Abstract
Prostaglandin E2 (PGE2), an important mediator of the inflammatory response, acts centrally to elicit sympathetic excitation. PGE2 acts on at least four E-class prostanoid (EP) receptors known as EP1, EP2, EP3, and EP4. Since PGE2 production within the brain is ubiquitous, the different functions of PGE2 depend on the expression of these prostanoid receptors in specific brain areas. The type(s) and location(s) of the EP receptors that mediate sympathetic responses to central PGE2 remain unknown. We examined this question using PGE2, the relatively selective EP receptor agonists misoprostol and sulprostone, and the available selective antagonists for EP1, EP3, and EP4. In urethane-anesthetized rats, intracerebroventricular (ICV) administration of PGE2, sulprostone or misoprostol increased renal sympathetic nerve activity, blood pressure, and heart rate. These responses were significantly reduced by ICV pretreatment with the EP3 receptor antagonist; the EP1 and EP4 receptor antagonists had little or no effect. ICV PGE2 or misoprostol increased the discharge of neurons in the hypothalamic paraventricular nucleus (PVN). ICV misoprostol increased the c-Fos immunoreactivity of PVN neurons, an effect that was substantially reduced by the EP3 receptor antagonist. Real-time PCR detected EP3 receptor mRNA in PVN, and immunohistochemical studies revealed sparsely distributed EP3 receptors localized in GABAergic terminals and on a few PVN neurons. Direct bilateral PVN microinjections of PGE2 or sulprostone elicited sympathoexcitatory responses that were significantly reduced by the EP3 receptor antagonist. These data suggest that EP3 receptors mediate the central excitatory effects of PGE2 on PVN neurons and sympathetic discharge.
Keywords: cardiovascular, prostaglandin E2, sympathetic nerve activity, brain, E-class prostanoid receptor
prostaglandin e2 (PGE2) is an important mediator of the effects of the proinflammatory cytokines on the central nervous system. Blood-borne proinflammatory cytokines induce cyclooxygenase-2 (COX-2) expression in the microvasculature of the blood-brain barrier, generating PGE2 that can diffuse into brain tissue to elicit a variety of responses including fever (21, 31, 32, 40) and activation of the hypothalamo-pituitary-adrenal axis (26, 34, 45). Recent studies from our laboratory suggest that this mechanism contributes to the augmented sympathetic nerve activity in rats with ischemia-induced systolic heart failure (12, 18, 48), in which plasma concentrations of proinflammatory cytokines are high.
Neither the prostanoid receptors that mediate the effects of PGE2 on sympathetic drive nor their central nervous system location(s) have been identified. There are at least four subtypes of E-class prostanoid (EP) receptors—EP1, EP2, EP3, and EP4—that mediate the responses to PGE2 (2, 30, 31, 35, 45), and their distribution is believed to determine the effects of PGE2 (35). One prior study (2), using intracerebroventricular (ICV) administration of EP agonists, suggested that the EP3 receptor mediates PGE2-induced increases in heart rate (HR), blood pressure (BP), and renal sympathetic nerve activity (RSNA).
The central administration of PGE2 activates multiple forebrain sites involved in cardiovascular and autonomic regulation (20). Among these is the paraventricular nucleus (PVN) of the hypothalamus, an important presympathetic nucleus (6, 7, 19) that has been implicated as a source of sympathetic excitation in rats with systolic heart failure (9–11, 23). Our previous work with that model has revealed a dramatic upregulation of COX-2 expression in the perivascular macrophages of the dense microvascular network of the PVN, in close proximity to excited PVN neurons. Preventing COX-2 expression reduced PVN neuronal excitation and sympathetic excitation, suggesting an important role for PGE2 (17, 48, 49). The present study examined the hypothesis that PGE2, acting on EP receptors in the PVN, contributes to the cardiovascular and sympathetic responses to centrally administered PGE2.
METHODS
Experiments at the University of Iowa were performed on male Sprague-Dawley rats weighing 300–350 g. Rats were housed in the University of Iowa Animal Care Facility and exposed to a normal 12-h:12-h light-dark cycle. All experimental protocols were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa and the Research and Development Committee of the Iowa City Department of Veterans Affairs Medical Center.
Experiments at Kyoto University were performed on male Sprague-Dawley rats (200–250 g), conformed to the guidelines of animal care by the Institute of Laboratory Animals, Faculty of Medicine, Kyoto University, and were approved by the Animal Research Committee, Graduate School of Medicine, Kyoto University (MedKyo11095).
Experimental Protocols
Electrophysiological and hemodynamic recording studies.
Anesthetized rats (n = 133) were instrumented with femoral arterial and venous catheters, renal nerve recording electrodes, and craniotomies as needed in each protocol for PVN neuronal recording and ICV injections or bilateral PVN tissue microinjections. There were five experimental protocols:
In 15 rats, PVN neuronal activity, RSNA, BP, and HR were recorded during ICV injections of PGE2 (0.5 nmol), the EP receptor agonist misoprostol (0.5 nmol) or vehicle [Veh, 1% DMSO in artificial cerebrospinal fluid (aCSF)].
In 64 rats, RSNA, BP, and HR were recorded during ICV injections of PGE2 (0.5 nmol), the EP receptor agonists misoprostol (0.5 nmol) or sulprostone (0.5 nmol), before and 10 min after ICV administration of the EP1 receptor antagonist (SC-19220, 10 nmol), the EP3 receptor antagonist (L-798106, 10 nmol) or the EP4 receptor antagonist (L-161982, 10 nmol).
In 12 rats, RSNA, BP, and HR were recorded during ICV injection of PGE2 (0.5 nmol) before and 10 min after bilateral PVN microinjection of L-798106 (2 nmol).
In 17 rats, RSNA, BP, and HR were recorded during bilateral PVN microinjection of PGE2 (0.2 nmol), PGE2 + L-798106 (2 nmol), sulprostone (0.2 nmol) or Veh (100 nl).
In 25 rats, RSNA, BP, and HR were recorded during bilateral PVN microinjection of PGE2 (0.2 nmol), sulprostone (0.2 nmol) or Veh (100 nl), before and 10 min after ICV pretreatment with the EP3 receptor antagonist (L-798106, 10 nmol).
To minimize the number of animals used, most rats underwent 1–3 interventions in variable order, with full recovery of recorded variables to baseline levels before each subsequent intervention, usually within about 90 min. In some experiments, more than one PVN unit was recorded and tested.
Immunohistochemical and immunofluorescent studies.
c-Fos expression in the PVN was examined in tissues collected from 22 rats euthanized 2 h after ICV administration of Veh + misoprostol (0.5 nmol), L-798106 (10 nmol) + misoprostol (0.5 nmol), or Veh alone.
EP3 receptor expression in the PVN was examined in tissues collected from four untreated euthanized rats using immunohistochemistry and double immunofluorescent staining for EP3 receptors and the 67-kDa form of glutamic acid decarboxylase (GAD67), a marker for GABAergic neuronal terminals.
Real-time PCR.
EP3 receptor mRNA expression in the PVN was examined in tissues collected from 10 untreated euthanized rats.
Electrophysiology, Hemodynamic Recording, and Microinjection
Rats were anesthetized with urethane (1.5 g/kg ip). The left femoral artery was cannulated for measurement of BP and the left femoral vein for delivery of drugs. The level of anesthesia was assessed by continuously recording BP and HR and periodically checking nociceptive reflex responses. Supplemental urethane (0.1–0.3 g/kg ip or iv) was administrated as needed.
Animals were intubated to facilitate spontaneous respiration and were placed in a stereotaxic head holder. Core temperature was kept at 37 ± 0.3°C with a heating blanket. Small craniotomies were performed to expose the brain for microinjection or recording in the PVN or for lateral ventricular injections.
ICV and PVN microinjections.
For ICV injections (49, 54), a 23-gauge stainless steel guide cannula was lowered into the right lateral cerebral ventricle using the following stereotaxic coordinates: −1.0 mm from bregma, 1.5 mm lateral to midline, and 3.5 mm ventral to dura (33). Injections (2 μl over 10 s) were made via a 29-gauge stainless steel cannula advanced beyond the tip of the guide cannula. At the conclusion of the study, Pontamine sky blue (2 μl) was injected to validate the position of the ICV cannula.
For bilateral PVN microinjections (52, 53), two 29-gauge guide cannulas were placed 1.8 mm posterior to bregma, one 0.4 mm lateral to midline, and the other 1.9 mm lateral to midline but angled 10° toward midline. Both cannula tips were advanced to a final position 6.6 mm ventral to dura, 1 mm above the PVN (33). A 35-gauge (128 μm outer diameter, and 51.2 μm inner diameter) stainless steel injection cannula was then advanced to a position 1.0 mm beyond the tip of each guide cannula. Bilateral simultaneous PVN microinjections (100 nl over 10 s) were made using 0.5-μl Hamilton microsyringes. At the end of experiments, 2% Pontamine sky blue (100 nl) was injected at the same locations for histological verification of the microinjection sites.
Electrophysiological recordings.
A craniotomy was performed to access the left PVN for extracellular single-unit recording, using glass micropipettes as previously described (51, 53). Stereotaxic coordinates for PVN recording were 1.6–2.1 mm posterior to bregma, 0.3–0.5 mm lateral to midline, and 7.0–8.0 mm ventral to dura (33). Pontamine sky blue was iontophoresed to mark the last recording site. Other recording sites were extrapolated from this point.
RSNA was recorded from the left renal nerve using previously reported methods (42, 43, 53, 55). In brief, the renal nerve was exposed through a left flank incision. A branch was dissected free from surrounding tissue and placed on bipolar silver wire recording electrodes and covered with silicon sealant. The animal was allowed to stabilize for at least 1 h before recording began. RSNA was amplified and displayed on an oscilloscope. The background noise level was determined by ganglionic blockade with hexamethonium (30 mg/kg iv) at the end of the experiment. Background noise was subtracted from the actual recorded activity to determine RSNA.
The BP signal, the rectified and integrated RSNA, and the PVN extracellular single-unit activity were fed into an online data acquisition system (Cambridge Electronics Design 1401 Plus) coupled with a computer. Mean BP (MBP) and HR were derived from the BP tracing. MBP, HR, integrated RSNA, and PVN single-unit discharges were averaged over 1-min intervals. A 5-min baseline was used as the control. Because of the baseline variations among animals, changes from baseline are reported instead of absolute values. RSNA is reported as a percent change from the baseline activity.
After the experiments were finished, rats were euthanized with an overdose of urethane. The brain was removed and fixed in a 10% formalin solution for 3 days and then sectioned (40 μm) on a cryostat. The sections were mounted on microscope slides and stained with 1% aqueous neutral red. Sites stained with Pontamine sky blue were identified with a light microscope, and the locations of other microinjection and recording sites were extrapolated with respect to this reference point. The recording and injection sites were plotted on representative schematic tracings of the PVN, based on the rat atlas of Paxinos and Watson (33).
Immunohistochemistry and Immunofluorescence
For c-Fos immunohistochemistry, rats were deeply anesthetized with urethane and transcardially perfused with 4% paraformaldehyde in 0.1 M PBS. Brains were removed and kept in the fixative overnight and then transferred to 30% sucrose in 0.1 M PBS overnight. The fixed forebrain region containing PVN was sliced into 16-μm coronal sections with a cryostat. Sections were mounted on slides and stored at −80°C for later studies. The sections were processed for c-Fos activity using the avidin-biotin-peroxidase complex technique as previously described (17, 46, 48). They were incubated overnight at 4°C with a primary rabbit anti-rat polyclonal anti-fos antibody (K-25, 1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA) and then for 1 h at room temperature with a secondary biotinylated anti-rabbit antibody (1:5,000, PK-6101, Vector, Burlingame, CA). The c-Fos positive neurons were colored with DAB kit (SK-4100, Vector). In each rat, the c-Fos positive neurons within a 100-μm2 window superimposed over ventrolateral parvocellular, dorsal parvocellular, medial parvocellular, and posterior magnocellular subnuclei on both sides of PVN were manually counted (47, 54). The number of c-Fos positive neurons used in the data analysis was determined by averaging the counts from two representative sections approximately −1.80 mm from bregma. The investigator counting c-Fos labeling was blinded to the experimental groups.
Immunohistochemistry for EP3 receptors was performed as previously described (28, 29). Briefly, rats were deeply anesthetized with chloral hydrate (280 mg/kg ip) and were transcardially perfused with 4% formaldehyde (pH 7.4). The brain was removed, postfixed in the fixative at 4°C for 8 h, and then cryoprotected with a 30% sucrose solution overnight. The tissue was cut into 20-μm- or 40-μm-thick frontal sections on a freezing microtome.
For single immunoperoxidase staining for EP3 receptors, the brain sections (thickness, 40 μm) were incubated overnight with an anti-rat EP3 receptor rabbit polyclonal antibody (1 μg/ml) (27) and then for 1 h with a biotinylated donkey antibody to rabbit IgG (10 μg/ml; Chemicon, Temecula, CA). The sections were further incubated for 1 h with avidin-biotinylated peroxidase complex (ABC-Elite; 1:50; Vector). Bound peroxidase was visualized by incubating the sections with a solution containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride (Sigma), 0.001% hydrogen peroxide, and 50 mM Tris·HCl (pH 7.6).
For double immunofluorescent staining for EP3 receptors and GAD67, a marker for GABAergic neuronal terminals, the brain sections (thickness, 20 μm) were incubated overnight with a mixture of an anti-EP3 receptor rabbit antibody (1 μg/ml) and an anti-GAD67 mouse monoclonal antibody (1:300; MAB5406, Chemicon). Subsequently, the sections were incubated for 1 h with a biotinylated donkey antibody to rabbit IgG (10 μg/ml) and then blocked with 10% normal rabbit serum. The sections were further incubated for 1 h with Alexa594-conjugated goat antibody to mouse IgG (10 μg/ml; Molecular Probes, Eugene, OR) and then with ABC-Elite (1:50). The sections were incubated for 13 min with fluorescein-labeled tyramide (1:50; Tyramide Signal Amplification systems, PerkinElmer, Boston, MA) according to the manufacturer's manual. The fluorescence signals for EP3 receptor and GAD67 immunoreactivities in the PVN were detected under a confocal laser-scanning microscope (LSM 5 PASCAL; Zeiss).
Real-Time PCR
Brain tissue was cut into 500-μm coronal sections. A punch biopsy was obtained from the PVN, including some immediately surrounding hypothalamus, using a 15-gauge needle stub (inner diameter, 1.5 mm) according to a previously described method (47, 49); a part of the cerebral cortex from the same coronal section was also punched to obtain control tissues. Tissues were homogenized in TriReagent (Molecular Research Center, Cincinnati, OH) to extract total RNA for EP3 receptor mRNA study. Following reverse transcription of total RNA, quantifications for mRNA expression of EP3 receptor and GAPDH were performed using the SYBR green real-time PCR method as previously described (16, 44). The sequences of primers used were as follows: EP3 receptor: forward primer, 5′-CACCACGGAGACGGCTATC-3′; reverse primer, 5′-AGGCGAACGGCGATTAGG-3′; (16) GAPDH: forward primer, 5′-AAGGTCATCCCAGAGCTGAA-3′; reverse primer, 5′-ATGTAGGCCATGAGGTCCAC-3′. (44). Real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA). The values were normalized by GAPDH, and the final concentration of mRNA was calculated using the formula x = 2−ΔΔCt (24), where x is fold difference relative to cerebral cortex.
Drugs Administered
Hexamethonium bromide, SC-19220, and urethane were purchased from Sigma (St. Louis, MO). Misoprostol, L-798106, L-161982, PGE2, and sulprostone were purchased from Tocris (Ellisville, MO). Misoprostol, L-798106, L-161982, SC-19220, and sulprostone were first dissolved in dimethyl sulfoxide (DMSO) and then diluted in aCSF to make a 1% final DMSO concentration. PGE2 was dissolved in aCSF for ICV injection or for PVN microinjection. The doses of drugs selected in this study were derived from previous reports from our laboratory and others and were optimized in preliminary experiments. Since the EP receptor agonists lack specificity for the EP3 receptor, two that are known to activate the EP3 receptor (misoprostol and sulprostone) were used. The EP1, EP3, and EP4 receptor antagonists are selective; a selective EP2 receptor antagonist is not commercially available.
Statistics
Statistical significance among multiple comparisons was determined by two-way repeated-measures ANOVA followed by post hoc Tukey's test. For other unpaired data, a Student's t-test was used for comparison between groups. Values are expressed as means ± SE. P < 0.05 was considered as statistically significance.
RESULTS
Sympathoexcitatory Effects of ICV Injection of PGE2, Misoprostol, and Sulprostone
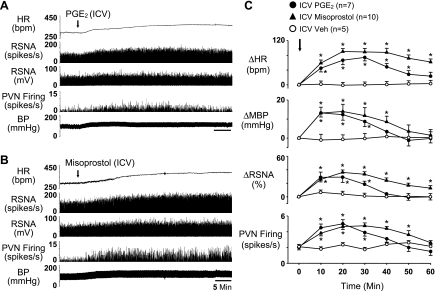
ICV administration of PGE2 (0.5 nmol) elicited significant (P < 0.05 vs. Veh) increases in PVN neuronal activity (2.2 ± 0.3 to 5.0 ± 0.4 spikes/s), RSNA (32.2 ± 8.2% from baseline), MBP (15.1 ± 1.2 from baseline of 95.7 ± 3.0 mmHg), and HR (77.8 ± 10.5 from baseline of 329.6 ± 9.8 beats/min) (Fig. 1, A and C). These responses started ∼1 to 2 min after injection, peaked at ∼15–20 min, and recovered about 60 min following injection. ICV injection of misoprostol (0.5 nmol) produced similar significant (P < 0.05 vs. Veh) increases in PVN neuronal activity (2.1 ± 0.3 to 4.7 ± 0.5 spikes/s), RSNA (36.6 ± 7.8%), MBP (14.1 ± 4.2 from baseline of 95.4 ± 2.9 mmHg), and HR (90.6 ± 12.1 from baseline 316.4 ± 10.3 beats/min) (Fig. 1, B and C). These responses (onset latency, amplitude) were similar to the responses to the ICV PGE2 injection, but longer lasting (>60 min). The RSNA, MBP, and HR responses to ICV sulprostone were similar to those elicited by PGE2 and misoprostol (Fig. 3).
Fig. 1.
Representative tracings (A and B) and grouped data (C) illustrating the sympathoexcitatory response to intracerebroventricular (ICV) administration of PGE2 (0.5 nmol) and the E-class prostanoid (EP) agonist misoprostol (0.5 nmol). ICV injection of these two agents elicited significant increases in paraventricular nucleus (PVN) neuronal activity and simultaneously recorded renal sympathetic nerve activity (RSNA), heart rate [HR; in beats/min (bpm)] and blood pressure (BP), or mean BP (MBP). Integrated (mV) and windowed (spikes/s) is shown. Arrows indicate time of drug injections. Values were expressed as means ± SE; n, number of interventions. *P < 0.05 vs. vehicle (Veh).
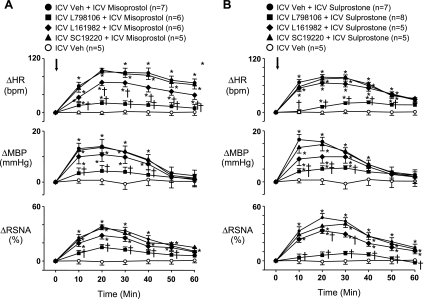
Fig. 3.
Group data showing the sympathoexcitatory responses to ICV misoprostol (0.5 nmol; A) and ICV sulprostone (0.5 nmol; B) were significantly diminished by pretreatment with the EP3 receptor antagonist L-798106, partially reduced by pretreatment with the EP4 receptor antagonist L-161982, and unaffected by pretreatment with the EP1 receptor antagonist SC-19220. Antagonist doses are same as in Fig. 2. Arrows indicate time of misoprostol and sulprostone injections. n, Number of interventions. *P < 0.05 vs. Veh; †P < 0.05 vs. Veh + misoprostol or Veh + sulprostone.
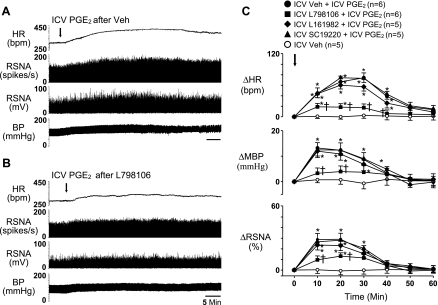
The RSNA, HR, and BP responses to PGE2, misoprostol, and sulprostone (Figs. 2 and 3) were all significantly (P < 0.05) diminished by pretreatment (10 min) with EP3 receptor antagonist L-798106. The HR response to misoprostol and the MBP and RSNA responses to sulprostone were slightly less in rats pretreated with the EP4 receptor antagonist L-161982. The EP1 receptor antagonist SC-19220 had no effects on any of the recorded variables.
Fig. 2.
Representative tracing (A and B) and group data (C) showing the sympathoexcitatory responses to ICV PGE2 were significantly diminished by ICV pretreatment with EP3 receptor antagonist L-798106 (10 nmol) but not affected by ICV pretreatment with the EP4 receptor antagonist L-161982 (10 nmol) or the EP1 receptor antagonist SC-19220 (10 nmol). Arrows indicate time of PGE2 injections. n, Number of interventions. *P < 0.05 vs. Veh; †P < 0.05 vs. Veh + PGE2.
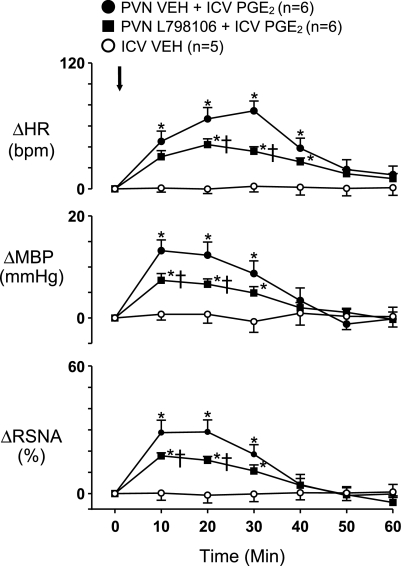
Bilateral PVN microinjection of L-798106 significantly reduced ICV PGE2-induced sympathoexcitatory responses (Fig. 4), but not as much as the ICV L-798106 injection. ICV injection of L-798106, L-161982, or SC-19220 or bilateral microinjection of L-798106 alone did not change baseline activity (data not shown). ICV injection of Veh (2 μl) had no effect on the measured variables.
Fig. 4.
Effects of bilateral PVN microinjection of the EP3 receptor antagonist L-798106 (2 nmol) on sympathoexcitatory responses to ICV PGE2 (0.5 nmol). Arrow indicates time of ICV PGE2 injection. n, Number of interventions. *P < 0.05 vs. Veh; †P < 0.05 vs. Veh + PGE2.
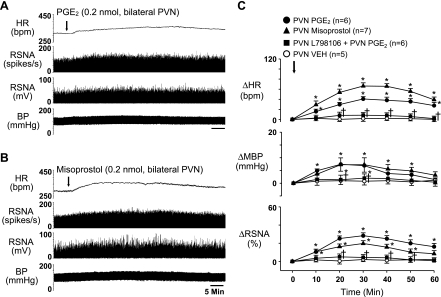
Bilateral PVN microinjection of PGE2 (0.2 nmol) increased RSNA (25.8 ± 3.4%), MBP (8.0 ± 2.1 from baseline of 94.3 ± 3.4 mmHg), and HR (40.6 ± 7.5 from baseline of 328.8 ± 11.7 beats/min) (Fig. 5, A and C), though to a lesser extent than the ICV PGE2 injections (Figs. 1–3). Responses to PVN PGE2 microinjections occurred in ∼2 to 3 min, peaked at ∼25–30 min, and lasted longer than 60 min. Bilateral PVN microinjection of the EP3 receptor antagonist L-798106 (2 nmol), immediately before bilateral microinjection of PGE2, prevented the PGE2-induced responses (Fig. 5C). Bilateral PVN microinjection of misoprostol elicited similar responses (Fig. 5, B and C), with larger increases in HR. Similarly, bilateral PVN microinjection of sulprostone increased RSNA (31.1 ± 3.0%), MBP (10.9 ± 1.3 from baseline of 98.2 ± 2.8 mmHg), and HR (55.2 ± 3.8 from baseline of 317.5 ± 9.7 beats/min) (Fig. 6, A and C). The sympathoexcitatory responses to bilateral PVN microinjection of PGE2 or sulprostone were significantly diminished by pretreatment with ICV L-798106 (Fig. 6, B and C).
Fig. 5.
Representative tracings (A and B) and grouped data (C) illustrating the sympathoexcitatory responses to bilateral PVN microinjections of PGE2 (A) and misoprostol (B). Both drugs produced significant increases of RSNA, HR and BP, or MBP. Bilateral PVN pretreatment with the EP3 receptor antagonist L-798106 (2 nmol) prevented the PGE2-induced responses. Arrows indicate time of agonist injections. n, Number of interventions. *P < 0.05 vs. Veh; †P < 0.05 vs. PGE2.
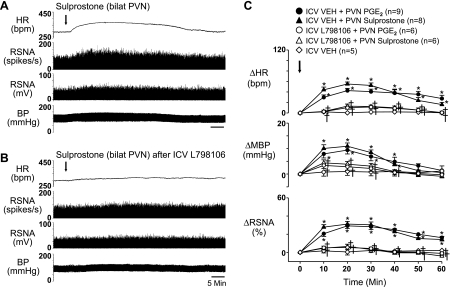
Fig. 6.
Representative tracings (A and B) and group data (C) showing that the sympathoexcitatory responses to bilateral (bilat) PVN microinjection of sulprostone (0.2 nmol) or PGE2 (0.2 nmol) were significantly diminished by pretreatment with ICV EP3 receptor antagonist L-798106 (10 nmol). Arrows indicate time of PGE2 and sulprostone injections. n, Number of interventions. *P < 0.05 vs. Veh; †P < 0.05 vs. Veh + PGE2 or Veh + sulprostone.
Microinjections of PGE2, sulprostone, and misoprostol into hypothalamic areas surrounding the PVN (1 mm lateral or dorsal) had no significant effects on RSNA, BP, or HR. Microinjection of the same volume (100 nl) of Veh in the PVN also had no effect on these variables.
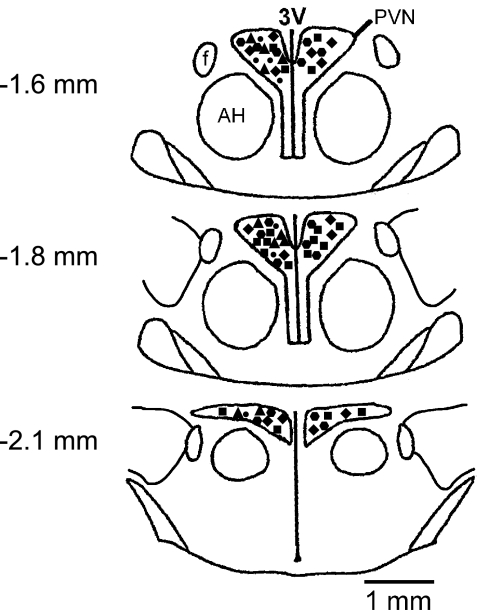
The single unit recording and microinjection sites in PVN are shown in Fig. 7. The PVN neurons recorded in this study were mainly located in the dorsal, ventrolateral, and medial parvocellular regions. There was no obvious anatomical cluster of neurons responsive to administered PGE2 or misoprostol within these areas. The microinjection sites were located throughout the PVN. No specific responsive regions were identified.
Fig. 7.
Schematic reconstruction of recording and microinjection sites within PVN regions. Neurons responsive to ICV PGE2 (●) and misoprostol (▴) and bilateral microinjection sites for PGE2 (■), misoprostol (♦), and sulprostone (hexagon). The distance from bregma is indicated. Schematics based on the Paxinos and Watson atlas of the rat brain (33). AH, anterior hypothalamus area, f, fornix; 3V, third ventricle.
All ICV injections were within the lateral cerebral ventricles, as confirmed by visualization of Pontamine sky blue in all cerebral ventricles.
Effects of ICV Injection of Misoprostol on c-Fos Activity in PVN Neurons
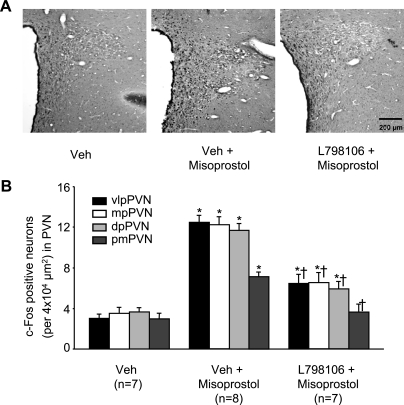
Two hours after ICV administration of misoprostol, c-Fos activity was significantly (P < 0.05) increased in the PVN, compared with Veh-treated rats (Fig. 8, A and B). Greater (3.5-fold) increases were observed in the parvocellular subnuclei (ventrolateral, dorsal, and medial) of the PVN than in magnocellular PVN (Fig. 8, A and B). Areas outside PVN, at the same stereotaxic level, had only scattered c-Fos activity, and there was no difference in these areas between groups.
Fig. 8.
Expression of c-Fos activity in the PVN of hypothalamus. A: representative sections showing that ICV misoprostol treatment increased c-Fos immunoreactivity in PVN neurons and that ICV pretreatment with the EP3 receptor antagonist L-798106 reduced misoprostol-induced c-Fos immunoreactivity in the PVN region. Third ventricle is to the left. B: quantitative assessment of the effects of misoprostol on c-Fos expression in ventrolateral pavocellular (vlp), medial parvocellular (mp), dorsal pavocellular (dp), and posterior magnocellular (pm) subnuclei of the PVN before and after pretreatment with L-798106. n, Number of rats. *P < 0.05 vs. Veh; †P < 0.05 vs. Veh + misoprostol.
In rats pretreated ICV with the EP3 receptor antagonist L-798106, ICV misoprostol-induced c-Fos activity was significantly (P < 0.05) less in both parvocellular and magnocellular PVN subnuclei (Fig. 8, A and B).
EP3 Receptor Expression in PVN
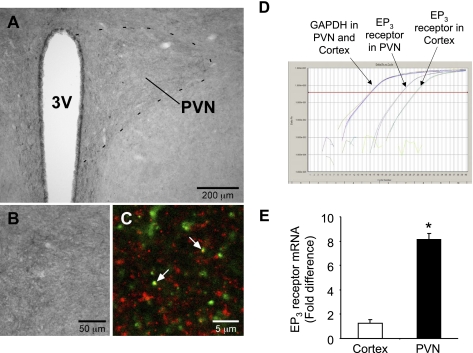
Immunoperoxidase staining visualized EP3 receptor immunoreactivity in the PVN using an anti-EP3 receptor antibody whose specificity in rat brain tissues was confirmed in our previous studies (27–29). The PVN and the periventricular hypothalamic nucleus were clearly identified with moderate to weak EP3 receptor immunoreactivity (Fig. 9A). EP3 receptor immunoreactivity in the PVN was relatively intense compared with that in the adjacent anterior hypothalamic area (Fig. 9A). EP3 receptor immunoreactivity in the PVN was mostly observed in neuropil, whereas cell bodies exhibiting the immunoreactivity were very few (Fig. 9B). No remarkable difference in the density of EP3 immunoreactivity was observed among the PVN subdivisions.
Fig. 9.
EP3 receptors in the PVN. A and B: immunoperoxidase staining for EP3 receptors. A dotted line in A delineates the PVN. B: magnified image of EP3 receptor immunoreactivity in the PVN. EP3 receptor immunoreactivity was predominantly localized in neuropil but was present in a few cell bodies in the PVN. C: confocal laser-scanning microscopy of double immunofluorescence labeling for EP3 receptor (green) and the 67-kDa form of glutamic acid decarboxylase (GAD67; red) in the PVN. This image was taken in the ventrolateral subdivision. Arrows indicate EP3 receptor-immunoreactive terminals that contain GAD67 immunoreactivity. D: representative progress curves done in duplicate for the real-time PCR amplification of EP3 receptor and GAPDH cDNA in the PVN and cerebral cortex at the same level. E: quantitative comparison of EP3 receptor mRNA expression in PVN vs. cerebral cortex. Values are means ± SE and expressed as fold difference relative to cerebral cortex; n = 10 rats for each group. *P < 0.05 vs. cortex.
To examine the possibility of localization of EP3 receptors in GABAergic neuronal terminals in the PVN, we performed double immunofluorescence staining for EP3 receptors and GAD67, a marker for GABAergic neuronal terminals. Confocal microscopy revealed that most EP3 receptor-immunoreactive processes and terminals in the PVN were negative for GAD67 immunoreactivity (Fig. 9C). However, we occasionally found EP3 receptor-immunoreactive terminals that contained GAD67 immunoreactivity (arrows, Fig. 9C). The density of such double-labeled terminals was not different among the PVN subdivisions.
EP3 mRNA expression was detectable by real-time PCR (cycle threshold below 35 cycles) in both the cerebral cortex and the PVN. The average cycle threshold value of EP3 receptor mRNA was 26 for the cerebral cortex and 23 for PVN tissues (Fig. 9D). The level of EP3 receptor mRNA in the PVN was significantly higher (∼8-fold) than that in the cerebral cortex (Fig. 9E).
DISCUSSION
The present study lends strong support to the hypothesis that EP3 receptors mediate the sympathetic response to centrally administered PGE2 and suggest a potential role for EP3 receptors in the hypothalamic PVN. The findings have important implications for pathophysiological states like hypertension and systolic heart failure in which proinflammatory cytokines (18, 46, 48) and angiotensin II (4) may induce the formation and release of PGE2 in the brain.
Centrally administered PGE2 is known to elicit a sympathoexcitatory response (1, 2, 14, 37, 53), but the receptors that mediate that response and their location(s) within the central nervous system have remained elusive. In the present study, ICV administration of two EP receptor agonists that can activate EP3 receptors, albeit nonselectively, elicited sympathoexcitatory responses similar to those elicited by ICV PGE2; ICV administration of a selective EP3 receptor antagonist, but not an EP1 or an EP4 receptor antagonist, largely prevented those effects. The other two available selective antagonists, to EP1 and EP4, had only minimal or no effect at all on sympathetic responses induced by ICV PGE2 or the two EP agonists. The lack of a selective antagonist for the EP2 receptor precluded our testing for its effects, but previous work with an EP2 agonist (2) suggested no role for the EP2 receptor in the sympathoexcitatory response to PGE2. Our results are consistent with that study (2) in which ICV administration of an EP3 receptor agonist mimicked the effects of ICV PGE2, increasing HR, arterial pressure, and RSNA, but the EP1, EP2, and EP4 agonists did not.
ICV injection of PGE2 excites neurons in multiple cardiovascular-related nuclei (20) including the PVN. In a heart failure model, in which CSF PGE2 levels are increased, COX-2 activity is dramatically increased in perivascular cells of the microvessels penetrating the PVN, and augmented COX-2 activity correlates with increased c-Fos expression among PVN neurons and increased sympathetic nerve activity (9, 17, 48, 49), e.g., reducing COX-2 activity by eliminating perivascular macrophages decreases c-Fos expression in the PVN and sympathetic activity. In the present study, ICV PGE2 and misoprostol excited PVN neurons, and misoprostol increased c-Fos expression diffusely in PVN, including in parvocellular regions of the PVN that harbor presympathetic neurons. PVN neuronal excitation occurred concomitant with the increases in RSNA, HR, and BP, consistent with central activation rather than a secondary baroreceptor-mediated response that would be expected to decrease rather than increase PVN neuronal activity (5). While direct bilateral microinjections of an EP3 antagonist into the PVN had only a partial effect to prevent the sympathoexcitation elicited by ICV PGE2, direct bilateral PVN microinjections of PGE2 and sulprostone after ICV administration of the EP3 antagonist failed to elicit the expected sympathetic responses. Moreover, bilateral PVN microinjections of the EP3 antagonist did prevent the hemodynamic and sympathetic responses to bilateral PVN microinjections of PGE2.
While these findings would seem to suggest that EP3 receptors within the PVN contribute to the central sympathoexcitatory responses to ICV PGE2, they do present a conundrum. The PVN is not among those nuclei that are heavily invested with EP3 receptors (8, 28). Our immunohistochemical studies confirmed previous work (28) demonstrating that EP3 receptors are sparsely present in the PVN, confined mostly to the neuropil, with no particular subnuclear localization. A few EP3 receptors appeared to be located on PVN neurons, perhaps accounting for the ability of real-time PCR to detect EP3 receptor mRNA, but again without any specific subnuclear localization.
The EP3 receptor is generally considered to couple to inhibitory GTP-binding proteins, although some EP3 receptor isoforms also couple to excitatory ones (22, 38). Since the activity of presympathetic PVN neurons is normally restrained by a potent GABAergic inhibition (50), we investigated the possibility that the EP3 receptors might be located on presynaptic GABAergic terminals in the PVN, where they might activate the PVN by disinhibiting GABAergic restraint. Such a role for EP3 receptors has been described in the regulation of supraoptic nucleus neurons (15). However, while our immunofluorescent studies show colocalization of some EP3 receptors with GABAergic terminals, most of the terminals containing EP3 receptors in the PVN were non-GABAergic. This observation might support an alternative possibility, that EP3 receptors in the PVN are located in excitatory neuronal terminals and can facilitate the release of excitatory neurotransmitters (e.g., glutamate) through coupling to excitatory GTP-binding proteins.
An EP3-mediated excitatory influence of PGE2 on PVN neurons has been demonstrated in a brain slice preparation (13), prompting the suggestion that PGE2 activates PVN neurons by inhibiting local peri-PVN GABAergic interneurons (13). We were unable to demonstrate a dense distribution of EP3 receptors in brain tissues immediately surrounding the PVN. However, because of the dense vascularity of the PVN, it is conceivable that direct microinjections of EP agonists into PVN may have spread to affect GABAergic neuronal populations in peri-PVN regions (13, 36, 39). Further studies are needed to clarify the site(s) at which PGE2 might act to disinhibit or otherwise excite PVN neurons.
The present study was performed in the context of our previous studies with a heart failure model, which manifests increased CSF PGE2 levels induced by circulating proinflammatory cytokines (18, 46, 48, 49). In addition, in normal rats an acute intracarotid injection of tumor necrosis factor-α induced a PGE2-mediated central sympathoexcitatory response. In these two settings, our data would suggest that increased CSF PGE2 might stimulate EP3 receptors, and thus sympathetic activity. Interestingly, a recent work has demonstrated a role for EP1 receptors in the subfornical organ in angiotensin II-induced hypertension in the rat (4). These results, examining the role of EP receptors in a different central nucleus and in a different pathophysiological model, speak to the potential pleiotropic influences of PGE2, acting on different receptors and regions of the central nervous system. Further studies are needed to address other potential sites and other receptor mechanisms by which PGE2 might mediate increases in sympathetic drive in cardiovascular disease states.
A limitation of this study is the lack of specificity of the EP agonists misoprostol and sulprostone. Sulprostone has greater affinity for the EP1 and EP3 receptors (3, 25, 41, 45), but both agonists have been shown to have some activity at multiple EP receptors (25, 30). Therefore, the interpretation of the present study hinges more on the selectivity of the receptor antagonists: the selective EP3 antagonist consistently provided the greatest inhibition of responses to PGE2 and the EP agonists, whether administered ICV or microinjected directly into the PVN.
Perspectives
PGE2 is a critical effector molecule for inflammatory processes affecting the brain. Its roles in the febrile response and in the activation of the hypothalamic-pituitary-adrenal axis are well recognized. The more recent recognition of an inflammatory component of cardiovascular diseases, particularly in hypertension and heart failure, has stimulated new interest in how inflammation might alter autonomic function. Our previous work in an experimental model of heart failure has demonstrated that cytokine-induced COX-2 activity in perivascular macrophages of the blood-brain barrier increases PGE2 in the brain, thereby augmenting sympathetic drive. The present study found that the sympathoexcitatory effects of ICV PGE2 are largely mediated by the EP3 receptor, with a significant contribution of EP3 receptors in the PVN. Emerging evidence suggests that PGE2 activation of EP1 receptors in the subfornical organ may also play an important sympathoexcitatory role in the context of angiotensin II-induced hypertension. Further delineation of the mechanisms by which PGE2 and its receptors effect changes in sympathetic drive may help identify new targets for therapeutic intervention in chronic cardiovascular diseases like heart failure and hypertension.
GRANTS
This work was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (to R. B. Felder); by the University of Iowa (to R. B. Felder); and by National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-073986 (to R. B. Felder). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or National Institutes of Health. This study was also supported by Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science Funding Program LS070 (to K. Nakamura); by Special Coordination Fund for Promoting Science and Technology (to K. Nakamura); and by Ministry of Education, Culture, Sports, Science and Technology of Japan Grants-in-Aid for Scientific Research 22689007 (to K. Nakamura) and 23790271 (to Y. Nakamura) and by grants from the Nakajima Foundation and Takeda Science Foundation (to K. Nakamura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ando T, Ichijo T, Katafuchi T, Hori T. Intracerebroventricular injection of prostaglandin E2 increases splenic sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 269: R662–R668, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Ariumi H, Takano Y, Masumi A, Takahashi S, Hirabara Y, Honda K, Saito R, Kamiya HO. Roles of the central prostaglandin EP3 receptors in cardiovascular regulation in rats. Neurosci Lett 324: 61–64, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Boie Y, Stocco R, Sawyer N, Slipetz DM, Ungrin MD, Neuschafer-Rube F, Puschel GP, Metters KM, Abramovitz M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol 340: 227–241, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Cao X, Wang G, Peterson J, Burmeister M, Sharma R, Iadecola C, Davisson R. Prostaglandin E2 (PGE2) type 1 receptors (EP1R) in the subfornical organ (SFO) contribute to slow-pressor angii sympathoexcitation and hypertension (HTN) via NADPH oxidase (Nox)-dependent signaling (Abstract). FASEB J 24: 1049.1, 2010 [Google Scholar]
- 5. Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. Distribution of the EP3 prostaglandin E(2) receptor subtype in the rat brain: relationship to sites of interleukin-1-induced cellular responsiveness. J Comp Neurol 428: 5–20, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol 95: 19–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felder RB, Francis J, Weiss RM, Zhang ZH, Wei SG, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann NY Acad Sci 940: 444–453, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284: R259–R276, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther 86: 216–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1beta on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 17: 498–508, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hoffman WE, Schmid PG. Cardiovascular and antidiuretic effects of central prostaglandin E2. J Physiol 288: 159–169, 1979 [PMC free article] [PubMed] [Google Scholar]
- 15. Ibrahim N, Shibuya I, Kabashima N, Sutarmo SV, Ueta Y, Yamashita H. Prostaglandin E2 inhibits spontaneous inhibitory postsynaptic currents in rat supraoptic neurones via presynaptic EP receptors. J Neuroendocrinol 11: 879–886, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Jovanovic N, Pavlovic M, Mircevski V, Du Q, Jovanovic A. An unexpected negative inotropic effect of prostaglandin F2alpha in the rat heart. Prostaglandins Other Lipid Mediat 80: 110–119, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–766, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand 177: 7–15, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lacroix S, Vallieres L, Rivest S. C-fos mRNA pattern and corticotropin-releasing factor neuronal activity throughout the brain of rats injected centrally with a prostaglandin of E2 type. J Neuroimmunol 70: 163–179, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Lazarus M. The differential role of prostaglandin E2 receptors EP3 and EP4 in regulation of fever. Mol Nutr Food Res 50: 451–455, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Legler DF, Bruckner M, Uetz-von Allmen E, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol 42: 198–201, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Malinowska B, Godlewski G, Buczko W, Schlicker E. EP3 receptor-mediated inhibition of the neurogenic vasopressor response in pithed rats. Eur J Pharmacol 259: 315–319, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, Mizoguchi A, Shichi H, Fukuda Y, Nakao K, Narumiya S. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci USA 100: 4132–4137, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Ichikawa A, Negishi M. Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett 260: 117–120, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol 421: 543–569, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience 161: 614–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci 9: 3046–3057, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP(1–4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol 428: 20–32, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Paxinos G, Watson C. The Rat Brain is Stereotaxic Coordinates. Sydney: Academic Press, 1986 [Google Scholar]
- 34. Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 26: 761–788, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223: 22–38, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 332: 123–143, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Saigusa T, Iriki M. Regional differentiation of sympathetic nerve activity during fever caused by intracerebroventricular injection of PGE2. Pflügers Arch 411: 121–125, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395: 281–284, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Walch L, de Montpreville V, Brink C, Norel X. Prostanoid EP(1)- and TP-receptors involved in the contraction of human pulmonary veins. Br J Pharmacol 134: 1671–1678, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei SG, Felder RB. Forebrain renin-angiotensin system has a tonic excitatory influence on renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 282: H890–H895, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension 52: 342–350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yalcin A. Quantification of thioredoxin mRNA expression in the rat hippocampus by real-time PCR following oxidative stress. Acta Biochim Pol 51: 1059–1065, 2004 [PubMed] [Google Scholar]
- 45. Yokotani K, Nishihara M, Murakami Y, Hasegawa T, Okuma Y, Osumi Y. Elevation of plasma noradrenaline levels in urethane-anaesthetized rats by activation of central prostanoid EP3 receptors. Br J Pharmacol 115: 672–676, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension 49: 511–518, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 101: 304–312, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 55: 652–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11β-Hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension 48: 127–133, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-α in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284: R916–R927, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens 28: 806–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]