Abstract
Previous studies demonstrated higher systolic intracellular Ca2+ concentration ([Ca2+]i) amplitudes result in faster [Ca2+]i decline rates, as does β-adrenergic (β-AR) stimulation. The purpose of this study is to determine the major factor responsible for the faster [Ca2+]i decline rate with β-AR stimulation, the increased systolic Ca2+ concentration levels, or phosphorylation of phospholamban. Mouse myocytes were perfused under basal conditions [1 mM extracellular Ca2+ concentration ([Ca2+]o)], followed by high extracellular Ca2+ (3 mM [Ca2+]o), washout with 1 mM [Ca2+]o, followed by 1 μM isoproterenol (ISO) with 1 mM [Ca2+]o. ISO increased Ser16 phosphorylation compared with 3 mM [Ca2+]o, whereas Thr17 phosphorylation was similar. Ca2+ transient (CaT) (fluo 4) data were obtained from matched CaT amplitudes with 3 mM [Ca2+]o and ISO. [Ca2+]i decline was significantly faster with ISO compared with 3 mM [Ca2+]o. Interestingly, the faster decline with ISO was only seen during the first 50% of the decline. CaT time to peak was significantly faster with ISO compared with 3 mM [Ca2+]o. A Ca2+/calmodulin-dependent protein kinase (CAMKII) inhibitor (KN-93) did not affect the CaT decline rates with 3 mM [Ca2+]o or ISO but normalized ISO's time to peak with 3 mM [Ca2+]o. Thus, during β-AR stimulation, the major factor for the faster CaT decline is due to Ser16 phosphorylation, and faster time to peak is due to CAMKII activation.
Keywords: phospholamban, phosphorylation, ryanodine receptor, sarcoplasmic reticulum calcium ion-adenosinetriphosphatase, calcium ion/calmodulin-dependent protein kinase
primary regulation of intracellular Ca2+ concentration ([Ca2+]i) uptake in the sarcoplasmic reticulum (SR) is performed by the SR Ca2+-ATPase (SERCA) (2). SERCA's function is modulated by the regulatory protein, phospholamban (PLB), which inhibits SERCA function (26). For example, transgenic mice with overexpression of PLB result in decreased SERCA activity and SR Ca2+ uptake, which results in a slower Ca2+ decline rate (19). The PLB-mediated inhibition of SERCA can be relieved by its dissociation. An increase in Ca2+ levels will cause this dissociation (20). Thus, increasing the peak amplitude of the [Ca2+]i transient will increase SERCA activity, which results in faster [Ca2+]i decline rates (3). PLB is also a major phosphoprotein in the myocyte. PLB can be phosphorylated on Ser16 through protein kinase A (PKA) or on Thr17 through Ca2+/calmodulin-dependent protein kinase (CAMKII). Phosphorylation at either site also results in the dissociation of PLB from SERCA. In fact, phosphorylation at these sites with β-adrenergic (β-AR) stimulation in PLB overexpression mice normalizes the Ca2+ decline compared with the wild type (WT) (19). Furthermore, PLB knockout mice result in increased SERCA activity and SR Ca2+ uptake, which results in faster Ca2+ decline rates (25). Interestingly, there was no further acceleration of the Ca2+ decline due to β-AR stimulation in these myocytes (24). In addition, a previous study in mice that had mutated PLB, which could not be phosphorylated (both Ser16 and Thr17 mutated to alanine), had no lusitropic effect during β-AR stimulation (5). Thus, PLB is an important mediator of Ca2+ decline during β-AR stimulation. However, the faster [Ca2+]i decline with β-AR stimulation may not only be due to PLB phosphorylation but the higher systolic Ca2+ levels as well. Unlike these mentioned studies, we matched Ca2+ transient peaks to best determine if the increase in systolic Ca2+ and/or PLB phosphorylation contributed to the lusitropic effect during β-AR stimulation.
Because lowering the [Ca2+]i is the initiating event that permits relaxation, clearly defining the processes contributing to Ca2+ resequestation in the SR is important. Relaxation of the cardiac muscle is governed by processes at the myofilament level (17, 18), and duration of activation is critically impacted by Ca2+ transient kinetics. Thus, governing of Ca2+ transient decline by the SR may have important clinical ramifications since relaxation is altered in many cardiomyopathies such as heart failure (31, 39).
Therefore, our purpose is to determine the major factor in the faster [Ca2+]i decline during β-AR stimulation (i.e., the high systolic levels of [Ca2+]i or PLB phosphorylation). We hypothesize that the high systolic [Ca2+]i levels seen during β-AR stimulation or high extracellular Ca2+ ([Ca2+]o) will be near the maximal velocity (Vmax) of the SERCA pump. Thus, matched systolic [Ca2+]i levels with high [Ca2+]o and β-AR stimulation will exhibit similar Ca2+ decline rates in the initial decline phase. In addition, PLB phosphorylation (i.e., β-AR stimulation) would then result in a faster Ca2+ transient decline during the final phase because of the leftward shift in the Michaelis constant at low [Ca2+]i levels. To test this hypothesis, each myocyte will be superfused with high [Ca2+]o (3 mM) and with the β-AR agonist isoproterenol (ISO) to obtain data under these conditions with matched levels of Ca2+ transient amplitude.
β-AR stimulation also results in positive chronotropy. It is known that increasing frequency of stimulation also results in faster Ca2+ decline rates. However, this is independent of PLB phosphorylation and occurs via a separate mechanism (34). Hence the chronotropic effect (i.e., increasing pacing frequency) was not investigated. To our knowledge, no one has measured [Ca2+]i decline rates comparing high [Ca2+]o and ISO in the same myocyte using matched Ca2+ transient amplitudes. A previous study examined the effects of ISO and high [Ca2+]o in the same trabeculas (7). However, this study was specifically focused on these interventions on developed pressure. There was some examination of Ca2+ decline, in which they observed a faster Ca2+ decline with ISO vs. 20 mM [Ca2+]o, but the amplitudes were not matched.
Our data suggest that, during β-AR stimulation, Ser16 phosphorylation is the major factor that drives the faster acceleration of the Ca2+ transient decline. Also, the faster Ca2+ transient time to peak is the result of CaMKII activation.
MATERIALS AND METHODS
Cardiomyocyte Isolation
Ventricular myocytes were isolated from mice (C57BL/6) (Jackson Laboratories, Bar Harbor, ME) as previously described (22). Briefly, the heart was cannulated and hung on a Langendorff apparatus. It was then perfused with Ca2+-free Tyrode solution for 4 min. The solution was then switched to Tyrode solution containing Liberase Blendzyme II (0.077 mg/ml) (Roche Applied Science, Indianapolis, IN). After 3–5 min, the heart was taken down, the ventricles were minced, and myocytes were dissociated by trituration. Subsequently, the myocytes were filtered, centrifuged, and resuspended in Tyrode solution containing 200 μmol/l Ca2+. Myocytes were used within 6 h of isolation. All of the animal protocols and procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
Measurement of myocyte Ca2+ transients.
Ca2+ transient measurements were performed as previously described (22). Briefly, myocytes were loaded at room temperature with fluo 4-AM (10 μmol/l; Molecular Probes, Eugene, OR) for 30 min, and then another 30 min were allowed for intracellular deesterification. The solution for deesterification was Tyrode solution containing 200 μmol/l Ca2+. The instrumentation used for cell fluorescence measurements was a Cairn Research Limited (Faversham, UK) epifluorescence system. [Ca2+]i was measured by fluo 4 epifluorescence with excitation at 480 ± 20 nm and emission at 535 ± 25 nm. The illumination field was restricted to collect the emission of a single cell. Data were expressed as ΔF/F0, where F is the fluorescence intensity, and F0 is the intensity at rest. Myocytes were stimulated at 1 Hz via platinum electrodes connected to a Grass Telefactor S48 stimulator (West Warwick, RI).
Western blot analysis.
Homogenized myocytes were used to measure specific phosphorylation at Ser16 and Thr17 (Badrilla, Leeds, UK) with phosphospecific antibodies and normalized to calsequestrin (ABR, Golden, CO) via Western blot analysis, as previously described (37).
Ca2+ uptake rate.
SR Ca2+ uptake rate was determined as described previously (32) by converting the RT50 (time to 50% of the Ca2+ transient decline) into a tau. This value was then used to fit a declining single exponential curve, which was then converted to a total cytosolic Ca2+ ([Ca]T) using well-characterized Ca2+-buffering parameters. Next, we took the derivative over time (d[Ca]T/dt). Finally, this derivative was fit into the Hill equation, and the uptake rate was calculated at 300 nm (roughly the RT50 [Ca2+]i value).
Solutions and drugs.
Normal Tyrode (NT) solution consisted of (in mmol/l): 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, and 5 HEPES, pH 7.4 adjusted with NaOH or HCl. ISO (1 μmol/l, a nonselective β-AR agonist) was prepared fresh each day. KN-93 (1 μmol/l, CAMKII inhibitor; EMD Chemicals, Gibbstown, NJ) was prepared each day from frozen aliquots. Zinterol (Zint, 100 nmol/l, β2-AR agonist; Tocris Biosciences, Ellisville, MO) All chemicals were from Sigma (St. Louis, MO) except where indicated.
Experimental protocol.
Our basic experimental protocol consisted of the myocyte first being perfused with control solution [NT with 1 mM Ca2+ concentration ([Ca2+])] until steady state was reached, and the myocyte was then superfused with the 3 mM [Ca2+] NT solution, which resulted in increased systolic Ca2+ levels. After steady state was reached, the solution was then switched back to control solution (1 mM [Ca2+] NT), resulting in the washout of 3 mM [Ca2+]o and the Ca2+ transient amplitude returning back to basal levels (∼3 min). The myocyte was then perfused with 1 μM ISO (with 1 mM [Ca2+]o), which increased myocyte systolic Ca2+ levels.
Statistics.
Data were presented as means ± SE. Differences between groups were evaluated for statistical significance (P < 0.05) by ANOVA for multiple groups or paired Student's t-tests for two groups.
RESULTS
To compare [Ca2+]i decline rates of comparable [Ca2+]i with low and high PLB phosphorylation, a NT solution made with 3 mM [Ca2+]o was used to achieve similar [Ca2+]i peaks as during β-AR stimulation with ISO. Variability of Ca2+ handling between cells being exposed to 3 mM [Ca2+]o and ISO was minimized by superfusing each myocyte with both solutions.
Ca2+ transient kinetics experimental protocol.
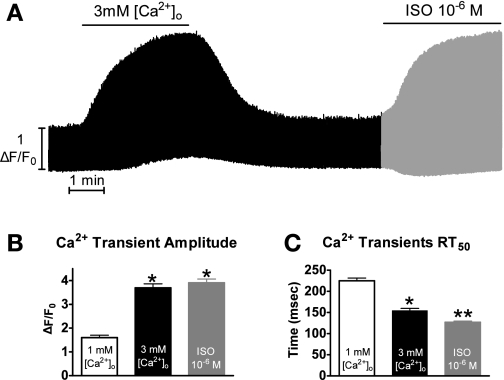
Shown in Fig. 1A is a representative experiment showing Ca2+ transients over time with the various solutions as explained in materials and methods.
Fig. 1.
Experimental protocol. A: representative time plot of the experimental protocol. B: pooled data (means ± SE) of maximum Ca2+ transient amplitude with 1 mM extracellular Ca2+ concentration ([Ca2+]o, open bar), 3 mM [Ca2+]o (black bar), or isoproterenol (ISO, gray bar). C: pooled data (means ± SE) of Ca2+ transient decline measured as the time to 50% relaxation (RT50). P < 0.05 vs. 1 mM [Ca2+]o (*) and vs. 1 and 3 mM [Ca2+]o (**) (n = 21 cells/10 hearts).
Shown in Fig. 1B, perfusing the myocytes with 3 mM [Ca2+]o and ISO resulted in a significantly increased maximal peak systolic [Ca2+] (1 mM [Ca2+]o: 1.6 ± 0.1; 3 mM [Ca2+]o: 3.6 ± 0.2; ISO: 3.9 ± 0.2 ΔF/Fo; n = 21 cells/10 hearts). The majority of myocytes reached a higher maximal peak systolic [Ca2+] with ISO compared with 3 mM [Ca2+]o (although not significant). Shown in Fig. 1C, perfusion with 3 mM [Ca2+]o and ISO also resulted in a significantly faster Ca2+ transient decline rate measured as RT50 (1 mM [Ca2+]o: 225 ± 7; 3 mM [Ca2+]o: 154 ± 5; ISO: 127 ± 3 ms). Interestingly, the ISO Ca2+ transient decay rate was significantly faster than the 3 mM [Ca2+]o.
Ca2+ transient kinetics with matched amplitudes.
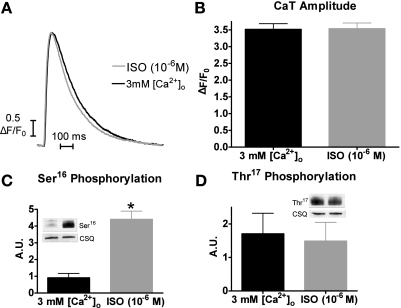
Although the maximum systolic Ca2+ levels were not statistically different between 3 mM [Ca2+]o and ISO, the slightly higher peak with ISO may have resulted in the faster Ca2+ transient decline. Thus, for better comparisons of Ca2+ transient decline rates, we used matched Ca2+ transient amplitude levels. Representative matched individual [Ca2+]i transient traces are shown in Fig. 2A. Shown in Fig. 2B are the matched peak values of 3 mM [Ca2+]o (3.5 ± 0.2 ΔF/Fo) and ISO (3.5 ± 0.2 ΔF/Fo).
Fig. 2.
Matched Ca2+ transient peaks and phospholamban (PLB) phosphorylation with 3 mM [Ca2+]o and ISO. A: representative trace of matched intracellular Ca2+ concentration ([Ca2+]i) amplitudes with 3 mM [Ca2+]o (black) or 1 μM ISO (gray). B: pooled data (means ± SE) of matched Ca2+ transient amplitude with 3 mM [Ca2+]o (black) or ISO (gray) (n = 21 cells/10 hearts). CaT, Ca2+ transient. C: PLB Ser16 phosphorylation with 3 mM [Ca2+]o or ISO (means ± SE) (n = 4). CSQ, calsequestrin; AU, arbitrary units. D: PLB Thr17 phosphorylation with 3 mM [Ca2+]o or ISO (means ± SE). *P < 0.05 vs. 3 mM [Ca2+]o (n = 4 myocyte homogenates).
Similar results were observed when we switched the order of solutions. That is, after initial equilibration at the basal condition of 1 mM [Ca2+]o, the myocytes were first superfused with ISO, washed out (∼15 min), and then perfused with 3 mM [Ca2+]o (data not shown). Thus, the effects of these interventions on Ca2+ transient kinetics are independent of the order of the solutions.
We also measured the Ser16 and Thr17 phosphorylation under similar experimental conditions (i.e., myocyte perfusion with 3 mM [Ca2+]o or ISO for 3 min, which is a similar time point in which we matched the Ca2+ transient amplitudes). Shown in Fig. 2C, myocytes perfused with ISO had significantly increased Ser16 phosphorylation compared with myocytes perfused with 3 mM [Ca2+]o [4.4 ± 0.5 vs. 0.9 ± 0.3 arbitrary units (AU) (n = 4)]. However, there was no difference in Thr17 phosphorylation between ISO and 3 mM [Ca2+]o [1.5 ± 0.6 vs. 1.7 ± 0.6 AU (Fig. 2D)]. Thus, with our experimental protocol and matching Ca2+ transient amplitudes, the only difference between ISO and 3 mM [Ca2+]o is the Ser16 phosphorylation levels.
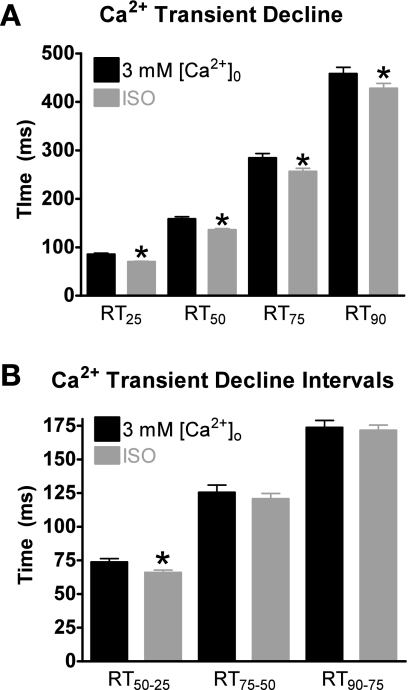
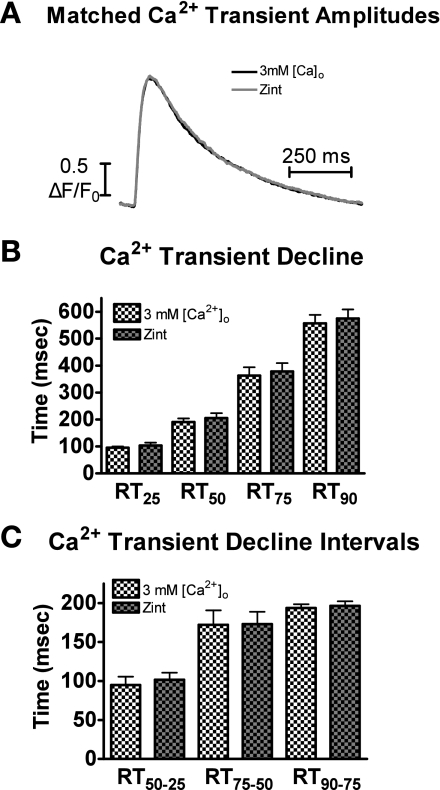
Using matched-amplitudes Ca2+ transient data, we explored the effects of 3 mM [Ca2+]o and ISO on Ca2+ decline by analyzing the time it takes the Ca2+ transient to decline by 25% (RT25), 50% (RT50), 75% (RT75), and 90% (RT90) from its peak amplitude. As shown in Fig. 3A, at each time point, the Ca2+ decline with ISO was significantly faster compared with 3 mM [Ca2+]o. We further analyzed the effects of ISO and 3 mM [Ca2+]o on the Ca2+ transient decline by examining various time intervals. By breaking the decline into intervals, we can better determine at which point in the [Ca2+]i transient PLB phosphorylation will result in different decline rates compared with matched systolic [Ca2+]i levels with 3 mM [Ca2+]o. Thus, we divided the declining Ca2+ transient into intervals: RT50–25, RT75–50, and RT90–75. For example, we subtracted the RT25 from the RT50 to get the RT50–25 interval. This is the time it took for Ca2+ to decline 25% from the peak amplitude to the 50% point. Data are shown in Fig. 3B. The RT50–25 interval was the only interval significantly different between 3 mM [Ca2+]o and ISO. Thus, the faster Ca2+ transient decline with ISO compared with 3 mM [Ca2+]o occurs only in the first 50%.
Fig. 3.
Effects of 3 mM [Ca2+]o and ISO on Ca2+ transient decline with matched peaks. A: pooled data (means ± SE) of Ca2+ decline with 3 mM [Ca2+]o (black) or ISO (gray). RT25, RT75, and RT90, the time it takes the Ca2+ transient to decline by 25, 75, and 90%, respectively, from its peak amplitude. B: pooled data (means ± SE) of Ca2+ transient decline time intervals with 3 mM [Ca2+]o or ISO. RT50-25, the time for Ca2+ to decline 25% from the peak amplitude to the 50% point; RT75-50, the time for Ca2+ to decline 50% from the peak amplitude to the 75% point; RT90-75, the time for Ca2+ to decline 75% from the peak amplitude to the 90% point. *P < 0.05 vs. corresponding 3 mM [Ca2+]o (n = 21 cells/10 hearts).
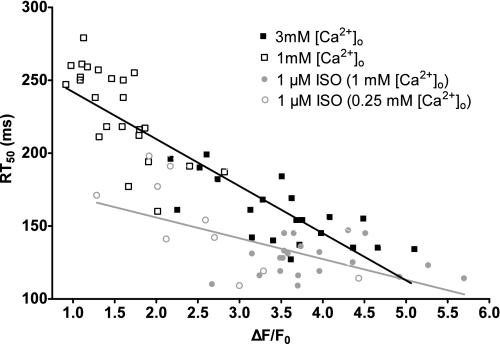
We also investigated the effects of 3 mM [Ca2+]o and ISO on diastolic Ca2+ levels. We observed no difference in diastolic Ca2+ values between 1 mM [Ca2+]o and 3 mM [Ca2+]o (103% of 1 mM [Ca2+]o). There was a slight but significant decrease in diastolic Ca2+ values with ISO compared with 1 mm [Ca2+]o (91% of 1 mM [Ca2+]o). We believe these results further strengthen our argument that Ser16 phosphorylation results in a greater Ca2+ decline, which results in a decreased diastolic [Ca2+]i. We further analyzed the relationship between the systolic Ca2+ levels and RT50. Shown in Fig. 4 are the maximal steady-state Ca2+ transient amplitudes plotted against their respective RT50. Myocytes with low PLB phosphorylation are shown in black (NT with 1 and 3 mM [Ca2+]o). Consistent with previous studies (3), the higher the peak [Ca2+]i, the faster the rate of decline. Myocytes with ISO (i.e., high Ser16 phosphorylation) are shown in gray. In addition to the 1 μM ISO (1 mM [Ca2+]o) group, we were also able to obtain smaller peak Ca2+ transient amplitudes by perfusing myocytes with 1 μM ISO and 0.25 mM [Ca2+]o. We then analyzed the slope of these lines. Our data show that the phosphorylation of Ser16 resulted in a weaker correlation (slope of −14.3 ± 3.4) compared with myocytes with low phosphorylated PLB (slope of −38.3 ± 2.8). Thus, our data suggest that the PLB phosphorylation is more effective at increasing the rate of [Ca2+]i decline and is much less dependent on peak Ca2+ transient amplitudes. Thus, with β-AR stimulation (and PLB phosphorylation), the cardiomyocyte is less dependent on the peak [Ca2+]i levels for the rate of Ca2+ decline.
Fig. 4.
Effects of PLB Ser16 phosphorylation on the relationship between maximal steady-state Ca2+ transient amplitude and decline. Individual values of peak [Ca2+]i vs. RT50 with low (black, r2 = 0.77) and high (gray, r2 = 0.37) Ser16 phosphorylation.
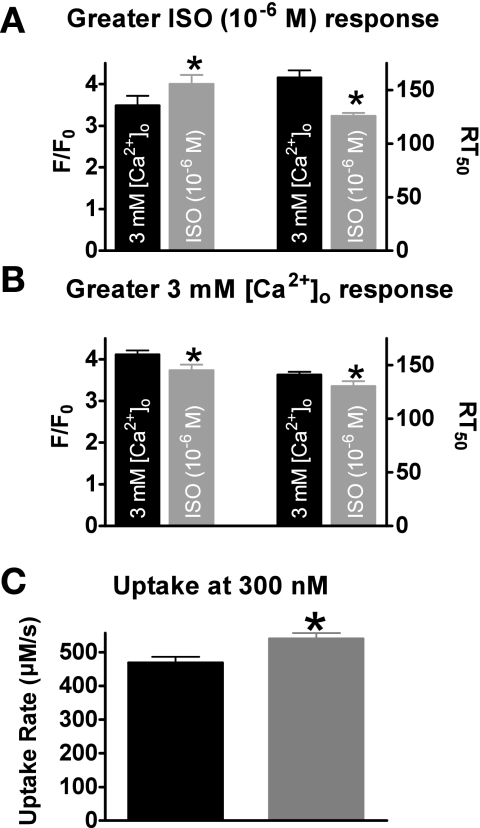
We further investigated this point by grouping the myocytes that had a higher maximum response to ISO together (n = 14) and the myocytes that had a higher response to 3 mM [Ca2+]o together (n = 7). Shown in Fig. 5A, when grouping the data, the myocytes that had a higher maximum peak systolic Ca2+ with ISO (4.0 ± 0.2 vs. 3.5 ± 0.2 ΔF/Fo, P < 0.05) also had a faster Ca2+ transient decline rate (126 ± 3 vs. 161 ± 7 ms, P < 0.05). It is unknown if the major factor in the faster Ca2+ decline is the higher systolic Ca2+ levels or PLB Ser16 phosphorylation. However, the myocytes that a higher maximum peak systolic Ca2+ with 3 mM [Ca2+]o (4.1 ± 0.1 vs. 3.7 ± 0.1 ΔF/Fo, P < 0.05) had a slower Ca2+ transient decline rate (141 ± 3 vs. 130 ± 5 ms, P < 0.05) (Fig. 5B). We also calculated Ca2+ uptake rates with 3 mM [Ca2+]i and ISO. Shown in Fig. 5C, ISO had a greater Ca2+ uptake rate at 300 nm [Ca2+]i (roughly the [Ca2+]i at the RT50) vs. 3 mM [Ca2+]i (540 ± 16 vs. 468 ± 28 μM/s, P < 0.05). Thus these data suggest that PLB Ser16 phosphorylation is the major factor responsible for the faster Ca2+ transient decline rate during β-AR stimulation.
Fig. 5.
Grouped Ca2+ transient amplitudes and declines. A: pooled data (means ± SE) of Ca2+ transient amplitude (left) and decline (right) in myocytes that had a higher response to ISO (n = 14). B: pooled data (means ± SE) of Ca2+ transient amplitude (left) and decline (right) in myocytes that had a higher response to 3 mM [Ca2+]o (n = 7). C: pooled data (means ± SE) of calculated Ca2+ uptake rate at 300 nM [Ca2+]i (*P < 0.05) vs. corresponding 3 mM [Ca2+]o (n = 21 cells/10 hearts).
Effects of β2-AR stimulation on Ca2+ transient decline rates with 3 mm [Ca2+]o and Zint.
The functional response to β-AR stimulation is primarily regulated through β1-AR and β2-AR receptors. β1-AR stimulation leads to high PLB Ser16 phosphorylation by PKA. Interestingly, β2-AR stimulation does not increase Ser16 PLB phosphorylation (23). To further test the contribution of Ser16 phosphorylation on Ca2+ decline, we performed our experimental protocol but substituted Zint, a β2-AR, agonist, for ISO. Matched Ca2+ transient amplitudes for 3 mM [Ca2+]o (2.2 ± 0.2 ΔF/Fo) and Zint (2.2 ± 0.2 ΔF/Fo) (n = 5 cells/3 hearts) were analyzed. Figure 6A displays representative matched Ca2+ transient traces of 3 mM [Ca2+]o and Zint. Shown in Fig. 6B are the Ca2+ transient decline rates (RT25, RT50, RT75, and RT90). We also analyzed the time intervals between 25–50%, 50–75%, and 75–90% (Fig. 6C), as described above. The data in Fig. 6, B and C, show that the Ca2+ transient decline is not significantly different at any time points or between intervals. Thus, with matched [Ca2+]i, β2-AR stimulation does not result in faster Ca2+ decline.
Fig. 6.
Effect of 3 mM [Ca2+]o and β-adrenergic type 2 receptor (β2-AR) agonist zinterol (Zint) on Ca2+ transient decline. A: representative traces of matched [Ca2+]i amplitudes with 3 mM [Ca2+]o (white) and Zint (gray). B: pooled data (means ± SE) of Ca2+ decline with 3 mM [Ca2+]o (white checkered) or Zint (gray checkered). C: pooled data (means ± SE) of Ca2+ transient decline time intervals with 3 mM [Ca2+]o or Zint. *P < 0.05 vs. corresponding 3 mM [Ca2+]o (n = 3 hearts/5 cells).
Effects of CAMKII inhibition on Ca2+ transient decline rates with 3 mm [Ca2+]o and ISO.
β-AR stimulation and high [Ca2+]o can lead to CAMKII activation and PLB Thr17 phosphorylation. Although we observed no difference in the Thr17 phosphorylation status with 3 mM [Ca2+]o and ISO, we wanted to determine if Thr17 phosphorylation played a role in the Ca2+ transient decline. Hence, we repeated our experiments in the presence of the CAMKII inhibitor KN-93 (1 μm/l).
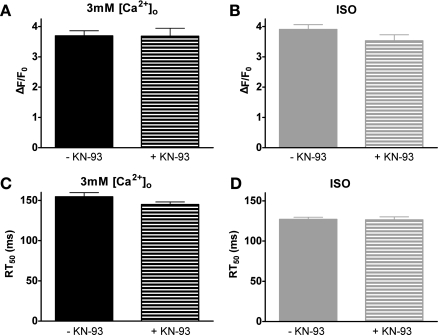
KN-93 resulted in no significant changes in maximum steady-state Ca2+ transient amplitudes (Fig. 7A) or RT50 (Fig. 7C) during superfusion with 3 mM [Ca2+]o.
Fig. 7.
Effects of the Ca2+/calmodulin-dependent protein kinase inhibitor KN-93. Pooled data (means ± SE) of 3 mM [Ca2+]o ± KN-93 on maximal steady-state Ca2+ transient amplitude (A) and corresponding RT50 (C). Pooled data (means ± SE) of ISO ± KN-93 on maximal steady-state Ca2+ transient amplitude (B) and corresponding RT50 (D) (−KN-93: n = 21 cells/10 hearts, +KN-93: n = 15 cells/4 hearts).
We also investigated the effect of ISO with KN-93 on maximum steady-state amplitudes (Fig. 7B) and RT50 (Fig. 7D). KN-93 did not have a significant effect on the response to ISO.
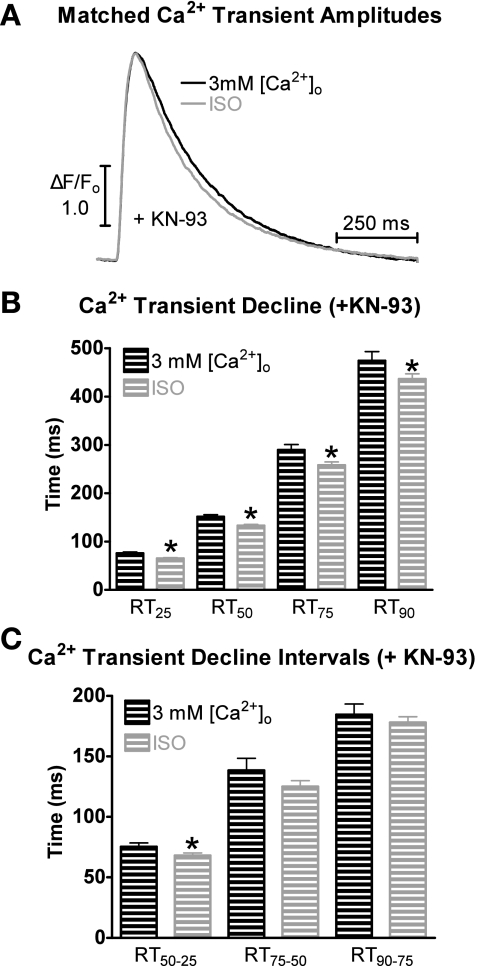
Figure 8A displays representative matched Ca2+ transient traces during superfusion with 3 mM [Ca2+]o and ISO in the presence of KN-93. Shown in Fig. 8B are the Ca2+ transient decline rates (RT25, RT50, RT75, and RT90) in the presence of KN-93. Results with and without KN-93 were similar, that is, the Ca2+ transient decline at each time point was faster with ISO superfusion compared with 3 mM [Ca2+]o.
Fig. 8.
Effects of 3 mM [Ca2+]o and ISO on Ca2+ transient decline in the presence of KN-93. A: representative traces of matched [Ca2+]i amplitudes with 3 mM [Ca2+]o + KN-93 (black) and ISO + KN-93 (gray). B: pooled data (means ± SE) of Ca2+ decline with 3 mM [Ca2+]o + KN-93 (black) or ISO + KN-93 (gray). C: pooled data (means ± SE) of Ca2+ transient decline time intervals with 3 mM [Ca2+]o + KN-93 or ISO + KN-93. *P < 0.05 vs. corresponding 3 mM [Ca2+]o (n = 15 cells/4 hearts).
We also analyzed the time intervals with KN-93 between 25–50%, 50–75%, and 75–90%, as described above. The data in Fig. 8C show that only the Ca2+ transient decline in the first 50% is significantly different. Thus, CAMKII inhibition (and PLB Thr17 phosphorylation) had no effect on the ISO- or 3 mM [Ca2+]o-induced Ca2+ decline. Thus, these data suggest that Ser16 is the major phosphorylation site responsible for the faster Ca2+ transient decline during β-AR stimulation.
Effects of 3 mm [Ca2+]o and ISO on Ca2+ transient time to peak.
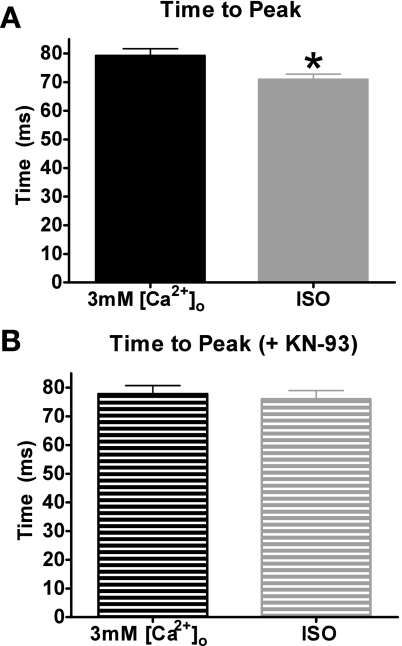
Ca2+ transient time to peak, the time it takes for the myocyte to reach its maximum systolic level, was compared with 3 mM [Ca2+]o vs. ISO (± KN-93). Shown in Fig. 9A, ISO reached its peak significantly faster than 3 mM [Ca2+]o (71 ± 2 vs. 79 ± 2 ms, P < 0.05). However, in the presence of KN-93, the time to peak with 3 mM [Ca2+]o and ISO is similar (78 ± 3 vs. 76 ± 3 ms) (Fig. 9B). Therefore, the faster time to peak during β-AR stimulation with ISO is likely because of the activation of CAMKII.
Fig. 9.
Effects of 3 mM [Ca2+]o and ISO on Ca2+ transient time to peak. A: pooled data (means ± SE) of Ca2+ transient time to peak with 3 mM [Ca2+]o (black) or ISO (gray). B: pooled data (means ± SE) of Ca2+ transient time to peak with 3 mM [Ca2+]o + KN-93 or ISO + KN-93. *P < 0.05 vs. 3 mM [Ca2+]o (−KN-93: n = 21 cells/10 hearts, +KN-93: n = 15 cells/4 hearts).
DISCUSSION
Our data show that, when we compared matched systolic [Ca2+]i levels with ISO and 3 mM [Ca2+]o, PLB Ser16 phosphorylation is the major factor for the faster Ca2+ decline. Interestingly, this faster decline was only observed during the first 50% of the decline. β-AR stimulation also resulted in a faster Ca2+ transient time to peak, which was prevented by CAMKII inhibition.
β-AR stimulation.
Stimulation of the β-AR pathway results in the classical positive inotropic and lusitropic effect (4), that is, in terms of Ca2+ handling, an increase in systolic [Ca2+]i levels with a faster time to peak and decline. In mouse myocytes, the majority (>93%) of the Ca2+ decline is the result of SR uptake via SERCA (2). SERCA is regulated by PLB. Ca2+ binding to SERCA results in the dissociation of PLB from SERCA, which will result in enhanced activation. The higher [Ca2+]i levels during β-AR stimulation should result in further dissociation of PLB from SERCA and faster Ca2+ decline. It has been observed that, with higher systolic Ca2+ levels, there is a faster Ca2+ transient decline (3). PLB is also a phosphoprotein and a key end target of the β-AR pathway. Thus, PKA phosphorylates PLB on Ser16 (21), and CAMKII phosphorylates PLB on Thr17. Phosphorylation of PLB also results in dissociation of PLB from SERCA. Hence, it is unknown which is the major factor accounting for the faster Ca2+ transient decline during β-AR stimulation: PLB phosphorylation or the greater systolic Ca2+ levels.
High extracellular Ca2+ vs. ISO.
We investigated Ca2+ transient kinetics with ISO and 3 mM [Ca2+]o. ISO and 3 mM [Ca2+]o increased peak systolic Ca2+ levels and increased the Ca2+ decline rate (Fig. 1). Interestingly, the decline rate with ISO was faster compared with 3 mM [Ca2+]o. To be assured that the faster decline observed with ISO was the result of PLB phosphorylation and not the small difference in systolic Ca2+ levels, we examined matched Ca2+ transient amplitudes (Fig. 2).
Using the matched amplitudes, we investigated the time it took the Ca2+ transient decline to reach 25, 50, 75, and 90% decline from its peak (Fig. 3A). Our data show that, at each time point, the Ca2+ decline rate was faster with ISO. We further analyzed these effects by examining various time intervals of the Ca2+ transient decline. We measured the interval from 25–50%, 50–75%, and 75–90% decline from its peak (Fig. 3B). Interestingly, our data show that ISO resulted in a faster decline rate during the initial 50% of the decline. These results do not match our hypothesis that PLB phosphorylation would result in a faster decline during the final phase of the decline. Therefore, we now propose that systolic Ca2+ levels in mouse myocytes, even during β-AR stimulation, do not reach the Vmax of the pump, but, during the first 50% of the decline, the dissociation constant between 3 mM [Ca2+]o and ISO is at its greatest difference at this point. Furthermore, we were able to show that, at 300 nm [Ca2+]i, ISO has a greater SR Ca2+ uptake rate compared with 3 mM [Ca2+]i (Fig. 5C). Thus, ISO and, more specifically, Ser16 phosphorylation, accelerates the SR Ca2+ uptake at [Ca2+]i levels at our RT50.
PLB phosphorylation with high extracellular Ca2+ and ISO.
As mentioned, β-AR stimulation leads to the phosphorylation of Ser16 by PKA. It has also been shown that β-AR stimulation and increased [Ca2+]o can activate CAMKII and PLB Thr17 phosphorylation (28, 35). Thus, it has been suggested that Thr17 phosphorylation is also involved in the faster Ca2+ decline rates. Our data show that there was a large increase in Ser16 phosphorylation with ISO compared with 3 mM [Ca2+]o; however, Thr17 phosphorylation levels were similar (Fig. 2). Because our Ca2+ transient amplitudes were matched, the only difference between our two datasets is the level of Ser16 phosphorylation. Thus, we believe our faster Ca2+ decline with ISO compared with 3 mM [Ca2+]o is the result of Ser16 phosphorylation.
Effect of PLB Ser16 phosphorylation on the relationship between systolic Ca2+ levels and [Ca2+]i decline rates.
We also examined the relationship between maximum systolic Ca2+ levels and the Ca2+ transient decline rate measured as RT50 (Fig. 4). Our data show that, with low Ser16 phosphorylation, as systolic Ca2+ levels increased there was a direct relationship for a faster Ca2+ transient decline rate. This is consistent with a previous study (3). There was a similar tendency when PLB Ser16 was phosphorylated. However, when we examined the slopes of each line, the ISO value was considerably less than the 3 mM [Ca2+]o value. Thus, the ISO group is less dependent upon Ca2+ transient peak. In addition, the r2 value for the ISO data indicated that we did not have a good fit. Thus, there is less of a correlation between Ca2+ transient amplitude and RT50 in the ISO group. Our data suggests that the Ca2+ transient decline rate with PLB Ser16 phosphorylation is much less dependent on systolic Ca2+ levels. We further analyzed this by grouping together the myocytes that had a higher response to 3 mM [Ca2+]o and grouping together the myocytes that had a higher response to ISO (Fig. 5). Interestingly, even though the 3 mM [Ca2+]o group had a significantly higher Ca2+ transient amplitude, this group had a significantly slower Ca2+ decline rate. Taken together, our data suggest that Ser16 phosphorylation is the major factor responsible for the faster Ca2+ transient decline rate with β-AR stimulation.
Effects of β2-AR stimulation on Ca2+ transient decline.
To further investigate the role of Ser16 phosphorylation on accelerating Ca2+ decline, we tested if β2-AR stimulation would change Ca2+ transient decline rates. β2-AR stimulation is known to increase Ca2+ transients via local regulation of Ca2+ entry, but, because of its coupling with Gi and its compartmentalization, β2-AR stimulation does not phosphorylate Ser16 PLB to the extent of β1-AR (23). Thus, if Ser16 phosphorylation is the driving force behind the accelerated Ca2+ decline, β2-AR stimulation should not result in faster Ca2+ decline with matching Ca2+ transient peaks. Our data demonstrate that this was indeed the case. That is, matched Ca2+ transient peaks from 3 mM [Ca2+]o and Zint had similar Ca2+ decline rates. Thus, this provided additional evidence that Ser16 phosphorylation is the driving force behind the accelerated Ca2+ decline.
Effects of CAMKII inhibition on Ca2+ transient decline.
Because we did observe Thr17 phosphorylation with ISO and 3 mM [Ca2+]o, we examined if this played any role in Ca2+ decline. The CAMKII inhibitor KN-93 had no effect on the maximal amplitude of the Ca2+ transient or the decline rate with ISO or 3 mM [Ca2+]o (Fig. 7). Although not significant, CAMKII inhibition blunted the Ca2+ transient amplitude with β-AR stimulation, consistent with previous data (6). We also examined the effects of Ca2+ transient decline with matched Ca2+ transient amplitudes during ISO and 3 mM [Ca2+]o perfusion with KN-93. We observed a similar effect, that is, even with CAMKII inhibition, ISO resulted in a faster Ca2+ decline during the first 50% (Fig. 8). Thus, under our experimental conditions, Thr17 phosphorylation does not play a role in enhancing the Ca2+ transient decline rate. Previous studies have found, for CAMKII-mediated PLB Thr17 phosphorylation, there must be concomitant phosphatase inhibition or acidosis (28, 35). Because we did not inhibit phosphatases with 3 mM [Ca2+]o, we would get minimal phosphorylation. During β-AR stimulation, there is an inhibition of the phosphatases (29). However, this phosphorylation step occurred much slower (∼5 min) than Ser16 phosphorylation. Given that we were using Ca2+ transients that had matched amplitudes, we picked ISO-stimulated Ca2+ transients before the peak was reached (∼2–3 min). With the short perfusion time and the lack of phosphatase inhibition or acidosis, CAMKII did not phosphorylate PLB and hence had no effect on ISO-stimulated Ca2+ transient decline. Furthermore, similar to our conclusions, these authors showed that the lusitropic effect of β-AR stimulation correlated with PLB Ser16 phosphorylation.
Other factors that may contribute to Ca2+ decline could be the Na+/Ca2+ exchanger (NCX) or troponin (Tn) I phosphorylation. NCX contributes to the Ca2+ transient decline (2). Studies have found that β-AR stimulation is able to enhance NCX current (30). However, this is controversial, since others did not observe this effect (10). Under our experimental conditions, we believe that NCX does not play a role in the effects of β-AR stimulation on Ca2+ transient decay. NCX plays a minor role in the Ca2+ decline rates in mouse myocytes (<5%); thus, this effect, if any, would be minor at best. Furthermore, with the use of the NCX knockout mouse, there was no difference in Ca2+ transient decline rates with β-AR stimulation in WT compared with knockout myocytes (14). In larger species (e.g., rabbit, human, etc.) NCX plays a greater role in the Ca2+ transient decline rate (∼30%) (2). Consequently, there may be a species difference on the role of NCX and regulation of Ca2+ transient decay during β-AR stimulation. However, in rabbit myocytes during β-AR stimulation, the SR accounts for ∼90% of the Ca2+ removal (36). Thus, the effect of NCX would still be minor. Therefore, during β-AR stimulation, we believe the major stimulus for the increased acceleration of the Ca2+ transient decline is PLB Ser16 phosphorylation.
Phosphorylation of TnI during β-AR stimulation desensitizes the myofilaments to Ca2+ and results in a greater disassociation of Ca2+ from TnC. This may contribute to the faster Ca2+ decline observed with ISO. However, a previous study has shown that TnI phosphorylation does not play a role in Ca2+ decline in an unloaded preparation (i.e., isolated myocytes) (24). Thus, we do not believe TnI phosphorylation plays a role in the faster Ca2+ decline with ISO under our experimental conditions.
Ca2+ transient time to peak with high extracellular Ca2+ and ISO.
We also measured the time it took the Ca2+ transient to reach its peak point (i.e., time from stimulation to peak). We compared the effects of ISO and 3 mM [Ca2+]o on Ca2+ transient time to peak. ISO perfusion resulted in a significantly faster Ca2+ transient time to peak, and this effect was prevented by CAMKII inhibition (Fig. 9). A previous study also observed that, with matched SR Ca2+ loads and L-type Ca2+ current, ISO also resulted in a faster rate of [Ca2+]i rise and implicated the ryanodine receptor (RyR) (11). Studies have shown that CAMKII can phosphorylate the RyR (12, 15, 27). Additionally, studies have shown that, during β-AR stimulation, CAMKII phosphorylates the RyR to increase its activity (6, 8). Our results with KN-93 may seem surprising in that CAMKII can affect Ca2+ transient upstroke but not decline. However, a previous study found that there was a time course disparity in Thr17 phosphorylation and RyR phosphorylation by CAMKII (16). That is, with increasing frequency of stimulation, PLB Thr17 phosphorylation did not change in the first 40 s and then linearly increased for 5 min. RyR Ser2814, the CaMKII site (although controversial), reached maximum phosphorylation after 90 s of increasing the frequency of stimulation. Although these measurements were performed using a different protocol than ours (force frequency vs. β-AR stimulation), there does appear to be a different time dependence to PLB and RyR CaMKII phosphorylation. Our data using β-AR stimulation are consistent with these results in that we observed an apparent effect of CaMKII on RyR function but not PLB function after 3 min. Thus, β-AR stimulation will activate CAMKII to likely increase RyR activity, which results in a faster Ca2+ transient time to peak. The faster time to peak may also be because of faster SR Ca2+ uptake. Nevertheless, a previous study found that transgenic mice overexpressing SERCA2a did not result in a faster Ca2+ transient time to peak (13). Our data are consistent with these observations, since our faster time to peak with ISO was prevented by CaMKII inhibition with KN-93. Yet, KN-93 had no effect on Ca2+ transient decline rates. Thus, if increased SERCA activity was responsible for the faster time to peak, this effect would not have been prevented by KN-93.
Our study also has implications for other mediators of PLB phosphorylation in addition to the β-AR/PKA pathway [i.e., phosphatases, SERCA activators via glutathionylation (1), nitroxyl-induced nitrosylation of PLB (9), reactive nitrogen species, etc.]. For example, nitric oxide (NO) is an important regulator of cardiac contraction (38). We have previously shown that neuronal NO synthase (NOS1) signaling targets PLB to modulate myocyte contraction (37). We have shown that NOS1 knockout myocytes had decreased PLB Ser16 phosphorylation, depressed Ca2+ transient amplitude, and a slower Ca2+ transient decline. It was unknown if the slower [Ca2+]i decline in these myocytes was because of the reduced PLB Ser16 phosphorylation and/or blunted Ca2+ transient amplitude. Our current results would suggest that the NOS1-mediated PLB Ser16 phosphorylation is the major factor on Ca2+ transient decline rather than the amplitude of the Ca2+ transient.
Some limitations of our study include only using one mouse strain. However, our previous work has shown no difference in force decline during β-AR stimulation in three different mouse strains (33). Thus, we believe that our observations are valid for most (if not all) mouse strains. β-AR stimulation also increases heart rate. Increasing pacing frequency by itself also accelerates the Ca2+ decline. However, this is independent of PLB phosphorylation and a separate mechanism (34). Thus, we did not investigate the effects of pacing. In addition, we only used one ISO concentration. We believe using lower ISO concentrations will produce the same qualitative results, but not as great quantitatively. Furthermore, we believe that performing these experiments will not change our conclusion that PLB Ser16 is the major factor for the faster Ca2+ transient decline during β-AR stimulation.
In conclusion, we have shown that the major factor for the faster Ca2+ decline during β-AR stimulation is PLB Ser16 phosphorylation, which only occurs during the initial 50%. β-AR stimulation also results in a faster Ca2+ transient time to peak that was dependent on CAMKII.
GRANTS
This research was supported by the American Heart Association (Established Investigator Award 0740040N, P. M. L. Janssen) and the National Heart, Lung, and Blood Institute (RO1HL-071893, T. R. Shannon, K02HL-094692, and R01HL-079283, M. T. Ziolo).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol Cell Physiol 268: C271–C277, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res 89: 373–375, 2001 [PubMed] [Google Scholar]
- 5. Brittsan AG, Ginsburg KS, Chu G, Yatani A, Wolska BM, Schmidt AG, Asahi M, MacLennan DH, Bers DM, Kranias EG. Chronic SR Ca2+-ATPase inhibition causes adaptive changes in cellular Ca2+ transport. Circ Res 92: 769–776, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Endoh M, Blinks JR. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res 62: 247–265, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Ferrero P, Said M, Sanchez G, Vittone L, Valverde C, Donoso P, Mattiazzi A, Mundina-Weilenmann C. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol 43: 281–291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry 47: 13150–13152, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Ginsburg KS, Bers DM. Isoproterenol does not enhance Ca-dependent Na/Ca exchange current in intact rabbit ventricular myocytes. J Mol Cell Cardiol 39: 972–981, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol 556: 463–480, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res 99: 398–406, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto K, Perez NG, Kusuoka H, Baker DL, Periasamy M, Marban E. Frequency-dependent changes in calcium cycling and contractile activation in SERCA2a transgenic mice. Basic Res Cardiol 95: 144–151, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun 376: 80–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huke S, Bers DM. Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII. J Mol Cell Cardiol 42: 590–599, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299: H1092–H1099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol 299: H1741–H1749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, 2nd, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest 97: 533–539, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem 272: 15061–15064, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Kohr MJ, Traynham CJ, Roof SR, Davis JP, Ziolo MT. cAMP-independent activation of protein kinase A by the Peroxynitrite Generator SIN-1 elicits positive inotropic effects in cardiomyocytes. J Mol Cell Cardiol In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases β-adrenergic stimulation in cardiomyocytes. Cardiovasc Res 77: 353–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, Xiao RP. G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem 274: 22048–22052, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278: H769–H779, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 75: 401–409, 1994 [DOI] [PubMed] [Google Scholar]
- 26. MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92: 904–911, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Mundina-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem 271: 33561–33567, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Neumann J, Gupta RC, Schmitz W, Scholz H, Nairn AC, Watanabe AM. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ Res 69: 1450–1457, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Perchenet L, Hinde AK, Patel KC, Hancox JC, Levi AJ. Stimulation of Na/Ca exchange by the beta-adrenergic/protein kinase A pathway in guinea-pig ventricular myocytes at 37 degrees C. Pflugers Arch 439: 822–828, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Periasamy M, Janssen PM. Molecular basis of diastolic dysfunction. Heart Fail Clin 4: 13–21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM. Murine strain differences in contractile function are temperature- and frequency-dependent. Pflugers Arch 452: 140–145, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Valverde CA, Mundina-Weilenmann C, Said M, Ferrero P, Vittone L, Salas M, Palomeque J, Petroff MV, Mattiazzi A. Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation. J Physiol 562: 801–813, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vittone L, Mundina-Weilenmann C, Said M, Mattiazzi A. Mechanisms involved in the acidosis enhancement of the isoproterenol-induced phosphorylation of phospholamban in the intact heart. J Biol Chem 273: 9804–9811, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Waggoner JR, Ginsburg KS, Mitton B, Haghighi K, Robbins J, Bers DM, Kranias EG. Phospholamban overexpression in rabbit ventricular myocytes does not alter sarcoplasmic reticulum Ca transport. Am J Physiol Heart Circ Physiol 296: H698–H703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol 294: C1566–C1575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and myocardial function. J Mol Cell Cardiol 45: 625–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation 109: 1886–1891, 2004 [DOI] [PubMed] [Google Scholar]