in certain conditions characterized by chronically elevated sympathetic tone, LF and LF-to-HF ratio may be unchanged or decreased, possibly due to decreased responsiveness of the sinus node to persistent sympathetic discharge (1). Accordingly, a more recent hypothesis relates LF with baroreflex function (5, 42). In our study, reductions in R2-M sBRS accompanied reductions in LF power and the LF-to-HF ratio, and sBRS was significantly correlated with raw values of LF, lending support to this hypothesis. For parasympathetic variability, two independent indexes (HF power, SD1:SD2) demonstrated a shift to increased parasympathetic control over beat-to-beat heart rate fluctuations in R2-M. Additionally, within R2-M, LVH was strongly correlated with only HRV indexes representing parasympathetic but not sympathetic variability, whereas both sympathetic and parasympathetic variability were moderately related to LVH when animals from all groups were included in the analysis. The correlations within R2-M were negative; R2-M with the highest parasympathetic variability demonstrated less LVH. Thus, it is conceivable that in this early stage of the disease, increases in parasympathetic variability are compensatory.
Independent of baseline BP, changes in both HRV and BP variability are associated with indicators of end organ damage such as LVH, diastolic dysfunction, arterial stiffness, ventricular arrhythmia, and proteinuria as well as increased morbidity and mortality in hypertensive and diabetic patients (1, 33, 41) and in rodent pathological models (15). Our finding of strong correlations between HRV indexes and LVH in R2-M is consistent with these previous reports, specifically those examining the relationship between HRV and hypertrophic cardiomyopathy (3). It is possible that other factors beyond HRV, such as local RAAS overactivation, may contribute to damage in the target organs we examined. Indeed, ANG II acting through its AT1R mediates cardiac hypertrophy directly and indirectly through autocrine and paracrine effects (30). Additionally, female sex and estrogen protect against cardiac remodeling (20). Furthermore, both sex (44) and RAAS (31) independently play a role in oxidative stress in tissues from hypertensive rodents, which is in accordance with our observation of female protection from effects of RAAS overactivation on NADPH oxidase activity and ROS overproduction in the heart. The exact mechanisms involved in HRV-associated target organ damage require further investigation.
Some basic principles of sexual dimorphism with respect to cardiac function in normal and healthy hypertensive humans (12) may also apply to the young rats studied here. Like women, the female rats studied here have smaller hearts and therefore lower stroke volumes than their male counterparts. Nonetheless, normal women tend to have a similar cardiac index (cardiac output normalized to body weight) as men, because young women (up to the age of 50) have higher heart rates than age-matched men. In the current experiments conscious SD-F had higher HR than SD-M. Ventilated, anesthetized female rats (undergoing PV loop) did not exhibit higher heart rates, but similar to women, they had nearly identical cardiac indexes compared with males regardless of strain. This regulation is achieved despite differences between SD and Ren2 rats in afterload or arterial load, as indicated by differences in BP and arterial elastance, respectively. Furthermore, we recently reported that the 10- to 11-wk-old R2-M exhibit early stage heart failure characterized by enhanced systolic contractility and diastolic dysfunction with preserved ejection fraction (17). Normal and healthy hypertensive women exhibit higher LV systolic function than men (12). Indeed, we detected higher Ees and Emax in SD and Ren2 female rats. No differences were observed in ejection fraction among SD and Ren2 rats of either sex. Although not evaluated in the current study, a correlation between diastolic dysfunction and HRV would be expected given our observed correlations between HRV indexes and LVH and the well-documented relationship between pathological LVH and diastolic dysfunction. Future studies are needed to evaluate this relationship.
It is important to consider that because hypertension was more severe in R2-M compared with R2-F, it is possible some of the observed sex differences in end organ damage can be ascribed to differences in BP. Indeed, much accumulated evidence exists for an association between mean BP and cardiovascular risk factors. However, HRV and BPV, independent of the mean BP, have been shown to play a role in organ damage and have prognostic value (1, 4, 10, 33, 41, 43). Several observations in this study suggest that differences in BP alone cannot fully account for differences between R2-M and R2-F. First, the nature of the relationship between HRV and SBP is very different between R2-M and R2-F. Within the R2-M, the most hypertensive rats had the lowest HRV, suggesting that decreased HRV may contribute to the hypertension. In contrast, within the R2-F the relationship is positive, such that HRV is greatest in female rats with higher BP. It is possible that at this stage of Ren2 hypertension (10 wk of age), decreased HRV in males is contributing to the hypertension, whereas the female compensatory response to rising BP is to increase HRV, which limits the development of hypertension and maintains BP values below that of the Ren2 male. Another indication that female sex, independent of BP, is protective relates to sex differences in the relationship between heart weight and HRV. Although BP is not different between male and female SD rats, only SD-M rats exhibit a negative correlation between heart weight and HRV. BP is elevated in R2-F, yet there is still no correlation between heart weight and HRV. Thus there is a sex component to the relationship between LVH and HRV that is independent of baseline arterial pressure.
In conclusion, we demonstrated the relative protection of females compared with males in development of hypertension, autonomic dysfunction, and associated end organ damage. Our data suggest a protective mechanism against RAAS overactivation in female rats. Given the fundamentally different ways that males and females maintain cardiovascular homeostasis, under chronic stress different sex-dependent vulnerabilities may arise, and these differences will mandate different therapeutic strategies. Table 4.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants 1R01-HL073101-02 and 1R01-HL107910-01 (to J. R. Sowers); Veterans Affairs Merit System Grant 0018 (to J. R. Sowers); and Veterans Affairs Career Development Award VA CDA-2 (to A. T. Whaley-Connell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Terry L. Carmack and Lisa D. Watkinson of the Truman Memorial Veterans′ Hospital Radiopharmaceutical Sciences Institute for surgical assistance and Brenda Hunter for editorial assistance.
REFERENCES
- 1. Anonymous. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17: 354–381, 1996 [PubMed] [Google Scholar]
- 2. Abdel-Rahman AR, Merrill RH, Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J Appl Physiol 77: 606–613, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy—relation to left ventricular mass and etiology. Am Heart J 151: 829–836, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 29: 334–339, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19: 1296–1303, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985 [PubMed] [Google Scholar]
- 7. Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng 48: 1342–1347, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 299: R439–R451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brotman DJ, Bash LD, Qayyum R, Crews D, Whitsel EA, Astor BC, Coresh J. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol 21: 1560–1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burger AJ, D′Elia JA, Weinrauch LA, Lerman I, Gaur A. Marked abnormalities in heart rate variability are associated with progressive deterioration of renal function in type I diabetic patients with overt nephropathy. Int J Cardiol 86: 281–287, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Campos LA, Plehm R, Cipolla-Neto J, Bader M, Baltatu OC. Altered circadian rhythm reentrainment to light phase shifts in rats with low levels of brain angiotensinogen. Am J Physiol Regul Integr Comp Physiol 290: R1122–R1127, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Celentano A, Palmieri V, Arezzi E, Mureddu GF, Sabatella M, Di Minno G, De Simone G. Gender differences in left ventricular chamber and midwall systolic function in normotensive and hypertensive adults. J Hypertens 21: 1415–1423, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 13: 446–452, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Contreras P, Canetti R, Migliaro ER. Correlations between frequency-domain HRV indices and lagged Poincare plot width in healthy and diabetic subjects. Physiol Meas 28: 85–94, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Cosson E, Valensi P, Laude D, Mesangeau D, Dabire H. Arterial stiffness and the autonomic nervous system during the development of Zucker diabetic fatty rats. Diabetes Metab 35: 364–370, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Crofton JT, Share L, Brooks DP. Pressor responsiveness to and secretion of vasopressin during the estrous cycle. Am J Physiol Regul Integr Comp Physiol 255: R1041–R1048, 1988 [DOI] [PubMed] [Google Scholar]
- 17. DeMarco VG, Johnson MS, Habibi J, Pulakat L, Gul R, Hayden MR, Tilmon R, Dellsperger KC, Winer N, Whaley-Connell AT, Sowers JR. Comparative analysis of telmisartan and olmesartan on cardiac function in the TG(mRen2)27 rat. Am J Physiol Heart Circ Physiol 300: H181–H190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol 51: 542–548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, Kario K. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens 27: 2265–2270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298: R1597–R1606, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Foley CM, Mueller PJ, Hasser EM, Heesch CM. Hindlimb unloading and female gender attenuate baroreflex-mediated sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 289: R1440–R1447, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Garcia-Espinosa MA, Shaltout HA, Olson J, Westwood BM, Robbins ME, Link K, Diz DI. Proton magnetic resonance spectroscopy detection of neurotransmitters in dorsomedial medulla correlate with spontaneous baroreceptor reflex function. Hypertension 55: 487–493, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldman RK, Azar AS, Mulvaney JM, Hinojosa-Laborde C, Haywood JR, Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol 296: R1419–R1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol 58: 591–610, 2007 [PubMed] [Google Scholar]
- 26. Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 26: 122–126, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Kamen PW, Krum H, Tonkin AM. Poincare plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin Sci (Lond) 91: 201–208, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Lemmer B, Witte K, Enzminger H, Schiffer S, Hauptfleisch S. Transgenic TGR(mREN2)27 rats as a model for disturbed circadian organization at the level of the brain, the heart, and the kidneys. Chronobiol Int 20: 711–738, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lerma C, Infante O, Perez-Grovas H, Jose MV. Poincaré plot indexes of heart rate variability capture dynamic adaptations after haemodialysis in chronic renal failure patients. Clin Physiol Funct Imaging 23: 72–80, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Malhotra R, Sadoshima J, Brosius FC, 3rd, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ Res 85: 137–146, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 93: 569–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maris ME, Melchert RB, Joseph J, Kennedy RH. Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol 32: 35–39, 2005 [DOI] [PubMed] [Google Scholar]
- 33. May O, Arildsen H. Long-term predictive power of heart rate variability on all-cause mortality in the diabetic population. Acta Diabetol 48: 55–59, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med 107: 158–161, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 344: 541–544, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Nishioka T, Callahan MF, Li P, Ferrario CM, Ganten D, Morris M. Increased central angiotensin and osmotic responses in the Ren-2 transgenic rat. Hypertension 33: 385–388, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95: 1441–1448, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med 86: 715–722, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poanta L, Porojan M, Dumitrascu DL. Heart rate variability and diastolic dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. In press [DOI] [PubMed] [Google Scholar]
- 42. Rahman F, Pechnik S, Gross D, Sewell L, Goldstein DS. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res 21: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rothwell PM. Does blood pressure variability modulate cardiovascular risk? Curr Hypertens Rep 13: 177–186, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol 34: 938–945, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Schiffer S, Pummer S, Witte K, Lemmer B. Cardiovascular regulation in TGR(mREN2)27 rats: 24h variation in plasma catecholamines, angiotensin peptides, and telemetric heart rate variability. Chronobiol Int 18: 461–474, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides 15: 919–926, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Soylu A, Yazici M, Duzenli MA, Tokac M, Ozdemir K, Gok H. Relation between abnormalities in circadian blood pressure rhythm and target organ damage in normotensives. Circ J 73: 899–904, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 34: 362–368, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 83: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Whaley-Connell A, Chowdhury N, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario CM, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol 291: F1308–F1314, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario CM, Sowers JR. Angiotensin-II mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-α mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007 [DOI] [PubMed] [Google Scholar]