Abstract
Exercise training has been shown to improve cardiac dysfunction in both patients and animal models of coronary artery disease; however, the underlying cellular and molecular mechanisms have not been completely understood. We hypothesized that exercise training would improve force generation in the myocardium distal to chronic coronary artery occlusion via altered intracellular Ca2+ concentration ([Ca2+]i) cycling and/or Ca2+ sensitization of myofilaments. Ameroid occluders were surgically placed around the proximal left circumflex coronary artery of adult female Yucatan pigs. Twenty-two weeks postoperatively, the myocardium was isolated from nonoccluded (left anterior descending artery dependent) and collateral-dependent (formerly left circumflex coronary artery dependent) regions of sedentary (pen confined) and exercise-trained (treadmill run, 5 days/wk for 14 wk) pigs. Force measurements in myocardial strips showed that the percent change in force at stimulation frequencies of 3 and 4 Hz relative to 1 Hz was significantly higher in exercise-trained pigs compared with sedentary pigs. β-Adrenergic stimulation with dobutamine significantly improved force kinetics in myocardial strips of sedentary but not exercise-trained pigs at 1 Hz. Additionally, time to peak and half-decay of intracellular Ca2+ (340-to-380-nm fluoresence ratio) responses at 1 Hz were significantly decreased in the collateral-dependent region of exercise-trained pigs with no difference in peak [Ca2+]i between groups. Furthermore, the skinned myocardium from exercise-trained pigs showed an increase in Ca2+ sensitivity compared with sedentary pigs. Immunoblot analysis revealed that the relative levels of cardiac troponin T and β1-adrenergic receptors were decreased in hearts from exercise-trained pigs independent of occlusion. Also, the ratio of phosphorylated to total myosin light chain-2, basal phosphorylation levels of cardiac troponin I (Ser23 and Ser24), and cardiac myosin binding protein-C (Ser282) were unaltered by occlusion or exercise training. Thus, our data demonstrate that exercise training-enhanced force generation in the nonoccluded and collateral-dependent myocardium was associated with improved Ca2+ transients, increased Ca2+ sensitization of myofilament proteins, and decreased expression levels of β1-adrenergic receptors and cardiac troponin T.
Keywords: coronary artery disease, myocardial function, calcium sensitivity, ischemic heart disease
coronary artery disease is the leading cause of morbidity and mortality in developed countries (28) and produces myocardial dysfunction in both animal models (29) and human patient populations (4). Exercise training improves myocardial function in healthy adults (16) as well as in animal models of coronary artery occlusion/stenosis (19, 30). Using the well-established porcine model of chronic coronary occlusion, previous investigators (30) have reported decreased left ventricular ejection fraction, myocardial ischemia, and decreased left ventricular wall thickness during the systolic phase of cardiac contraction. Additional experimental evidence suggests that exercise training partially corrects the reduction of left ventricular wall thickness in addition to enhancing the collateral circulation in the ischemic myocardial regions in this porcine model as well as in human patients (7, 20). However, the cellular and molecular mechanisms by which exercise training improves cardiac performance in chronic occlusion/stenosis remain to be determined.
Cardiac myocyte contractility is primarily linked to intracellular Ca2+ concentration ([Ca2+]i), and studies (11, 19) using rodent models of coronary artery ligation have suggested that exercise training restores cardiac myocyte contractility by improving intracellular Ca2+ handling. Korte et al. (21) demonstrated that altered phosphorylation and changes in expression levels of myofilament proteins after exercise training led to improved Ca2+ sensitization and cardiac contractility in a diabetic swine model. Furthermore, differential expression of cardiac troponin T (cTnT) isoforms have been associated with altered Ca2+ sensitivity and force production in skinned cardiac muscle fiber bundles (13). Hence, we hypothesized that exercise training would improve force generation in the myocardium distal to chronic coronary artery occlusion via altered [Ca2+]i cycling and/or Ca2+ sensitization of myofilaments. To test our hypothesis, we determined frequency-stimulated [Ca2+]i generation in the myocardium from nonoccluded and collateral-dependent regions of sedentary and exercise-trained animals. In addition, myofilament sensitivity to Ca2+ was determined from skinned preparations, and immunoblot analyses were carried out to investigate the associated changes in regulatory proteins.
MATERIALS AND METHODS
Animal model and exercise training protocol.
All animal protocols were in accordance with the “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” and were approved by the Institutional Animal Care and Use Committee of Texas A&M University in accordance with American Association for Accreditation of Laboratory Animal Care procedures. Furthermore, all methods conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23). Adult female Yucatan miniature swine (age: 9–12 mo, body weight: 25–44 kg, Sinclair Research Center, Auxvasse, MO) were surgically instrumented with an ameroid occluder around the proximal left circumflex coronary (LCx) artery as previously described (14, 15). Animals were preanesthetized with glycopyrrolate (0.004 mg/kg im) and midazolam (0.5 mg/kg im). Anesthesia was induced with ketamine (20 mg/kg im) and maintained with 2–3% isoflurane and 97% O2 throughout aseptic surgery. Overall, mortality in this study was 5.7% of instrumented pigs due to sudden cardiac death occurring at the approximate time that the LCx artery typically becomes completely occluded. Eight weeks postoperatively, pigs were randomly divided into sedentary (pen confined, n = 26) and exercise-trained (n = 23) groups that initiated a 14-wk (5 days/wk) progressive treadmill exercise training protocol as previously described (14, 15). Citrate synthase enzyme activity was determined as previously described (33).
Preparation of myocardial segments and myocyte isolation.
Animals were anesthetized with ketamine (25 mg/kg), xylazine (2.25 mg/kg), and thiopental sodium (20 mg/kg) and terminated by removal of the heart. Hearts were immediately placed in ice-cold Krebs-Henseleit buffer. For experimental purposes, the left ventricular myocardial wall was isolated from both the nonoccluded (left anterior descending artery supplied) and collateral-dependent (formerly LCx supplied) regions.
The myocyte isolation technique was modified from previously described methods (18). Briefly, a section of the ventricular wall (1 cm3) was removed, placed in cold relaxing solution (2 mM EGTA, 5 mM MgCl2, 4 mM ATP, 10 mM imidazole, and 100 mM KCl; pH 7.0), cut into smaller pieces, and gently crushed with a cold mortar and pestle. Cells were released by gentle trituration with a fire-polished glass pipette, filtered through a sterile mesh (200-μm pore size), and resuspended in cold relaxing solution for further use. Cells were allowed to settle to the bottom of a petri dish, and cardiac myocyte width and length were measured on an inverted microscope (Olympus) at ×40 magnification. Myocyte length represented the distance between adjacent intercalated disks. Myocyte width measured the distance perpendicular to the linear axis between the cell margins at the center of the myocyte.
Force measurements in left ventricular myocardial strips.
Nonoccluded and collateral-dependent myocardial sections (1 × 0.8 × 0.5 cm) were isolated from the whole heart. Subsequently, myocardial strips (1.5 ± 0.5 mm in length, 0.3 ± 0.05 mm in width, and 0.2 ± 0.1 mm in depth) were dissected from these myocardial sections as previously described (9). The width of the myocardial strip was kept within the maximal diffusion distance to maintain the delivery of oxygen and nutrients to myocytes at the muscle core to prevent ischemia (35). Myocardial strips were mounted within a muscle measurement suite (Scientific Instruments) and superfused at the rate of 1.5 ml/min continuously with Tyrode solution [containing (in mM) 136.9 NaCl, 5.0 KCl, 1.8 CaCl2, 1.5 MgCl2, 0.4 NaH2PO4, 11.9 NaHCO3, and 5.0 d-glucose; pH 7.4]. The frequency of stimulation was increased stepwise, i.e., 0.2, 0.3, 0.5, 1, 2, 3, and 4 Hz (3-ms pulse duration), at 34°C to determine the force-frequency relationship. To avoid ischemia within the core, myocardial strips were continually perfused with 95% O2-5% CO2, and strips manifesting a positive force-frequency relationship over frequencies of stimulation (0.2, 0.3, and 0.5 Hz) were selected for further experimentation. Next, myocardial strips were superfused with Tyrode solution containing dobutamine (10−6 M), and changes in force kinetics were analyzed at 1 Hz. The maximal rate of contraction (+dF/dtmax; in mN·mm−2·s−1) and maximal rate of relaxation (−dF/dtmin; in mN·mm−2·s−1) were calculated as previously described (31, 40). In addition, the time to peak force (Fmax) and time to half-relaxation of force {[(Fmax − Fmin)/2] + Fmin, where Fmin represents minimum force} were calculated in the absence (control) and presence of dobutamine.
Frequency-stimulated [Ca2+]i (fura-2) in cardiac fibers at 1 Hz.
Frequency-stimulated [Ca2+]i measurements were monitored continuously using a digital oscilloscope suite, National Instruments analog-to-digital board, and Labview software, as previously described (40). A mercury lamp and filter wheel provided alternating ultraviolet pulses of 340 and 380 nm at 250 Hz with a pulse duration of 1.5 ms to illuminate the strip. The combination of a microscope, dichroic mirror, filter, and photomultiplier tube was used to collect the fura-2 fluorescence. Final data for estimates of intracellular free Ca2+ were expressed as the fluorescence ratio (340/380 nm) because of uncertainties in extrapolating in vitro calibrations to in situ measures, as previously reported (37). In addition, the time to peak ratio and time to half-decay of the ratio were calculated as previously described (31, 40).
pCa2+-tension relationships in skinned preparations.
Skinned cardiac fiber preparations were completed as described previously (12). Small sections (1 cm3) of nonoccluded and collateral-dependent myocardial regions of sedentary and exercise-trained pigs were placed in high-relaxing solution of the following composition (in mM): 53.3 KCl, 10 EGTA, 20 MOPS, 1 free MgCl2, 5.4 MgATP, and 12 creatine phosphate. The pH of the solution was adjusted to 7.0 with KOH. Cardiac fiber bundles (∼150–250 μm in width and 2–3 mm in length) were prepared in cold (4°C) high-relaxing solution. Fiber bundles were mounted between a micromanipulator and force transducer and were skinned in pCa 6.5 solution containing 1% Triton X-100. The skinning procedure was terminated when developed tension in response to pCa 6.5 attained a plateau (typically 35–40 min). Bundles were washed with high-relaxing solution and then subsequently bathed in low-relaxing solution. Compared with high-relaxing solution, low-relaxing solution contained 0.1 mM EGTA. A resting sarcomere length of 2.2 μm was then established from laser diffraction patterns (17). Isometric tension was recorded after development in solutions of varying pCa values at room temperature. The pCa2+-tension relationship was determined and fitted to a Hill equation with nonlinear analysis to derive the pCa50 and Hill coefficient using Prism software (version 4.0c).
Immunoblot and phosphoprotein analyses.
Protein lysate was prepared from myocardial segments isolated from nonoccluded and collateral-dependent regions of sedentary and exercise-trained animals as previously described (42). Equal amounts of protein (10 μg) were separated on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Urea-glycerol gels were prepared to analyze the phosphorylated-to-total ratio of myosin light chain-2 (MLC-2), as previously described (27). The membrane was blocked and incubated overnight at 4°C in blocking buffer with the primary antibodies for cardiac troponin I (cTnI; 19C7, Advanced Immunochemical, 1:2,000), phosphorylated (p)-cTnI (p-cTnI; 8921, Abcam, 1:1,250), cardiac myosin binding protein-C (cMyBP-C; SC-50115, Santa Cruz Biotechology, 1:500), p-cMyBP-C (ALX-215-057, Enzo, 1:500), cTnT (SAB2102502, Sigma, 1:500), the β1-adrenergic receptor (β1-AR; SC-568, Santa Cruz Biotechnology, 1:100), and MLC-2 (3672, Cell Signaling, 1;1,500). The incubation duration for GAPDH (6C5, Advanced Immunochemical, 1:2,000) was 45 min at room temperature to confirm equal protein loading. The membrane was incubated with secondary antibody (species-specific anti-IgG, 1:25,000) conjugated to horseradish peroxidase for 45 min at room temperature. Peroxidase activity was detected using the Super Signal West Dura substrate (Pierce). The signal density of protein bands was quantified using Fuji LAS 3000 Image Analyzer and Multigauge software and was normalized to GAPDH protein band intensity. For phosphorylated and total protein (cTnI and cMyBP-C) detection, the membrane was initially probed for phosphorylated protein and then stripped with Restore stripping buffer (Thermo Scientific) and subsequently reprobed for total protein for cTnI and cMyBP-C.
Statistical analysis.
Body weight, heart-to-body weight ratio, and citrate synthase values between sedentary and exercise-trained pigs were compared using Student's t-tests. Myocyte length and width measurements, percent changes in force-frequency relationships, and peak fura-2 ratios as well as the time to peak and time to half-decay, shifts in pCa50 values, Hill's coefficients, and immunoblots were analyzed using two-way ANOVA followed by a Bonferroni post hoc test when a main effect was identified. Force-frequency relationships were compared using two-way ANOVA with repeated measures as previously described (24).The force kinetic data (+dF/dtmax, −dF/dtmin, time to peak force, and time to half-relaxation of force) in the absence and presence of dobutamine were analyzed using paired t-tests. For all analyses, P values of ≤0.05 were considered significant. Values are presented as means ± SE of animals studied.
RESULTS
Efficacy of the exercise training program.
The effectiveness of the 14-wk exercise training program was demonstrated by significant increases in skeletal muscle oxidative enzyme activity and increased heart-to-body weight ratio in exercise-trained animals compared with sedentary animals. Citrate synthase activity increased significantly (P < 0.05 for all comparisons) in the deltoid muscle (46.7 ± 1.9 vs. 36.2 ± 1.1 μmol·min−1·g−1) and lateral (38.6 ± 2.0 vs. 29.8 ± 1.1 μmol·min−1·g−1) and long (35.1 ± 2.4 vs. 27.1 ± 1.7 μmol·min−1·g−1) heads of the triceps brachii muscle in exercise-trained pigs (n = 11) compared with sedentary pigs (n = 13), respectively. At the time of death, body weights were significantly lower in exercise-trained pigs compared with sedentary pigs (31.9 ± 0.7 vs. 35.0 ± 1.0 kg). The heart-to-body weight ratio was significantly greater in exercise-trained pigs compared with sedentary pigs (5.4 ± 0.1 vs. 4.4 ± 0.1 g/kg).
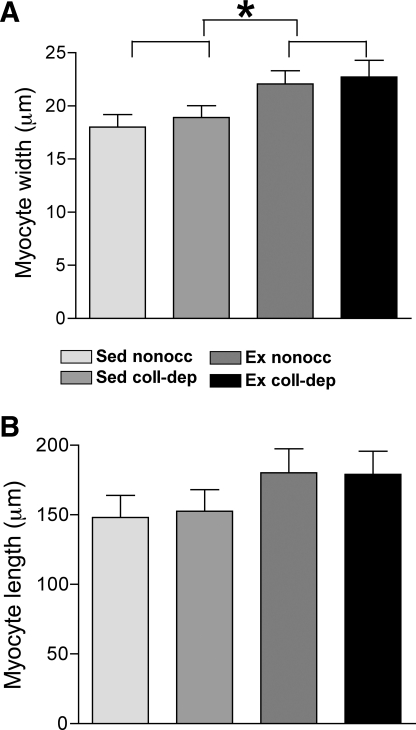
Cardiac myocytes isolated from nonoccluded and collateral-dependent regions of exercise-trained animals displayed significantly increased cellular width compared with those from sedentary animals (Fig. 1A). Cardiac myocytes isolated from the nonoccluded and collateral-dependent myocardium exhibited a 22% and 20% increase in cellular width in cells from exercise-trained pigs compared with cells from sedentary pigs, respectively. Cellular length of cardiac myocytes tended to be increased (P = 0.06) in exercise-trained compared with sedentary animals (Fig. 1B).
Fig. 1.
Exercise training-mediated hypertrophy of cardiac myocytes. Isolated cardiac myocytes were measured for width (A) and length (B). Values are means ± SE. For each experimental group, 20–25 cells/region were studied from 8–10 pigs for a total of 180–220 myocytes. Sed, sedentary; Ex, exercise trained; nonocc, nonoccluded myocardial region; coll-dep, collateral-dependent myocardial region. *P ≤ 0.05, Ex vs. Sed groups.
Changes in force development at higher frequencies were greater in myocardial strips from exercise-trained pigs.
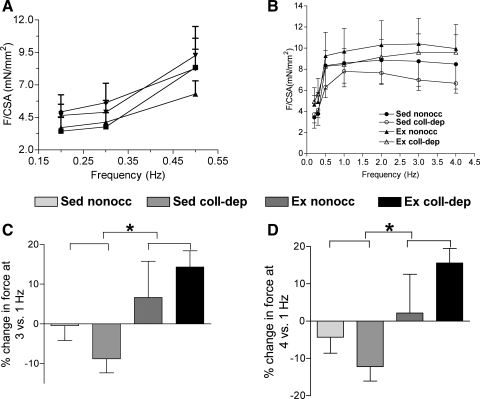
As shown in Fig. 2A, myocardial strips from nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs exhibited positive force-frequency relationships at lower stimulation frequencies (0.2–1.0 Hz). Only strips manifesting a positive force-frequency relationship over the lower frequencies of stimulation were used for further experimentation. These force-frequency relationships were not statistically different between groups. Figure 2B shows the force-frequency relationship for myocardial strips isolated from the experimental groups. However, the percent change in force development, observed from 1.0 to 3.0 Hz (Fig. 2C) and from 1.0 to 4.0 Hz (Fig. 2D), was significantly higher in myocardial strips from exercise-trained pigs compared with sedentary pigs.
Fig. 2.
Force-frequency relationship at 34°C. A: force measurements at stimulation frequencies of 0.2, 0.3, and 0.5 Hz. B: force measurements at different stimulation frequencies are shown for the experimental groups. Percent changes in force generation at 3 versus 1 Hz (C) and 4 versus 1 Hz (D) in strips from Ex pigs were greater than those from Sed pigs. For each experimental group, 11 animals were studied. Values are means ± SE. * P ≤ 0.05, Ex vs. Sed groups.
The response to dobutamine was compromised in nonoccluded regions of exercise-trained animals.
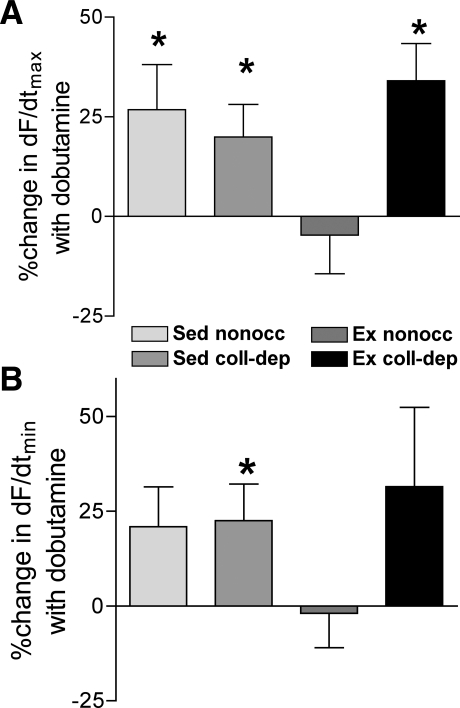
+dF/dtmax and −dF/dtmin were calculated in the absence (control) and presence of dobutamine. Furthermore, the data were normalized to the control for each experiment and reported as the percent change from the control. Values for +dF/dtmax and −dF/dtmin were not statistically different under control conditions in myocardial strips isolated from the nonoccluded and collateral-dependent myocardium of sedentary and exercise-trained pigs (+dF/dtmax: exercise-trained pigs, 39.2 ± 7.7 mN/s in the collateral-dependent myocardium and 74.9 ± 16.0 mN/s in the nonoccluded myocardium, and sedentary pigs, 57.8 ± 11.1 mN/s in the collateral-dependent myocardium and 90.9 ± 21.2 mN/s in the nonoccluded myocardium; −dF/dtmax: exercise-trained pigs, 31.3 ± 5.2 mN/s in the collateral-dependent myocardium and 48.9 ± 10.3 mN/s in the nonoccluded myocardium, and sedentary pigs, 39.5 ± 7.5 mN/s in the collateral-dependent myocardium and 56.2 ± 12.0 mN/s in the nonoccluded myocardium, n = 15–20 pigs/group). Treatment with dobutamine significantly improved +dF/dtmax in nonoccluded and collateral-dependent myocardial regions of sedentary animals and the collateral-dependent region of exercise-trained pigs. In contrast, the nonoccluded region of exercise-trained pigs showed no significant improvement in +dF/dtmax with dobutamine treatment (Fig. 3A). Furthermore, dobutamine treatment significantly increased −dF/dtmin in the collateral-dependent myocardium of sedentary pigs compared with control conditions (Fig. 3B). Both the nonoccluded myocardium of sedentary pigs and collateral-dependent myocardium of exercise-trained pigs revealed a tendency (P = 0.08 and 0.07, respectively) for an increase in −dF/dtmin in the presence of dobutamine. However, the nonoccluded myocardium of exercise-trained animals demonstrated no improvement in −dF/dtmin (Fig. 3B).
Fig. 3.
Effects of dobutamine on the rate of maximal contraction (+dF/dtmax) and rate of relaxation (−dF/dtmin) at 1 Hz. Percent changes in +dF/dtmax (A) and −dF/dtmin (B) by dobutamine (10−6 M) were calculated as described in methods. Values are means ± SE. For each experimental group, 15–20 animals were studied. *P ≤ 0.05, dobutamine vs. control.
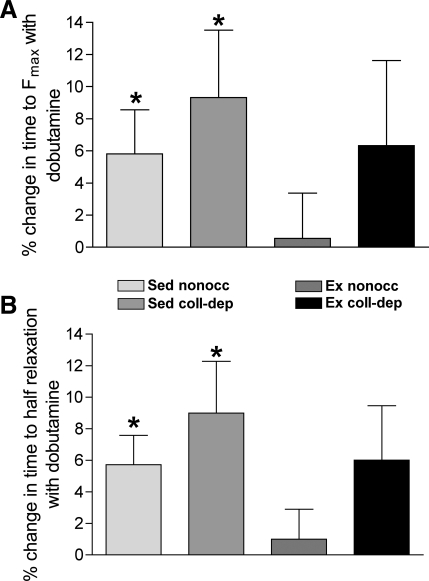
In addition, the nonoccluded and collateral-dependent myocardium of sedentary pigs demonstrated a significantly shorter duration to peak force and to half-relaxation in the presence of dobutamine compared with control conditions, whereas both the time to peak force generation and time to half-relaxation were not significantly altered by dobutamine in both the nonoccluded and collateral-dependent regions of exercise-trained animals (Fig. 4, A and B, respectively).
Fig. 4.
Effects of dobutamine on the time to peak force and time to half-relaxation at 1 Hz. Percent changes in time to peak force (A) and time to half-relaxation (B) were significantly decreased by dobutamine treatment in myocardial strips isolated from nonoccluded and collateral-dependent regions of Sed compared with Ex pigs. Values are means ± SE; 15–20 animals/group. *P ≤ 0.05, dobutamine vs. control.
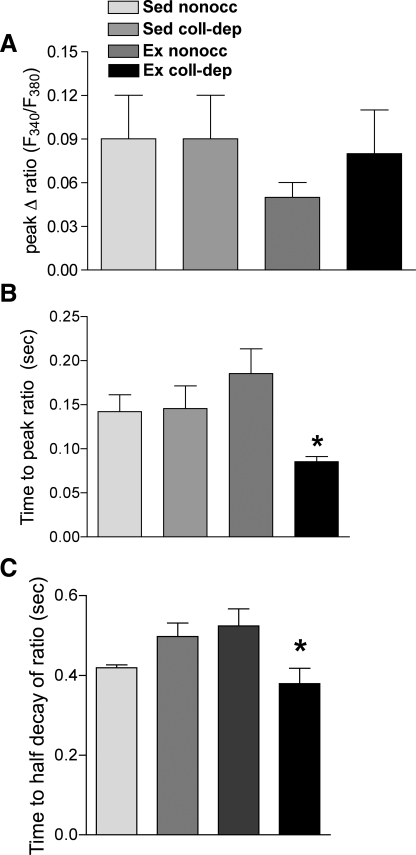
Frequency-stimulated [Ca2+]i levels in isolated myocardial strips.
The peak cytosolic Ca2+ response to frequency stimulation was calculated by the subtraction of basal Ca2+ levels from the peak Ca2+ response to generate the peak change in the fura-2 ratio. No differences were observed in the peak change in the fura-2 ratio between the experimental groups (Fig. 5A). However, the time to peak (Fig. 5B) and time to half-decay (Fig. 5C) of the fura-2 signal were significantly decreased in the collateral-dependent region of exercise-trained animals compared with other experimental groups.
Fig. 5.
Ratio of fluorescence at 340 to 380 nm (F340/F380) versus time at 1 Hz. The net change in the ratio was unaltered (A), whereas the time to peak ratio (B) and time to half-decay of the ratio (C) were significantly reduced in isolated myocardial strips from the collateral-dependent region of Ex animals. Values are means ± SE; 8–13 pigs/group. *P ≤ 0.05.
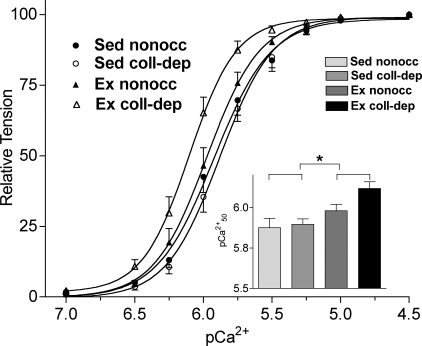
The skinned myocardium from exercise-trained pigs exhibited an increase in Ca2+ sensitivity.
Figure 6 shows the pCa2+-tension relationship of nonoccluded and collateral-dependent myocardial strips of exercise-trained and sedentary animals. Force generated by myofilaments from exercise-trained pigs was more sensitive to Ca2+ compared with myofilaments from sedentary pigs, as demonstrated by the significant leftward shifts in pCa50 values for strips from exercise-trained pigs (Fig. 6, inset; exercise-trained pigs: 6.11 ± 0.04 in the collateral-dependent region and 5.99 ± 0.04 in the nonoccluded region; sedentary pigs: 5.89 ± 0.03 in the collateral-dependent region and 5.87 ± 0.06 in the nonoccluded region, n = 13–14 pigs/group, P = 0.01). The level of cooperativity between the cardiac myofilaments, as measured by the Hill coefficient, tended to be higher in exercise-trained pigs (exercise-trained pigs: 3.1 ± 0.4 in the collateral-dependent region and 3.0 ± 0.2 in the nonoccluded region; sedentary pigs: 2.8 ± 0.1 in the collateral-dependent region and 2.6 ± 0.2 in the nonoccluded region). Additionally, no changes were observed among experimental groups in basal tension at pCa 7.0 (exercise-trained pigs: 0.14 ± 0.05 mN/mm2 in the collateral-dependent region and 0.13 ± 0.04 mN/mm2 in the nonoccluded region; sedentary pigs: 0.16 ± 0.03 mN/mm2 in the collateral-dependent region and 0.17 ± 0.03 mN/mm2 in the nonoccluded region) or maximal tension at pCa 4.5 (exercise-trained pigs: 2.35 ± 0.61 mN/mm2 in the collateral-dependent region and 2.50 ± 0.57 mN/mm2 in the nonoccluded region; sedentary pigs: 3.02 ± 0.33 mN/mm2 in the collateral-dependent region and 2.97 ± 0.41 mN/mm2 in the nonoccluded region).
Fig. 6.
pCa2+-tension relationships in skinned cardiac fibers. Force was normalized to the corresponding maximum force at pCa 4.5. Inset: pCa50 values for the experimental groups. Values are means ± SE; 13–14 pigs/group. *P ≤ 0.05, Ex vs. Sed groups.
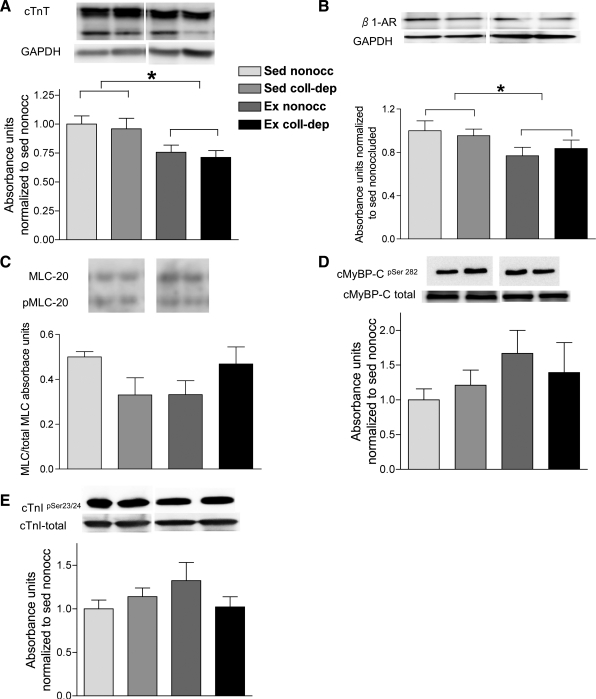
cTnT and β1-AR protein expression levels were decreased in hearts from exercise-trained pigs.
Myofilament protein analyses indicated that the relative levels of cTnT were significantly lower in exercise-trained pigs compared with sedentary pigs (Fig. 7A). Additionally, a significantly lower protein content of β1-ARs was observed in exercise-trained pigs (Fig. 7B). However, the ratio of p-MLC-2 to total MLC-2 and phosphorylation levels of cMyBP-C (Ser282) and cTnI (Ser23 and Ser24) were not statistically different between nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs (Fig. 7, C–E).
Fig. 7.
Effects of chronic occlusion and exercise training on myofibrillar proteins. A–E: representative blots for cardiac troponin T (cTnT; 10–12 pigs/group; A), β1-adrenergic receptors (β1-ARs; 5–6 pigs/group; B), phosphorylated (p)-myosin light chain-2 (MLC-2) to total MLC-2 (4–6 pigs/group; C), p-cardiac myosin binding protein-C (cMyBP-C; pSer282) to total cMyBP-C (4–6 pigs/group; D), and p-cardiac troponin I (cTnI; pSer23/24) to total cTnI (12 pigs/group; E). Proteins of interest were quantified by densitometry analysis, normalized to GAPDH, and expressed relative to the density of proteins in the nonoccluded myocardium of Sed pigs. Values are means ± SE. *P ≤ 0.05, Ex vs. Sed groups.
DISCUSSION
The present study is the first to combine the investigation of functional, cellular, and molecular adaptations of cardiac function with exercise training in a porcine model of chronic coronary occlusion, an animal model that is highly clinically relevant to human cardiovascular pathophysiology. Our data showed an increase in force generation at higher frequencies of stimulation in the myocardium from exercise-trained pigs, independent of chronic occlusion, suggesting an improvement in myocardial function during stress compared with sedentary animals. An increase in Ca2+ sensitivity appears to underlie the increase in force generation in myocardial strips from exercise-trained pigs. Interestingly, dobutamine increased +dF/dtmax and −dF/dtmin in myocardial strips isolated from both nonoccluded and collateral-dependent regions of sedentary pigs compared with exercise-trained pigs. These data were associated with decreased protein levels of β1-ARs and cTnT in the myocardium from exercise-trained pigs. We also revealed a faster time to peak and faster time to half-decay of [Ca2+]i in myocardial strips isolated from the collateral-dependent region of exercise-trained pigs at a stimulation frequency of 1 Hz. In addition, +dF/dtmax in the presence of dobutamine was increased in myocardial strips isolated from both nonoccluded and collateral-dependent regions of sedentary pigs and from the collateral-dependent region of exercise-trained pigs in contrast to strips isolated from the nonoccluded region of exercise-trained pigs.
To mimic the increase in heart rate attained during exercise training bouts, we stimulated myocardial strips at frequencies of 3.0 and 4.0 Hz. The resting heart rate of the pig is ∼ 65 beats/min (1.0 Hz) (7), whereas during the exercise training bouts used for this study, heart rates attained ∼220–230 beats/min (3.0–4.0 Hz; C. L. Heaps, unpublished observations). The results from force-frequency experiments (Fig. 2) indicated that myocardial strips from exercise-trained pigs demonstrate progressively increased force generation from 0.2 to 4.0 Hz. In contrast, the increment in force generation continues up to 2.0 Hz in strips from sedentary animals, and further increases in frequency of stimulation resulted in a decline in force generation. Differences in force generation were observed at higher, but not lower, frequencies of stimulation between treatment groups, such that myocardial strips from sedentary pigs increase cardiac contractility at lower frequency of stimulation or heart rate (60–100 beats/min), but at higher heart rates, such as those observed during exercise, the myocardium of sedentary pigs was unable to maintain the increase in force generation. In contrast, the myocardium from exercise-trained animals continued to generate higher contractile force as the frequency of stimulation was increased, suggesting an exercise training-mediated adaptation that reveals improvements in myocardial function during stress.
Previous studies (2, 25) have shown desensitization of the β-adrenergic signaling pathway in exercise-trained animals. The cardiac β-adrenergic signaling pathway, via PKA activation, influences cellular Ca2+ homeostasis by regulating the function of L-type Ca2+ channel proteins, ryanodine receptors, Ca2+ removal mechanisms such as sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), phospholamban, the Na+/ Ca2+ exchanger, and myofilament proteins (5). Thus, β-adrenergic stimulation causes an increase in cardiac contractility (inotropy), heart rate (chronotropy), and cardiac relaxation (lusitropy). Our results demonstrate that myocardial strips from sedentary pigs responded to dobutamine treatment by manifesting an increase in the contractile parameters. In contrast, the myocardium from exercise-trained pigs generally exhibited little change in contractile properties in response to dobutamine, suggesting a desensitization of the β-adrenergic response that is supported by our findings of decreased β1-AR protein levels in the myocardium from both nonoccluded and collateral-dependent regions of exercise-trained pigs. Consistent with our data, Barbier et al. (2) also showed that protein levels of myocardial β1-ARs were decreased by exercise training, whereas β2-AR levels were unchanged and β3-AR levels increased. A previous report (34) showing decreased inotropic responses of β2-ARs after voluntary exercise training in isolated cardiac myocytes as well as decreased mRNA levels of β2-ARs in the ventricular myocardium provides additional support for our finding of desensitization to dobutamine stimulation after exercise training.
Despite our general finding of no effect of dobutamine in the myocardium from exercise-trained pigs, myocardial strips from the collateral-dependent region of exercise-trained pigs did show a significant increase in +dF/dtmax in response to dobutamine. Thus, the collateral-dependent region of exercise-trained pigs appears unique in manifesting an increase in +dF/dtmax in the presence of dobutamine, suggesting less β-adrenergic desensitization. Additionally, this partial responsiveness to β-adrenergic signaling occurred despite a significant decrease in β1-AR protein levels. We speculate that the responsiveness of the collateral-dependent region of exercise-trained pigs to dobutamine is perhaps due to unique regional adaptations of the β-adrenergic signaling pathway or other mechanisms, such as those associated with the faster Ca2+ kinetics observed in the collateral-dependent region. Unique adaptations in the collateral-dependent myocardial region of exercise-trained pigs have been previously noted in this model (8). Although not measured in our study, previous reports (23, 38) have suggested that differential expression of β-ARs, such as β2- and/or β3-ARs, altered phosphorylation status of β-ARs by β-AR kinase 1 or G protein-coupled kinase 2, and PKA may contribute to desensitization to β-adrenergic stimulation. Furthermore, β-adrenergic signaling and Ca2+-handling pathways are intricately linked, since downstream effectors of β-adrenergic signaling include the L-type Ca2+ channel, the ryanodine receptor, and phospholamban. Thus, adaptations in Ca2+-handling proteins, as suggested by our finding of a faster time to peak and time to half-decay of [Ca2+]i in the collateral-dependent myocardium of exercise-trained pigs, may have also contributed to the partial responsiveness to β-adrenergic signaling.
Independent of the potential contribution of Ca2+ handling to β-adrenergic signaling, the adaptations in the Ca2+ signal in the collateral-dependent region of exercise-trained pigs likely also contributed directly to the enhanced force generation in response to frequency stimulation. Despite no significant differences in the peak [Ca2+]i signal between myocardial treatment groups, the faster kinetics may have contributed to the increased force generation in the collateral-dependent myocardium of exercise-trained pigs. We speculate that changes in the density or phosphorylation of the L-type Ca2+ channel, or a closer structural organization of the L-type Ca2+ channel and ryanodine receptor, could have potentially contributed to a faster time to peak [Ca2+]i in the collateral-dependent myocardium of exercise-trained pigs. Similarly, the faster time to half-decay may be attributable to faster [Ca2+]i reuptake by SERCA, potentially via enhanced phosphorylation of phospholamban. Thus, faster influx was potentially compensated by faster reuptake of [Ca2+]i in this region with no net change in [Ca2+]i. These findings also explain the observed recovery of the response to dobutamine in this region. Yet, the mechanisms for increased force generation observed at higher frequency based on [Ca2+]i changes are unclear. Our additional findings suggest that increased Ca2+ sensitivity in the myocardium of exercise-trained pigs potentially contributes to the observed augmentation in force generation in the collateral-dependent and nonoccluded myocardium at higher stimulation rates of exercise-trained pigs.
Findings in the literature regarding exercise training-induced adaptations in cTnT protein levels are highly variable. Previous studies (1, 21, 26) have reported increased expression of cTnT isoform 2 with decreased ATPase activity and increased Ca2+ sensitivity in the failing human heart and diabetic cardiomyopathy. In addition, Nassar et al. (26) showed that Ca2+ sensitization was associated with an increase in cTnT isoform 2 protein and a reduction in cTnT isoform 4 protein in neonatal rabbits. In another study (18), exercise training mediated no change in cTnT isoforms in hearts from control pigs. Our results revealed decreased protein levels of cTnT by exercise training in both nonoccluded and collateral-dependent regions. We speculate that the unique adaptations observed between these studies may be related to variable animal models as well as different states of health or disease.
We also analyzed the role of other myofilament proteins, such as MLC-2, cTnI, and cMyBP-C, that affect Ca2+ sensitivity in the underlying setting of exercise training. A previous study (18) has shown that the relative phosphorylation levels of cTnI and cMyBP-C are increased in exercise-trained animals; however, we did not observe significant changes in these proteins in our model. On the other hand, we did not examine the phosphorylation status of additional sites for cTnI (Ser149) (22) and cMyBP-C (Ser273, Ser302, and Ser307) (3), which may contribute to Ca2+ sensitization. Recent findings have indicated that a decrease in p-MLC-2 is linked with impaired cardiac function (36) and an increase in the Ca2+ sensitivity of force generation (10). However, our results indicated no change in the ratio of p-MLC-2 to total MLC-2 between our experimental groups. Additionally, a previous study (6) demonstrated an association of force-generating capacity with total myofilament density. Whether these findings contribute to the increased force generation observed in our model remains to be determined.
Clinical implications and conclusions.
Despite experimental and clinical evidence that exercise training enhances myocardial contractility in diseased hearts (16, 18, 19, 30), the underlying cellular and molecular adaptations have only begun to be studied (8, 21, 39). Our data using the exercise training-induced adaptations in a porcine model of chronic occlusion demonstrated that increased force development in the collateral-dependent region after exercise training is associated with Ca2+ sensitization of the myofilaments as well as faster intracellular Ca2+ kinetics during stimulation. Furthermore, despite reduced β1-AR protein levels with exercise training in both the nonoccluded and collateral-dependent myocardium, the collateral-dependent region was responsive to dobutamine stimulation, whereas the nonoccluded region was not. Taken together, these data suggest that faster Ca2+ kinetics may have contributed to the partial responsiveness to dobutamine in the collateral-dependent region compared with the nonoccluded region. Thus, our results provide additional insights in the understanding of the underlying mechanisms that contribute to the beneficial effects of exercise training, particularly in patients of coronary artery disease.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-064931 (to C. Heaps) and KO2-HL-86650 (to M. Muthuchamy), American Heart Association Grant-In-aid 11GRNT7890010 (to M. Muthuchamy), and a Texas A&M College of Veterinary Medicine and Biomedical Sciences postdoctoral grant (to V. Sarin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors greatly appreciate the technical and surgical expertise of Mildred Mattox and the excellent technical contributions of Jana Havey and Jeff Bray. Furthermore, the authors thank Dr. Jerome Trzeciakowski for expert suggestions on statistical analysis.
REFERENCES
- 1. Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle/ Circ Res 69: 1226–1233, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Barbier J, Rannou-Bekono F, Marchais J, Berthon PM, Delamarche P, Carre F. Effect of training on β1, β2, β3 adrenergic and M2 muscarinic receptors in rat heart. Med Sci Sports Exerc 36: 949–954, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48: 866–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bito V, van der Velden J, Claus P, Dommke C, Van Lommel A, Mortelmans L, Verbeken E, Bijnens B, Stienen G, Sipido KR. Reduced force generating capacity in myocytes from chronically ischemic, hibernating myocardium. Circ Res 100: 229–237, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol 56: 656–665, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Boluyt MO, Cirrincione GM, Loyd AM, Korzick DH, Parker JL, Laughlin MH. Effects of gradual coronary artery occlusion and exercise training on gene expression in swine heart. Mol Cell Biochem 294: 87–96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collado MC, Beleta J, Martinez E, Miralpeix M, Domenech T, Palacios JM, Hernandez J. Functional and biochemical evidence for diazepam as a cyclic nucleotide phosphodiesterase type 4 inhibitor. Br J Pharmacol 123: 1047–1054, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J Appl Physiol 91: 309–315, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gaffin RD, Gokulan K, Sacchettini JC, Hewett T, Klevitsky R, Robbins J, Muthuchamy M. Charged residue changes in the carboxy-terminus of α-tropomyosin alter mouse cardiac muscle contractility. J Physiol 556: 531–543, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomes AV, Guzman G, Zhao J, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem 277: 35341–35349, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948–1956, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984–H1992, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hermansen L, Saltin B. Oxygen uptake during maximal treadmill and bicycle exercise. J Appl Physiol 26: 31–37, 1969 [DOI] [PubMed] [Google Scholar]
- 17. Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol 329: 527–540, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hinken AC, Korte FS, McDonald KS. Porcine cardiac myocyte power output is increased after chronic exercise training. J Appl Physiol 101: 40–46, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisloff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol 43: 354–361, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolibash AJ, Bush CA, Wepsic RA, Schroeder DP, Tetalman MR, Lewis RP. Coronary collateral vessels: spectrum of physiologic capabilities with respect to providing rest and stress myocardial perfusion, maintenance of left ventricular function and protection against infarction. Am J Cardiol 50: 230–238, 1982 [DOI] [PubMed] [Google Scholar]
- 21. Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol 98: 461–467, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Leosco D, Rengo G, Iaccarino G, Golino L, Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M, Galasso G, Altobelli GG, Conti V, Matrone G, Cimini V, Ferrara N, Filippelli A, Koch WJ, Rengo F. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res 78: 385–394, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res 28: 303–311, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Mohan RM, Choate JK, Golding S, Herring N, Casadei B, Paterson DJ. Peripheral pre-synaptic pathway reduces the heart rate response to sympathetic activation following exercise training: role of NO. Cardiovasc Res 47: 90–98, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Nassar R, Malouf NN, Kelly MB, Oakeley AE, Anderson PA. Force-pCa relation and troponin T isoforms of rabbit myocardium. Circ Res 69: 1470–1475, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Perrie WT, Perry SV. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J 119: 31–38, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart hisease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth DM, White FC, Bloor CM. Regional myocardial dysfunction during coronary occulsion in conscious swine. Cardiovasc Pathol 1: 97–103, 1996 [Google Scholar]
- 30. Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 82: 1778–1789, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Sarin V, Gaffin RD, Meininger GA, Muthuchamy M. Arginine-glycine-aspartic acid (RGD)-containing peptides inhibit the force production of mouse papillary muscle bundles via α5β1 integrin. J Physiol 564: 603–617, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solaro RJ, Sheehan KA, Lei M, Ke Y. The curious role of sarcomeric proteins in control of diverse processes in cardiac myocytes. J Gen Physiol 136: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srere PA. Citrate synthase. Methods Enzymol 13: 3–11, 1969 [Google Scholar]
- 34. Stones R, Natali A, Billeter R, Harrison S, White E. Voluntary exercise-induced changes in β2-adrenoceptor signalling in rat ventricular myocytes. Exp Physiol 93: 1065–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol 558: 927–941, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJ. Myosin light chain composition in non-failing donor and end-stage failing human ventricular myocardium. Adv Exp Med Biol 538: 3–15, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wagner-Mann C, Bowman L, Sturek M. Primary action of endothelin on Ca release in bovine coronary artery smooth muscle cells. Am J Physiol Cell Physiol 260: C763–C770, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Wallukat G. The β-adrenergic receptors. Herz 27: 683–690, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54: 162–174, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Wu X, Chakraborty S, Heaps CL, Davis MJ, Meininger GA, Muthuchamy M. Fibronectin increases the force production of mouse papillary muscles via α5β1 integrin. J Mol Cell Cardiol 50: 203–213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yumoto F, Lu QW, Morimoto S, Tanaka H, Kono N, Nagata K, Ojima T, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Nishita K, Tanokura M, Ohtsuki I. Drastic Ca2+ sensitization of myofilament associated with a small structural change in troponin I in inherited restrictive cardiomyopathy. Biochem Biophys Res Commun 338: 1519–1526, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Zhou M, Widmer RJ, Xie W, Jimmy Widmer A, Miller MW, Schroeder F, Parker JL, Heaps CL. Effects of exercise training on cellular mechanisms of endothelial nitric oxide synthase regulation in coronary arteries after chronic occlusion. Am J Physiol Heart Circ Physiol 298: H1857–H1869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]