Abstract
The histidine-rich calcium binding protein (HRC) Ser96Ala polymorphism was shown to correlate with ventricular arrhythmias and sudden death only in dilated cardiomyopathy patients but not in healthy human carriers. In the present study, we assessed the molecular and cellular mechanisms underlying human arrhythmias by adenoviral expression of the human wild-type (HRCWT) or mutant HRC (HRCS96A) in adult rat ventricular cardiomyocytes. Total HRC protein was increased by ∼50% in both HRCWT- and HRCS96A-infected cells. The HRCS96A mutant exacerbated the inhibitory effects of HRCWT on the amplitude of Ca2+ transients, prolongation of Ca2+ decay time, and caffeine-induced sarcoplasmic reticulum Ca2+ release. Consistent with these findings, HRCS96A reduced maximal sarcoplasmic reticulum calcium uptake rate to a higher extent than HRCWT. Furthermore, the frequency of spontaneous Ca2+ sparks, which was reduced by HRCWT, was increased by mutant HRCS96A under resting conditions although there were no spontaneous Ca2+ waves under stress conditions. However, expression of the HRCS96A genetic variant in cardiomyocytes from a rat model of postmyocardial infarction heart failure induced dramatic disturbances of rhythmic Ca2+ transients. These findings indicate that the HRC Ser96Ala variant increases the propensity of arrhythmogenic Ca2+ waves in the stressed failing heart, suggesting a link between this genetic variant and life-threatening ventricular arrhythmias in human carriers.
Keywords: histidine-rich calcium binding protein, human mutation, dilated cardiomyopathy, stress condition
arrhythmic events such as ventricular tachycardia or ventricular fibrillation are the most common causes of sudden cardiac death in dilated cardiomyopathy patients (12). During progression of cardiac remodeling, the expression levels of many Ca2+ cycling proteins are altered (14), which contribute to defective Ca2+ handling in failing hearts. Among these alternations, the current density of Na+/Ca2+ exchanger (NCX) is enhanced (4, 22) and Ca2+ leak from the sarcoplasmic reticulum (SR) is elevated, resulting in disturbances of rhythmic cardiac contraction, either through early afterdepolarization or delayed afterdepolarization (DAD).
SR Ca2+ load has been suggested to play an important role in regulating SR Ca2+ leak, especially in failing hearts (13). Along these lines, the expression level of histidine-rich calcium binding protein, which has structural analogy to calsequestrin (CSQ), has been found reduced in experimental and human heart failure. Interestingly, HRC appears to directly interact with both triadin (16, 25) and sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a; Ref. 3) and has been implicated as a regulator of both SR Ca2+ release and uptake. Transgenic models with HRC overexpression revealed decreased Ca2+ transient amplitude and prolonged transient decay time (8), implicating HRC as a regulator of Ca2+ cycling in vivo.
Studies on identification of human mutations in key SR Ca2+ cycling genes have provided further insights on the functional role of these proteins in the heart. Actually, several point mutations in the ryanodine receptor (RyR; Refs. 9, 18) and CSQ (21, 23) have been linked to sudden cardiac death and the underlying mechanism involved defective RyR activity. Generally, stress conditions or exercise related catecholaminergic polymorphic ventricular tachycardia have been associated with increased functional activity of RyR channels, which contributed to increased SR Ca2+ leak and disturbed intracellular Ca2+ homeostasis leading to ventricular arrhythmias. However, parallel studies on human mutations in junctin, triadin, and HRC, known as effective regulators of RyR activity and SR Ca2+ release, are not currently available.
Recently, a genetic variant of HRC, Ser96Ala, was shown to have a correlation with ventricular arrhythmogenesis in dilated cardiomyopathy (DCM; Ref. 2). Although HRCS96A occurred with comparable frequencies in DCM patients and normals, this variant was associated with a history of ventricular tachycardia or ventricular fibrillation before screening entry. Furthermore, the percentage of homozygous HRCS96A DCM patients with an implantable cardioverter-defibrillator was almost double of the number of HRCS96A heterozygotes, which suggested a strong dosage effect of this polymorphism on ventricular arrhythmias. To assess the underlying mechanisms, we expressed the human wild-type and S96A variant of HRC in normal or heart failure rat myocytes. Our results demonstrate that the single substitution of Ser96Ala modulates HRC function in normal cardiomyocytes, while it elevates the occurrence of Ca2+ waves in heart failure cells under stress conditions.
MATERIALS AND METHODS
Recombinant adenoviral constructs.
Human HRC cDNA was purchased from Open Biosystems. cDNA expressing HRC S96A was generated using the Quik-change-site-directed mutagenesis II kit (Stratagene). Human HRC cDNA was firstly cloned into the pShuttle vector and then transferred into the Adeno-X viral DNA using the Ad-Easy XL system (Stratagene). Adenoviruses containing the cDNA sequence of wild-type HRC (Ad.HRCWT), mutant HRC (Ad.HRCS96A), or greenfluorescent protein (Ad.GFP) were assembled and amplified in HEK-293 cells. In the final step, the viruses were purified, using the adenovirus mini purification kit (Virapur) and tittered, using the Adeno-X rapid titer kit (Clonetech).
Myocardial infarction-induced heart failure and echocardiographic evaluation.
After echocardiographic assessment of baseline function, male Wistar rats were subjected to left descending coronary artery ligation as previously described (15). Left ventricular echocardiography was performed to monitor the heart function with a General Electric Vivid 7 dimension instrument equipped with a linear i13L transducer. There was a progressive increase in left ventricular end-diastolic volume. Eight weeks following the surgery, cardiac ejection fraction was reduced to 32%, and this model was defined as a postmyocardial infarction heart failure model. All surgical procedures described in this study were approved by the Institutional Animal Care and Use Committee of Peking University.
Adenoviral infection of cardiomyocytes.
Cardiomyocytes were isolated from either normal or failing rat hearts as previously described (8). After 2 h of being cultured on coverslips or in dishes precoated with 10 μg/ml laminin, myocytes were infected with correspondent adenoviruses. Experiments were performed 48 h after infection and ∼100% of cells appeared infected.
HEK 293 cell culture and coimmunoprecipitation of HRC with SERCA2.
HEK 293 cells (ECACC, Salisbury, UK) were maintained in DMEM supplemented with 10% FBS (Invitrogen, Carlsbad, CA). Full-length human SERCA2 (NIH Mammalian Gene Collection Clone ID 5503508; Invitrogen) and GFP-HRC S96 or GFP-HRC A96 constructs were transiently transfected in HEK293 cells with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Forty-eight hours after transfection, cells were harvested and lysed in 50 mM Tris·HCl, 150 mM NaCl, and 1% NP40 with protease inhibitors. Cell lysates were precleared with 30 μl protein G-Sepharose (Amersham Biosciences Europe, Uppsala, Sweden) for 1 h at 4°C. The precleared cell lysates were then incubated with 4 μg of SERCA2 antibody (Affinity Bioreagents) and 30 μl protein G-Sepharose at 4°C overnight. The beads were washed twice with lysis buffer and were analyzed by Western blots with either anti-SERCA (Affinity Bioreagents) or anti-HRC (Sigma-Aldrich) antibodies.
SR Ca-uptake rates.
Initial rates of SR Ca uptake were determined in cardiomyocyte homogenates after 48 h of infection, with the use of oxalate and 45CaCl2, as previously described (17). Briefly, 250 μg of homogenate were incubated at 37°C in a reaction buffer containing: 40 mmol/l imidazole pH 7.0, 95 mmol/l KCl, 5 mmol/l NaN3, 5 mmol/l MgCl2, 0.5 mmol/l EGTA, and 5 mmol/l potassium oxalate. The initial uptake rates were determined over a range of Ca concentrations (pCa 8 to 5). Ca uptake into SR vesicles was initiated by addition of 5 mmol/l ATP, and aliquots were filtered through a 0.45-μm Millipore filter after 0, 30, 60, and 90 s. The specific Ca-uptake values, Ca affinity (EC50), and maximal Ca-uptake rates (Vmax) were analyzed by nonlinear regression, using the OriginLab 5.1 program.
Quantitative immunoblotting.
Cultured cardiomyocytes were harvested and lysed as previously described (8). An appropriate amount of protein sample was separated by SDS-PAGE and transferred to a nitrocellulose membrane. After being blocked with 5% nonfat milk, the membranes were incubated with the corresponding primary antibodies: phospholamban (1:5,000), CSQ (1:1,000), actin (1:5,000; Affinity Bioreagents), SERCA2a (1:1,000; homemade polyclonal rabbit anti-SERCA antibody), junctin (1:1,000; Santa Cruz Biotechnology), triadin (1:1,000; Thermo Scientific), and HRC (1:5,000; a generous gift from Dr. Woo Jin Park). The corresponding horseradish peroxidase linked secondary antibody was purchased from GE Healthcare. Membranes were then developed using SuperSignal West Pico chemiluminescent substrate from Thermo Scientific. Protein loading was normalized to endogenous actin level and quantitated by using AlphaEaseFC software (Alpha Innotech, San Leandro, CA).
Ca2+ cycling measurements.
Cardiomyocytes were loaded with Rhod-2 AM (10 μmol/l, 30 min; Invitrogen), and Ca2+ transients as well as Ca2+ sparks were acquired with Zeiss LSM 510 confocal microscope under line=scan mode. To measure Ca2+ transients, myocytes were paced at 0.5 Hz in solution containing (in mmol/l): 137 NaCl, 20 HEPES, 10 d-glucose, 5.4 KCl, 1.2 MgCl2, 1.2 NaH2PO4, and 1 CaCl2 1 (pH 7.4). To assess SR Ca2+ load, caffeine-induced Ca2+ release was initiated by rapid application of 20 mM caffeine after steady-state field stimulation. Stress condition was induced with 1 μM isoproterenol treatment and 1-Hz field stimulation. Rhod-2 was excited at 543 nm, and emitted fluorescence was collected at >560 nm.
For resting Ca2+ measurement, myocytes were loaded with indo-1 AM (10 μmol/l, 30 min) and excited with ultraviolet light. The emission fluorescence was recorded by a CCD camera (Luca; Andor Technology) at 405 nm (F405) and 485 nm (F485) separated by an emission splitter. Resting Ca2+ was estimated by the ratio of F405/F485. All experiments were performed at room temperature. Image processing and data analysis were performed using IDL program (Research Systems).
Statistical analysis.
All values were expressed as means ± SE. Statistical comparisons among three groups were evaluated with one-way ANOVA. In all analyses, P values <0.05 were considered statistically significant.
RESULTS
Overexpression of human HRCWT or HRCS96A in adult rat cardiomyocytes.
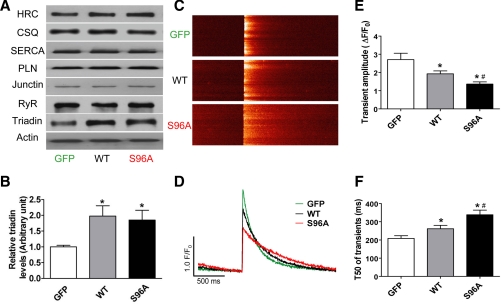
Rat cardiac myocytes were infected with Ad.GFP, Ad.HRCWT, or Ad.HRCS96A. At 48 h postinfection, quantitative immunoblotting revealed 50% increases in total HRC levels of HRCWT and HRCS96A groups (Fig. 1A). Interestingly, the protein level of triadin was increased by twofold in HRCWT or HRCS96A, compared with GFP (Fig. 1B), while the expression levels of other key SR Ca2+ cycling proteins, such as SERCA2a, CSQ, phospholamban, junctin, and RyR, did not show any alternations (Fig. 1A).
Fig. 1.
Effects of human histidine-rich calcium binding protein wild type (HRCWT) and HRCS96A overexpression on intracellular Ca2+ transients. A: quantitative immunoblotting showed total HRC protein was increased by ∼50% in both the human Ad.HRCWT- and Ad.HRCS96A-infected cells, compared with Ad.GFP. There were no alternations in the expression levels of sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), calsequestrin (CSQ), phospholamban (PLN), ryanodine receptor (RyR), and junctin between these groups. B: triadin protein level was increased in HRCWT and HRCS96A, compared with GFP. C: confocal line-scan images of Ca2+ transients under 0.5-Hz field stimulations. D: corresponding time courses of Ca2+ transients. E and F: statistical results of Ca2+ transient amplitude and decay time (T50, time from peak amplitude to 50% decrease of Ca2+ transients). n = 30 to 36 cells from 6–7 hearts for each group. *P < 0.05, compared with GFP. #P < 0.05, compared with HRCWT.
Regulation of intracellular Ca2+ transients by HRCWT and HRCS96A.
To assess the functional significance of human HRC protein expression in rat cardiomyocytes, intracellular Ca2+ transients were recorded during field stimulations. Figure 1, C and D, is a typical example of line-scan images and their corresponding time courses from Ad.GFP-, Ad.HRCWT-, or Ad.HRCS96A-infected cells, respectively. Overexpressing HRCWT resulted in a marked decrease (29%) in Ca2+ transient amplitude, compared with the GFP group, in agreement with earlier studies (8) on mouse HRC overexpression. Interestingly, expression of the human variant, HRCS96A at similar levels as HRCWT, caused a further reduction in the Ca2+ transient amplitude (50% decrease compared with GFP; Fig. 1E). The transient half-decay time (T50) was also delayed 25% with human HRCWT overexpression, while the HRCS96A induced additional decelerated Ca2+ decline (62% compared with GFP; Fig. 1F).
These results on human HRC further confirm previous findings (8) on the functional role of this protein in the heart and suggest that Ala substitution at position Ser96 may increase the inhibitory activity of HRC on SR Ca2+ uptake.
SR Ca2+ load and fractional release.
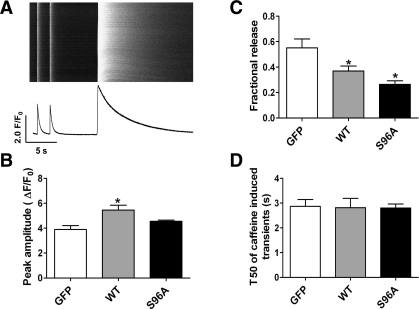
Previous studies (16) have shown that HRC shares structural similarity with CSQ and may play a role as a Ca2+ storage protein. We (8) have also shown that acute overexpression of mouse HRC in rat cardiomyocytes increased SR Ca2+ load. In the present study, HRCWT- or HRCS96A-infected myocytes were subjected to 20 mM caffeine after steady-state 0.5-Hz field stimulations (Fig. 2A). Expression of HRCWT resulted in a dramatic increase in SR Ca2+ load (40%), while expression of HRCS96A did not alter SR Ca2+ load, compared with the GFP control (Fig. 2B). We also determined the ratio of electrically evoked Ca2+ transients to caffeine associated SR Ca2+ load, referred to as fractional release, to assess the fraction of Ca2+ within the SR that responds to electrical stimulation. This fractional Ca2+ release was decreased by 38 and 52% upon HRCWT and HRCS96A expression, respectively (Fig. 2C). Moreover, the half-decay time of caffeine-induced Ca2+ transient did not change among the three groups, which was an index of similar Na+/Ca2+ exchanger activity (Fig. 2D).
Fig. 2.
Effects of human HRCWT and HRCS96A on sarcoplasmic reticulum (SR) Ca2+ storage. A: typical line-scan image and its time course of steady-state Ca2+ transients under 0.5-Hz field stimulation, followed by caffeine-induced SR Ca2+ release. B: statistics of caffeine-induced SR Ca2+ release amplitude. C: field stimulation evoked Ca2+ transient expressed as percentage of caffeine-induced SR Ca2+ store (fractional release) in each group. D: T50 of caffeine-induced SR Ca2+ release. n = 16–18 cells for each group *P < 0.05, compared with GFP. P < 0.05, compared with HRCWT.
Variant of S96A HRC on its interaction with SERCA2 and modulation of SERCA2 function.
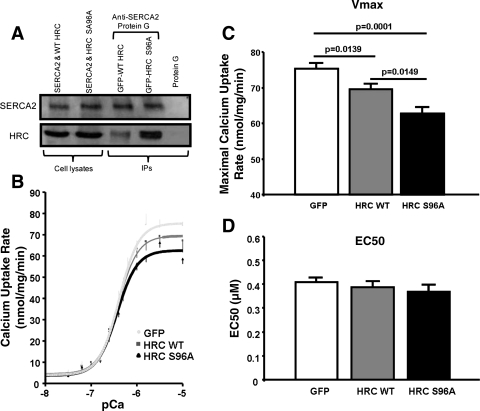
To determine whether HRCS96A and HRCWT have different binding affinities for SERCA2, we generated full-length human HRCWT and HRCS96A and cotransfected HEK293 cells with either of these constructs together with full-length human SERCA2. We then coimmunoprecipitated the SERCA2/HRC complex and observed that the HRC levels were almost twofold higher from the HRCS96A cell lysates, compared with the HRCWT cells, while the levels of SERCA2 were similar between these two groups (Fig. 3A). Furthermore, to assess the effects of HRCWT and HRCS96A on SERCA2 function, oxalate-supported SR Ca-transport rates were determined. HRCWT overexpression resulted in significantly decreased maximal calcium uptake rates compared with GFP, while the HRCS96A caused a further reduction (Fig. 3, B and C). However, the EC50 of SERCA2 for calcium was similar among the three groups (Fig. 3D).
Fig. 3.
Interaction of human HRCWT and HRCS96A with SERCA2 and alterations of SR Ca transport. A: anti-SERCA2 antibody, coupled to protein G-agarose beads, was used for coimmunoprecipitation of HRC from HEK293 cells, coexpressing SERCA2 and HRCWT or HRCS96A. Top: immunoblotting with SERCA2 antibody; bottom: immunoblotting with HRC antibody. B: oxalate-supported SR calcium uptake rates in homogenates from HRCWT- or HRCS96A-infected cells. C and D: maximal calcium uptake rates and the EC50 of SERCA2 for Ca, respectively (n = 4–6 preparations, each in duplicate).
Modulation of spontaneous Ca2+ sparks and resting Ca2+ by HRCWT and HRCS96A.
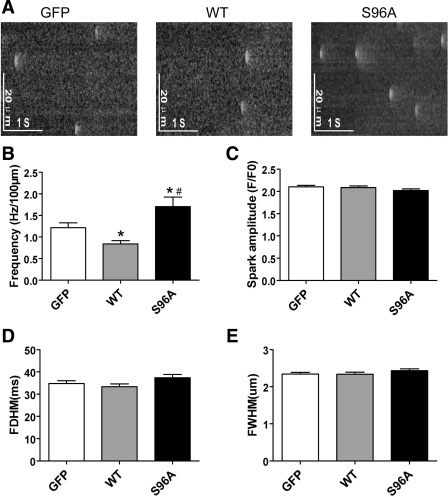
To further understand the effects of HRCS96A on the Ca2+ release properties, we measured spontaneous Ca2+ sparks in resting intact cardiomyocytes. HRCS96A and HRCWT had opposite effects on the occurrence of Ca2+ sparks. HRCWT expression diminished the frequency by 31%, compared with GFP control. In contrast, HRCS96A induced hyperactive Ca2+ sparks, increasing the spark frequency by 40% (Fig. 4, A and B). Pooled data demonstrated unchanged spatial and temporal properties of sparks among the three groups (Fig. 4, C–E).
Fig. 4.
Effects of human HRCWT and HRCS96A on spontaneous Ca2+ sparks properties. A: representative line-scan images of Ca2+ sparks acquired in cells infected with either Ad.GFP, Ad.HRCWT, or Ad.HRCS96A. B–E: cumulative data on Ca2+ spark frequency (B), amplitude (C), full duration at half-maximal (FDHM; D) as well as full width at half maximal (FWHM; E), respectively. n = 177 to 186 sparks for each group. *P < 0.05, compared with GFP. #P < 0.05, compared with HRCWT.
Resting Ca2+ levels were estimated as the ratio of F405/F485 measured by indo-1. HRCS96A elicited elevated resting Ca2+ compared with GFP, while no significant difference was found between HRCWT and GFP (Supplemental Fig. S1; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website).
Spontaneous Ca2+ waves during rhythmic pacing in failing cardiomyocytes.
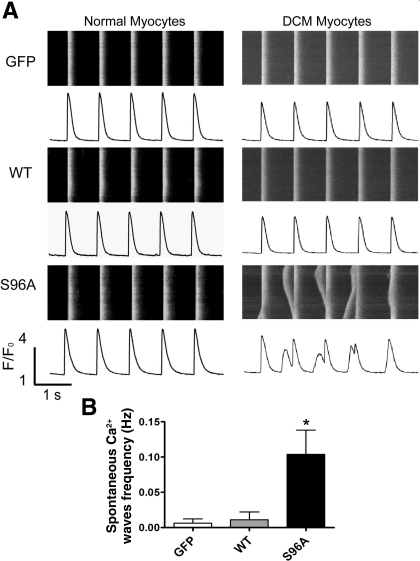
To assess the effects of HRCS96A expression under stress conditions, cardiomyocytes were subjected to 1-Hz electrical field stimulation in the presence of 1 μM isoproterenol. It is interesting to note that almost no spontaneous Ca2+ waves were observed in GFP, HRCWT, or HRCS96A expressing cardiomyocytes under these conditions (Fig. 5A, left).
Fig. 5.
Spontaneous Ca2+ waves during rhythmic pacing in cultured heart failure (HF) myocytes expressing HRCS96A. A: typical line-scan images and corresponding time courses of Ca2+ transients from Ad.GFP, Ad.HRCWT, or Ad.HRCS96A infected normal or HF myocytes, which were subjected to 1-Hz field stimulation in the presence of 1 μM isoproterenol. B: analysis of spontaneous Ca2+ wave frequency in HF cells. n = 18–30 cells for each group. *P < 0.05, compared with GFP.
To further investigate the function of the HRC genetic variant under DCM conditions, we generated a rat model with myocardial infarction-induced heart failure as previously described (1). Heart failure was verified by the increased end-diastolic volume as well as decreased ejection fraction at 8 wk after infarction surgery. Other cardiac morphological and functional parameters, such as left ventricular inner dimension at end diastole and systole, end-systolic volume, and weight-to-body weight ratio, were also determined (Table 1). Cardiomyocytes isolated from the rat failing hearts exhibited an average cell length of 160.9 ± 2.5 μm (n = 223), compared with 121.7 ± 1.3 μm (n = 150) for normal hearts. The normal and heart failure myocytes were infected with Ad.GFP, Ad.HRCWT, or Ad.HRCS96A for 48 h, and the basal Ca2+ cycling properties were determined. Generally, these three viruses gave similar alterations in heart failure cells, as in normal myocytes (Supplemental Fig. S2, A and B). Then the cardiomyocytes were subjected to 1-Hz field stimulation in the presence of 1 μM isoproterenol. Notably, only the HRCS96A cells developed dramatic Ca2+ disturbances, manifested as spontaneous extrasystolic Ca2+ waves, while the GFP and HRCWT myocytes did not (Fig. 5). These oscillations of intracellular Ca2+ transients were considered as the underlying cause of delayed afterdepolarizations, which could induce malignant arrhythmias. Isoproterenol stimulation of the heart failure myocytes resulted in elevations of SR Ca content in the Ad.GFP (28%)-, Ad.HRCWT (16%)-, or Ad.HRCS96A (18%)-infected groups (Supplemental Fig. S2C). However, it is worth noting that the SR Ca content of HRCWT was still significantly higher than GFP as well as HRCS96A. Thus these results indicate that expression of HRCS96A may destabilize the RyR in failing cardiomyocytes, leading to increased SR Ca2+ leak, which may be the underlying mechanism of sudden death in human carriers.
Table 1.
Echocardiographic parameters before and 8 wk after left descending coronary artery ligation
| Before Surgery | 8 wk After Surgery | |
|---|---|---|
| LVIDd, mm | 7.8 ± 0.2 | 10.1 ± 0.1* |
| LVIDs, mm | 4.6 ± 0.2 | 8.8 ± 0.2* |
| EDV, ml | 1.04 ± 0.07 | 2.12 ± 0.06* |
| ESV, ml | 0.24 ± 0.03 | 1.46 ± 0.10* |
| HW/BW, mg/g | 2.9 ± 0.1 | 3.5 ± 0.2* |
| EF, % | 77.3 ± 1.2 | 31.5 ± 3.4* |
Data are means ± SE. LVIDd and LVIDs, left ventricular inner dimension at end diastole and systole; EDV, end-diastolic volume; ESV, end-systolic volume; HW/BW, heart weight-to-body weight ratio; EF, ejection fraction.
P < 0.05, compared with before surgery.
DISCUSSION
Molecular mechanisms of arrhythmia in heart failure.
In end-stage DCM patients, beyond contractile dysfunction, malignant arrhythmias constitute a major risk factor for sudden cardiac death (12). These arrhythmias stem mainly from nonreentry mechanisms, such as early afterdepolarization and delayed afterdepolarization, which are all linked to impaired cardiomyocyte Ca2+ cycling. During heart failure, elevated SR Ca2+ leakage enhances the occurrence of spontaneous Ca2+ sparks and Ca2+ waves (6), which may activate the inward current through NCX and trigger cell membrane depolarization. Actually, the expression levels and activity of several key calcium transport proteins are altered during heart failure, which may be responsible for the SR leak. For example, the protein levels of junctin, triadin, and HRC, which are components of the macromolecular complex regulating RyR activity, are diminished in human failing hearts (8, 10). Accordingly, downregulation of junctin and triadin in genetic knockout animal models is associated with arrhythmia under stress conditions (7, 24). In addition, the elevated NCX expression and function in heart failure may lead to a greater inward current and further depolarization of cell membrane at a given SR Ca2+ release. Indeed, studies in human failing hearts or heart failure animal models have demonstrated the role of NCX in inducing arrhythmias (4, 22). However, the overall remodeling of molecular and cellular networks in heart failure is complicated and the exact mechanisms for sudden cardiac death are not yet entirely clarified.
One of the downregulated SR proteins during heart failure is HRC, a SR luminal protein. Previous studies have provided insights into its regulatory role on SR calcium homeostasis during physiological and pathological conditions. Briefly, adenoviral-mediated acute HRC overexpression in cultured rat cardiomyocytes (8) showed diminished Ca2+ transient amplitude although the SR load was increased. Chronic HRC overexpression in vivo led to cardiac hypertrophy and dilated ventricular remodeling, when the mice were subjected to transverse aortic constriction surgery (11). Thus it was hypothesized that decreased HRC expression during heart failure may provide a compensatory mechanism in the deteriorated Ca2+cycling.
Effects of S96A on HRC function in normal hearts.
The present study explored the mechanisms underlying a recently discovered genetic variant of HRCS96A, which was shown to be associated with arrhythmia in DCM patients (2). Expression of human wild-type HRC reduced Ca2+ transient amplitude and diminished spontaneous Ca2+ spark frequency, while the SR load was increased. This suggests that HRCWT may immobilize SR luminal Ca2+ through its buffering property and inhibit Ca2+ release or it may function as a luminal regulator and stabilize RyR. Compared with HRCWT, human HRCS96A increased the frequency of Ca2+ sparks (nearly twofold) in the setting of reduced Ca2+ transient amplitude and lower SR load. This apparent paradox pointed to the possibility that HRCS96A may enhance RyR activity from the SR luminal side, increasing uncontrolled Ca2+ release and induced Ca2+ instability, supporting the existence of a luminal gating mechanism relatively independent of the cytosolic gating mechanism (26). The modulation of RyR function could be mediated by direct interaction of HRC with triadin, a component of the RyR Ca2+ release complex. Interestingly, the triadin protein levels were significantly increased by either HRCS96A or HRCWT expression. A previous study (20) has shown that acute overexpression of triadin in rat cardiomyocytes is associated with lower SR Ca2+ load and increased open probability of RyRs in lipid bilayers as well as increased frequency of spontaneous Ca2+ sparks, leading to arrhythmias. However, the triadin levels were increased to a similar extent in HRCS96A and HRCWT, suggesting that the aberrant SR Ca release was not mediated by triadin overexpression under our experimental conditions.
In addition, HRCS96A diminished the Ca2+ transient decline time even more than HRCWT, indicating an increased inhibitory effect by the S96A variant on SERCA2a activity. Actually, the reduced Ca reuptake by the SR may be due to increased inhibition of the maximal SR calcium transport rate by HRCS96A, consistent with its stronger binding to SERCA2, compared with HRCWT. Collectively, these phenotypes point to HRC as a nodal point, bridging Ca2+ release and Ca2+ uptake in the heart (3). Furthermore, the S96A substitution may modulate this regulatory role of HRC.
To test if this genetic variant is linked to arrhythmia, we challenged the cardiomyocytes with 1 μM isoproterenol under 1-Hz field stimulation. However, these conditions did not trigger any after contractions in the HRCS96A cells, suggesting that this single amino acid substitution is not sufficient to introduce arrhythmogenesis in the normal heart.
S96A-HRC is linked to arrhythmias in heart failure.
Further studies were carried out in cardiomyocytes from a heart failure rat model. When challenged with 1 μM isoproterenol and 1-Hz field stimulation, the variant HRCS96A was associated with a significantly elevated Ca2+-wave frequency. This may be a plausible mechanism underlying arrhythmias in HRC S96A carriers under heart failure conditions, when their catecholamine levels are elevated. So why does HRCS96A elicit arrhythmias in failing cardiomyocytes and not in normal cells? The hypothesis is that heart failure alters the properties of SR Ca2+ release as evidenced by enhanced Ca2+ spark frequency (Fig. 4B vs. Supplemental Fig. S3A). As a result, the inconspicuous effects of HRCS96A on extra-systolic Ca2+ release during rhythmic pacing in normal cardiomyocytes become dominant under heart failure. Since Ca2+ sparks appear to occur at the majority of the sites for Ca2+ wave initiation (5), the dramatically elevated Ca2+ spark frequency may exceed the stable threshold of RyR local control and elicit arrhythmic Ca2+ waves, which could be the triggering mechanism of arrhythmias in human carriers. Thus the HRCS96A turns out to be a context-sensitive human mutation, which becomes arrhythmogenic in heart failure condition.
However, a limitation of the present study is that expression of human HRCS96A occurred in the presence of endogenous rat HRC, while the heterozygous or homozygous HRCS96A patients have only human HRC present and at a lower overall expression level than our study. Thus it will be valuable to generate knockin HRCS96A mice and obtain more detailed information on how this genetic variant regulates SR Ca2+ cycling in vivo. These mice can be subjected to stress conditions including heart failure, which may closer mimic the conditions of human carriers and elucidate the underlying mechanisms of the observed arrhythmias.
Conclusions.
In summary, we have suggested a link between the human HRC S96A variant and clinical arrhythmias in DCM patients carrying this polymorphism. Expression of human HRCS96A elevated the activity of RyRs in normal as well as failing cardiomyocytes, while it enhanced the disturbances of rhythmic Ca2+ transients under stress conditions in heart failure. These arrhythmogenic events may serve as the underlying mechanism of life-threatening ventricular arrhythmias in human carriers.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-26057, HL-64018, and HL-77101 (to E.G. Kranias), the Leducq Foundation (to E. G. Kranias), the European Community's Seventh Framework Programme FP7/2007-2013 under Grant Agreement No. HEALTH-F2-2009-241526, EUTrigTreat (to E. G. Kranias), and the Chinese National Natural Science Foundation, Major Basic Research Development Programs of China (2007CB512100 and 2011CB809100 to H. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Sarah E. K. Figueira for technical and secretarial assistance.
REFERENCES
- 1. Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation 110: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Arvanitis DA, Sanoudou D, Kolokathis F, Vafiadaki E, Papalouka V, Kontrogianni-Konstantopoulos A, Theodorakis GN, Paraskevaidis IA, Adamopoulos S, Dorn GW, Kremastinos DT, II, Kranias EG. The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur Heart J 29: 2514–2525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol 293: H1581–H1589, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97, Suppl 1: I36–42, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol Cell Physiol 270: C148–C159, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD, Franzini-Armstrong C, Knollmann BC. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci USA 106: 7636–7641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol 287: H1705–H1711, 2004 [DOI] [PubMed] [Google Scholar]
- 9. George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res 93: 531–540, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Muller FU, Schluter KD, Schmitz W, Neumann J. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol 293: H728–H734, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, Park WJ, Dorn GW, Bers DM, II, Kranias EG. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol 40: 653–665, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Grimm W, Maisch B. Sudden cardiac death in dilated cardiomyopathy–therapeutic options. Herz 27: 750–759, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res 101: 802–810, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol 92, Suppl 1: 87–93, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Hochman JS, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation 65: 1446–1450, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC [histidine-rich Ca(2+)-binding protein] and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem 276: 39533–39538, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 75: 401–409, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103: 196–200, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res 96: 651–658, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 98: 1151–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Verkerk AO, Veldkamp MW, Baartscheer A, Schumacher CA, Klopping C, van Ginneken AC, Ravesloot JH. Ionic mechanism of delayed afterdepolarizations in ventricular cells isolated from human end-stage failing hearts. Circulation 104: 2728–2733, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Viatchenko-Karpinski S, Terentyev D, Gyorke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Gyorke S. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res 94: 471–477, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, Wang HS, II, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation 115: 300–309, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272: 23389–23397, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Zhou Q, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Unique action of carvedilol and its novel analogs: suppression of arrhythmogenic store-overload-induced Ca2+ release (SOICR). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.