Abstract
The emerging paradigm for Na+ current in heart failure (HF) is that its transient component (INaT) responsible for the action potential (AP) upstroke is decreased, whereas the late component (INaL) involved in AP plateau is augmented. Here we tested whether Navβ1- and Navβ2-subunits can modulate INaL parameters in normal and failing ventricular cardiomyocytes (VCMs). Chronic HF was produced in nine dogs by multiple sequential coronary artery microembolizations, and six dogs served as a control. INa and APs were measured by the whole cell and perforated patch-clamp in freshly isolated and cultured VCMs, respectively. INaL was augmented with slower decay in HF VCMs compared with normal heart VCMs, and these properties remained unchanged within 5 days of culture. Post-transcriptional silencing SCN1B and SCN2B were achieved by virally delivered short interfering RNA (siRNA) specific to Navβ1 and Navβ2. The delivery and efficiency of siRNA were evaluated by green fluorescent protein expression, by the real-time RT-PCR, and Western blots, respectively. Five days after infection, the levels of mRNA and protein for Navβ1 and Navβ2 were reduced by >80%, but mRNA and protein of Nav1.5, as well as INaT, remained unchanged in HF VCMs. Navβ1-siRNA reduced INaL density and accelerated INaL two-exponential decay, whereas Navβ2-siRNA produced an opposite effect in VCMs from both normal and failing hearts. Physiological importance of the discovered INaL modulation to affect AP shape and duration was illustrated both experimentally and by numerical simulations of a VCM excitation-contraction coupling model. We conclude that in myocytes of normal and failing dog hearts Navβ1 and Navβ2 exhibit oppositely directed modulation of INaL.
Keywords: action potential, in silico simulation
the emerging paradigm for Na+ current (INa) in chronic heart failure (HF) is that its transient component (INaT) responsible for the action potential (AP) upstroke and excitation propagation is decreased, whereas the late component (INaL) involved in AP plateau is augmented (21, 23, 25, 42, 45, 48). Molecular mechanisms of these HF-related INa alterations are still understudied. Numerous studies indicate a possible role of Navβ auxiliary subunits to modulate Na+ channel (NaCh) expression and function (27), but implications of Navβ in INaL modulation have not been studied in detail, especially in HF. Our previous studies in a canine chronic HF model showed that the protein level of the main NaCh isoform expressed in the heart, Nav1.5, underlying INaL (18), is reduced but remains unchanged for Navβ1- and Navβ2-subunits, making these β-subunits relatively upregulated (48). Thus an intriguing possibility could be that differential expression of Nav1.5 and Navβ-subunits in normal and failing hearts can contribute, at least in part, to INaL alterations observed in HF.
In the present study using a combination of experimental and numerical modeling approaches, we tested a hypothesis that Navβ1 and Navβ2 can modulate INaL in ventricular cardiomyocytes (VCMs) isolated from adult failing dog hearts. We performed post-transcriptional silencing of SCN1B and SCN2B genes by the sequence-directed RNA interference using short interfering RNA (siRNA). We took advantage of the established adult dog VCMs culture model that preserves INaL and INaT over 5 days, i.e., sufficient time for gene silencing and membrane protein turnover (18). We found that silencing of SCN1B and SCN2B genes significantly lessens or enhances INaL, respectively. Physiological significance of these β-subunits–related modulations of INaL was illustrated by the concomitant AP changes both experimentally and in silico.
MATERIALS AND METHODS
HF Model
The study conforms to the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health and was approved by the Animal Care and Use Committee of the Henry Ford Health System. Chronic HF that is similar by a vast array of functional and pathophysiological parameters (35) to that in humans was produced in nine dogs by multiple sequential coronary artery microsphere embolizations as previously described (36). Six normal dogs serve as a control. At the time of harvesting the heart (∼3 mo after last embolization), left ventricular (LV) ejection fraction was ∼25%.
Cell Culture and Transfection
Midmyocardial VCMs were enzymatically isolated from the apical LV midmyocardial slices as reported previously (21). The yield of viable rod-shaped, Ca2+-tolerant VCMs varied from 40% to 70%. VCMs were cultured for 5 days [the time frame required for the gene silencing (18)], as described previously (23). During this time, VCMs manifested slightly rounded edges, i.e., well-known characteristics for the cultured adult cardiomyocytes (9, 31). Adenovirus-delivered siRNAs did not further affect morphology of VCMs (Supplemental Fig. S1). We added 10 μl of 5 × 108 virus particle/ml directly to the medium of culture dish containing ∼15 × 104 cells and cultured them further during the next 4 days (5 days total). Effectiveness of transfection was visually monitored in individual cardiomyocytes (Nikon Diaphot 200) by green fluorescent protein (GFP) fluorescence (Fig. 2A, inset, and Supplemental Fig. S1).
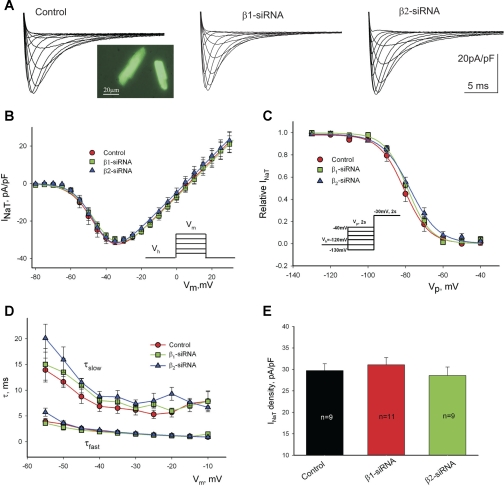
Fig. 2.
Post-transcriptional silencing of Navβ1- or Navβ2-subunits expression does not affect the whole cell transient sodium current, INaT, in patch-clamped cultured left ventricular cardiac myocytes from dogs with chronic HF. A: representative raw traces of INaT were recorded at different membrane potentials (Vm) in cells 5 days after infection with the virus containing control nonsilencing siRNA (control, left), β1-siRNA (middle), or β2-siRNA (right). Image of cells expressing green fluorescent protein (GFP) is shown at left inset. B: average data for peak INaT-voltage relationship obtained in cultured cells for 5 days with virally delivered control siRNA, Navβ1-siRNA, and Navβ2-siRNA. Solid lines show theoretical curves fit to current-voltage (I-V) to evaluate steady-state activation (SSA) parameters (Supplemental Eq. S2, solid dashed and dotted lines, respectively). C: average experimental data of steady-state inactivation (SSI) data points along with the fit to a Boltzmann function (Supplemental Eq. S3, solid, dashed, and dotted lines, respectively). No statistically significant difference was found when theoretical SSI curves were compared (F test). D: decay time constants of INaT (double exponential fit; Supplemental Eq. S1) were evaluated at the different membrane potentials. E: maximum density of INaT remained unchanged in these conditions. Data in B–D represents means ± SE and were pooled from 5 to 8 cells. Detailed statistics for all SSA and SSI parameters of the theoretical fits shown in B and C are presented in Table 1. Voltage-clamp protocols are shown in B and C, insets. Vh, holding potential; Vp, prepulse.
siRNA Design
Double-stranded hairpin siRNAs corresponding to the previously sequenced dog Navβ1 and Navβ2 (GenBank DQ061859 and AY263393, respectively) were designed as recommended (8). The details and sequences of silencing and nonsilencing control siRNAs used in this study are given in Supplemental Table S1. A Blast search for these sequences against the GenBank database was performed and did not reveal matches with any other gene.
Real-time PCR and Western Blot Analysis
To quantify mRNA levels, we used the fluorescence-based (cyber green) kinetic real-time PCR performed using 7500 Fast Real-Time PCR System Applied Biosystems sequence detection system. The relative mRNA abundance expressed as arbitrary units was calculated using the expression levels of all transcripts normalized to GAPDH mRNA. This value was then normalized to mRNA levels measured from VCMs transfected with the nonsilencing siRNA for the appropriate transcript (2−ΔΔCt method). The sets of primers are given in Supplemental Table S2. Membrane protein preparations were obtained from ∼3 × 106 myocytes as previously described (Ref. 48; Supplemental Material for details). Antibody was detected with Western blot chemoluminescence reagent (NEN Life Science Product). Western blots were considered specific if the peptide epitope reduced the band intensity. The resulting images of Western blots were scanned, and relative densities of bands were quantified using SigmaGel or ImageJ software, which includes a background subtraction algorithm. The primary polyclonal anti-Navβ1 and ant-Navβ2 antibodies were obtained from Cell Applications (San Diego, CA) and Alomone Labs (Jerusalem, Israel), respectively. Representative full Western blots with these antibodies are shown in the Supplemental Fig. S2. Polyclonal calsequestrin antibody (Abcam) was used for the protein loading control.
Electrophysiology
INa and AP were recorded using a conventional whole cell and perforated patch-clamp techniques, respectively. The details of a double exponential fit to decay kinetics of the INaT and INaL as well as evaluation of the steady state activation (SSA) and steady state inactivation (SSI) voltage dependency is given in the Supplemental Material (Supplemental Equations S1, S2, and S3, respectively).
Numerical AP Model
To address physiological significance of INaL modulation by β-subunits, we used a modified excitation-contraction (E-C) coupling model of normal and failing canine ventricular myocyte developed previously by Winslow et al. (46). Our model modification to include INaL equations has been recently published (44).
All experimental procedures and numerical modeling details (including numerical values of parameters used in the present study) are available in the Supplemental Material (expanded Supplemental Methods section).
Statistical Analysis
Statistics are reported as means ± SE with n representing the number of cells. Multiple comparisons between treatment groups were made using one-way ANOVA followed by the Bonferroni's post hoc test. The significance of changes of theoretical SSA and SSI fits was evaluated with an F test (StatMost, Data-Most, Salt Lake City, UT) for tabulated values predicted by the model (Supplemental Eqs. S2 and S3) at a confidence level of 0.95. Differences for both experimental data and model predictions were considered statistically significant for P < 0.05.
RESULTS
Efficacy of siRNAs to Silence SCN1B and SCN2B Genes
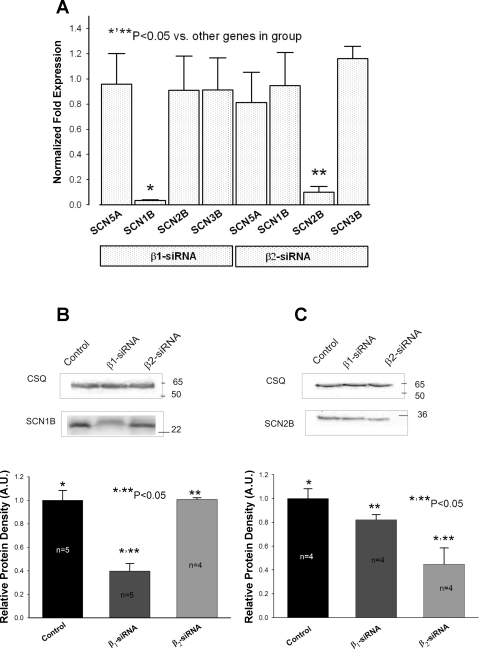
The efficacy of virally delivered siRNAs to silence concomitant SCN1B and SCN2B genes in cultured VCMs was assessed by changes in both mRNA transcript levels and membrane protein levels of Navβ1 and Navβ2 (Fig. 1). The expression of the reporter gene GFP was controlled both visually and by RT-PCR (Fig. 2A and Supplemental Fig. S1). We found that after 4 to 5 days of culture, 100% of cells expressed GFP confirming successful viral transfection. Level of mRNA transcripts encoding Navβ1 and Navβ2 (Fig. 1A) dramatically decreased in VCMs treated with the corresponding siRNA compared with those treated with control nonsilencing siRNA. The expression of the main pore forming cardiac subunit Nav1.5, or other auxiliary β-subunits, not targeted by the siRNA, remained unchanged in these cells (Fig. 1A). Parallel to mRNA, membrane protein levels for both Navβ1 (Fig. 1C) and Navβ2 (Fig. 1D) were also dramatically reduced in response to the siRNA treatment. These results confirm efficacy and specificity of the virally delivered siRNAs to silence SCN1B and SCN2B genes in cultured adult dog VCMs.
Fig. 1.
Efficacy of virally delivered Navβ1-short interfering RNA (siRNA) and Navβ2-siRNA to post-transcriptionally silence SCN1B and SCN2B genes in ventricular cardiac myocytes from dogs with chronic heart failure (HF). A: SCN5A, SCN1B, SCN2B, and SCN3B mRNA expression levels detected by the real-time PCR and normalized to GAPDH mRNA and to the respective mRNA levels detected in cells transfected with nonsilencing siRNA (2−ΔΔCt method) for each gene. *,**Significantly reduced mRNA level compared with other genes in the group (P < 0.05, ANOVA followed by Bonferroni's post hoc test). Data gathered from 4 to 7 transfections. B and C, top: representative Western blot of Navβ1 (SCN1B) and Navβ2 (SCN2B) subunits (bottom bands) and housekeeping protein calsequestrin (CSQ; top bands) for normalization in cells treated with virally delivered siRNA. Control stands for nonsilencing siRNA. B and C, bottom: bar graphs, in arbitrary units (AU), of the relative protein density, which demonstrates dramatic reduction of Navβ1 or Navβ2 in response to the transfection with concomitant siRNA when normalized to the CSQ, and then normalized to the quantity of the immunoreactive protein in cells transfected with nonsilencing siRNA. Membrane proteins were isolated from at least 3 transfections for each condition (n is shown at the bars). Control in B and C stands for the control, nonsilencing siRNA. *,**Protein levels that were significantly reduced compared with the control (P < 0.05, P < 0.05, ANOVA followed by Bonferroni's post hoc test). In this and in the rest of the figures Navβ1 and Navβ2 were abbreviated to β1 and β2, respectively.
Post-transcriptional Silencing of SCN1B and/or SCN2B Genes Does Not Affect INaT
We tested whether reduced expression of Navβ1 or Navβ2 can affect INaT. Figure 2A shows representative family of current traces at different depolarization steps to evaluate the current voltage (I-V) relationship in cells transfected with control nonsilencing siRNA (Fig. 2, left) and with siRNAs designed to silence either SCN1B (Fig. 2, middle) or SCN2B (Fig. 2, right) genes. We found that I-V, SSA (Fig. 2B), SSI, (Fig. 2C), two-exponential decay kinetics (Fig. 2D), and density for INaT (Fig. 2E) remained unchanged in VCMs with silenced SCN1B or SCN2B genes, respectively. Statistical data for SSA and SSI parameters for these experiments are given in Table 1.
Table 1.
Voltage dependence of SSA and SSI for INa in ventricular cardiomyocytes from normal dogs and dogs with chronic heart failure
| SSI Parameters |
SSA Parameters |
|||||
|---|---|---|---|---|---|---|
| Conditions | V1/2A, mV | kA, mV | n | V1/2G, mV | kG, mV | n |
| Normal heart, INaT | ||||||
| Control siRNA | −82.0 ± 0.6 | −5.9 ± 0.8 | 7 | −41.3 ± 0.1 | 5.2 ± 0.4 | 5 |
| Navβ1-siRNA | −81.9 ± 0.7 | −6.2 ± 0.6 | 5 | −40.9 ± 1.2 | 5.9 ± 0.2 | 5 |
| Navβ2-siRNA | −82.1 ± 1.8 | −6.1 ± 1.1 | 5 | −40.7 ± 1.8 | 6.0 ± 0.8 | 5 |
| Failing heart, INaT | ||||||
| Control siRNA | −81.6 ± 1.7 | −6.7 ± 0.7 | 11 | −40.3 ± 1.5 | 6.4 ± 0.4 | 10 |
| Navβ1-siRNA | −82.2 ± 1.4 | −5.9 ± 0.9 | 9 | −40.2 ± 1.1 | 6.1 ± 0.3 | 10 |
| Navβ2-siRNA | −81.9 ± 1.5 | −6.4 ± 0.7 | 11 | −39.9 ± 1.7 | 5.6 ± 0.4 | 9 |
| Normal heart, INaL | ||||||
| Control siRNA | −81.7 ± 0.5 | −6.2 ± 0.5 | 7 | −37.8 ± 1.2 | 6.1 ± 0.8 | 5 |
| Navβ1-siRNA | −80.2 ± 1.6 | −6.4 ± 0.4 | 5 | −40.1 ± 0.8 | 5.3 ± 0.4 | 5 |
| Navβ2-siRNA | −80.9 ± 1.4 | −6.2 ± 0.8 | 5 | −41.0 ± 0.7 | 5.1 ± 0.6 | 5 |
| Failing heart, INaL | ||||||
| Control siRNA | −80.2 ± 0.8 | −6.3 ± 0.5 | 15 | −38.5 ± 1.9 | 5.4 ± 0.7 | 11 |
| Navβ1-siRNA | −82.0 ± 1.2 | −6.1 ± 0.4 | 19 | −39.2 ± 3.1 | 5.6 ± 0.9 | 6 |
| Navβ2-siRNA | −81.6 ± 1.2 | −6.6 ± 0.4 | 16 | −39.1 ± 1.1 | 5.6 ± 0.5 | 12 |
Values are means ± SE; n, cell number. Steady-state inactivation and steady-state activation (SSI and SSA) parameters were obtained from Na+ current (INa) data fit to the Supplemental Eqs. S2 and S3, respectively. We did not find statistically significant differences between parameters within the experimental groups evaluated by the ANOVA followed by Bonferroni's post hoc test. siRNA, short interfering RNA; INaT, transient Na+ component; INaL, late Na+ component; V1/2G and kG, midpoint and the slope of the respective Boltzmann function underlying the NaCh SSA; V1/2A an kA, midpoint and slope of the relationship of SSI parameters.
Modulation of INaL by Navβ1 and Navβ2 Subunits
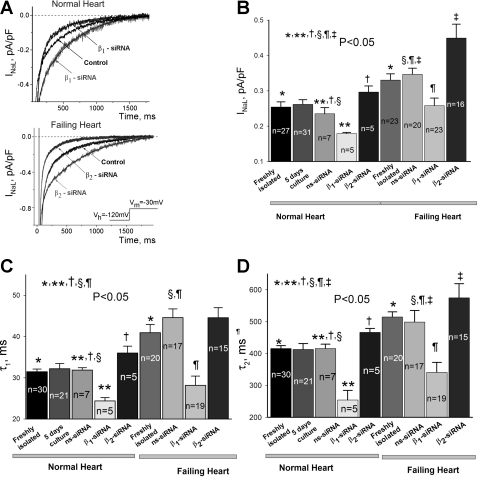
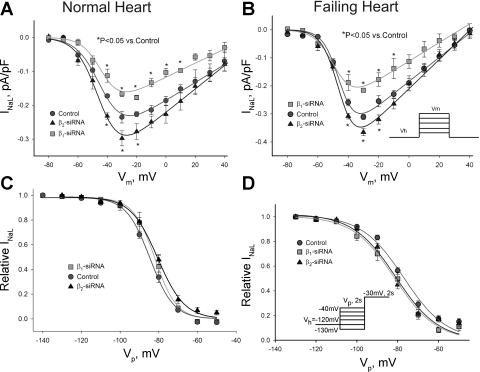
At first we tested whether primary cell culture affects the established paradigm that INaL is augmented and slower in failing compared with normal hearts (25, 42, 45). It is evident from Fig. 3, B–D, that within 5 days culture INaL remained robust in both VCMs from normal and failing hearts, and the paradigm was preserved. Moreover, our infection procedure did not alter INaL parameters in failing VCMs when freshly isolated and treated with virus containing nonsilencing siRNA were compared (Fig. 3, B–D). In contrast with INaT, we found a significant but opposite change in INaL in response to reduced expression of Navβ1 or Navβ2 by the siRNAs (Fig. 3). As evident from the figure, in VCMs isolated from both normal and failing hearts, reduction of Navβ1 expression resulted in INaL decay acceleration and density reduction. In contrast, INaL decay was delayed and density was increased in VCMs with reduced levels of Navβ2. Figure 4A shows I-V relationship for these experiments. Clearly, reduction of Navβ1 or Navβ2 expression reduced or increased density of INaL within the wide range of membrane potentials including potentials corresponding to the AP plateau, respectively. SSA (Fig. 4A) and SSI (Fig. 4B) parameters remained unchanged in these conditions (statistical data are given in Table 1).
Fig. 3.
Navβ1 and Navβ2 subunits modulate late Na+ current (INaL) decay kinetics and density in ventricular cardiac myocytes from both normal dogs and from dogs with chronic heart failure. Shown are results of post-transcriptional silencing of SCN1B and SCN2B genes with the corresponding siRNAs. A: representative raw traces of INaL were recorded in cells from the normal (top) and failing (bottom) hearts cultured for 5 days after transfection with the control nonsilencing siRNA (control) and with Navβ1-siRNA or Navβ2-siRNA (indicated by the arrows), respectively. Exponential fits (Supplemental Eq. S1) are shown by solid lines and are superimposed with the experimental traces. Bottom, inset: voltage protocol. Statistical data for current density (B), and decay time constants (C and D) evaluated by the double exponential fit (Supplemental Eq. S1) in freshly isolated myocytes and after 5 days in culture of normal hearts, in freshly isolated myocytes of failing dog hearts and after 5 days in culture infected by the nonsilencing (ns-siRNA) or silencing siRNA, respectively, are shown. There was no statistically significant difference between INaL parameters when freshly isolated and cultured myocytes were compared for both normal and failing hearts. All INaL parameters remain significantly different when normal and failing hearts were compared for both freshly isolated and cultured cells infected by the ns-siRNA (*, §). Infection of myocytes of normal and failing heart with adenovirus containing Navβ1- or Navβ2-siRNA caused statistically significant (**, ¶) reduction or increase (†, ‡) in the INaL density (B) or decay time constants for both normal and failing hearts, respectively. Statistical significance, P < 0.05, was evaluated by the ANOVA followed by Bonferroni's post hoc test. Data represent means ± SE; cell numbers are indicated at the bars.
Fig. 4.
Post-transcriptional silencing of SCN1B and SCN2B genes does not affect the SSA and SSI of INaL in ventricular cardiac myocytes from normal dogs (NH) and dogs with chronic heart failure (HF). A and B: INaL-voltage relationship in cultured cells from NH and HF transfected with nonsilencing siRNA (control), with Navβ1-siRNA and Navβ2-siRNA, respectively. Solid lines show theoretical curves of SSA (Supplemental Eq. S2). In response to Navβ1-siRNA and Navβ2-siRNA a normalized maximum Na+ conductance (Gmax) was significantly (P < 0.05) reduced (2.08 ± 0.07, n = 5, NH; 3.56 ± 0.03, n = 5, HF) or increased (3.45 ± 0.04, n = 4, NH; 6.56 ± 0.03, n = 12, HF), in comparison with control (2.67 ± 0.05, n = 5, NH; 5.37 ± 0.04 pS/pF, n = 11, HF), respectively. C and D: data points of SSI for INaL along with the Boltzmann function fit (solid lines, Supplemental Eq. S3) for NH and HF, respectively. Detailed statistical comparison of all SSA and SSI parameters are given in Table 1. Data represent means ± SE pooled from 5 to 8 cells. *Statistical significant difference (P < 0.05, ANOVA followed by Bonferroni's post hoc test) compared with control in A. B and D insets shows voltage-clamp protocols.
Navβ1 and Navβ2 Subunits Modulate AP Duration
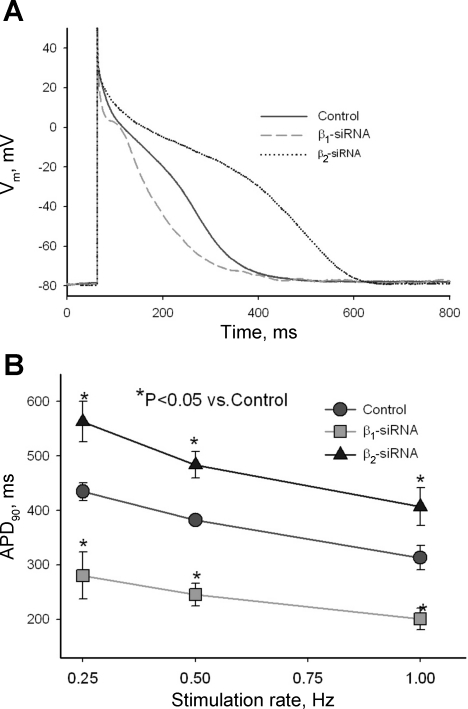
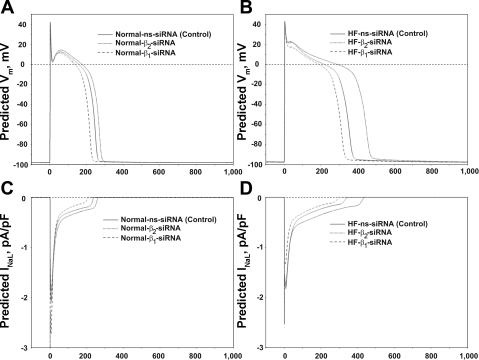
We recorded APs in response to different stimulation rates (0.25, 0.5, and 1.0 Hz) in VCMs treated with control nonsilencing siRNA and with siRNAs directed to silence either SCN1B or SCN2B genes. Figure 5A shows representative traces of APs recorded in these conditions. Statistical data on APD modulation by SCN1B and SCN2B genes is shown in Fig. 5B. Reduced expression of Navβ1 caused significant shortening of APD at all pacing rates tested. In contrast, reduction of Navβ2 expression significantly increased APD. Because ion currents change during primary culture of adult VCMs (30, 31) (see Study Limitations for inherent constrains of electrophysiological studies of the cultured VCMs), the effect of INaL modulation by the β-subunits on APs of the ventricular myocytes of the failing heart needs to be further evaluated. For this purpose we used E-C coupling model of canine ventricular myocytes (46) with new formulations of INaL (44), in which we included experimentally measured INaL modulation by β-subunits (the model details are given in the Supplement Material). Figure 6 shows simulated AP traces and simultaneous INaL traces in control and after silencing of Navβ1 or Navβ2 subunits in VCMs of both normal and failing hearts at a stimulation rate of 1.0 Hz, respectively. Simulated APs become notably shortened or prolonged in response to INaL changes caused by the reduction of the Navβ1 or Navβ2 subunit expression, respectively.
Fig. 5.
Effects of post-transcriptional silencing of Navβ1 and Navβ2 subunits on action potential (AP) of cardiac myocytes from dogs with chronic HF. A: superimposed typical AP traces recorded at stimulation rate 0.25 Hz in cells transfected with control nonsilencing siRNA (control), with Navβ1-siRNA and Navβ2-siRNA, respectively. B: statistical data for AP duration at 90% (APD90) of repolarization assessed at 3 different stimulation rates of 0.25, 0.5, and 1.0 Hz. Data represent means ± SE pooled from 5 to 15 cells obtained from at least 3 different transfections after 5 days in culture. *Statistical significant difference (P < 0.05, ANOVA followed by Bonferroni's post hoc test) compared with control.
Fig. 6.
Numerical model simulations of dog ventricular myocyte APs (A and B) and simultaneous INaL dynamics (C and D) from normal and failing hearts (normal vs. HF, respectively) in control and in response to SCN1B or SCN2B gene silencing. All simulations presented in the figure are based on the respective changes of INaL parameters measured experimentally by patch-clamp (see Supplemental Material for the modeling details).
DISCUSSION
Result Summary and Possible Importance
For the first time, we report a highly effective RNA interference-based method for post-transcriptional silencing SNCB1 and/or SNCB2 genes in cultured adult cardiac myocytes isolated from the left ventricles of normal dogs and dogs with chronic HF. The Navβ1-siRNA and Navβ2-siRNA used in this study were virally delivered and caused ∼80% reduction of expression of SNCB1 and/or SNCB2 genes assessed by the mRNA transcript levels and the membrane protein content. The whole cell transient current, INaT, was not affected by the reduced expression of either Navβ1 or Navβ2 but caused a significant loss-of-function or gain-of-function of INaL, respectively. Post-transcriptional SNCB1 and/or SNCB2 gene silencing effects on INaL were similar for normal and failing hearts. Our experiments and in silico simulations predict notable AP duration changes within modulation range of INaL by both β-subunits measured experimentally, thus indicating that this modulation could be a physiologically important mechanism of AP regulation in both normal and failing myocardium (Figs. 5 and 6). We also simulated INaL dynamics underlying the AP changes (Fig. 6, C and D). Please note that simulated INaL profile during APs well corresponds to the respective experimentally measured INaL profile reported previously in canine VCMs (3).
Many prior studies have demonstrated an important role of INaL. It was shown that INaL reduction by 50–60% by tetrodotoxin (TTX), saxitoxin, counter-current injection, or a specific INaL blocker ranolazine, i.e., in the range similar to Navβ1-siRNA-related modulation (Figs. 3 and 4), causes significant physiological effects in freshly isolated human and dog HF VCMs (21, 41, 42, 44), specifically 1) reduced AP duration and duration variability, 2) eliminated EADs, 3) improved single cell contractility, and 4) reduced diastolic Ca2+ accumulation. Therefore, in this regard overall INaL decrease produced by silencing SCN1B reported in the present study is expected to be physiological significant. However, silencing SCN2B gene causes overall INaL increase, i.e., similar to that reported for HF (Figs. 3, 4, and 5; Refs. 41, 44, and 45). Therefore, targeting SCN1B but not SCN2B gene with the aim to modulate INaL in HF is likely to be considered as a plausible target for the future gene therapy.
Cultured Adult Cardiomyocytes as a Model to Study NaCh Regulation
Primary cultures of cardiomyocytes are a useful model for broad reasons: 1) it produces a substantial amount of viable homogeneous population of terminally differentiated cells free of extracellular matrix and neurohumoral factors and 2) it is suitable for patch-clamp experiments and molecular biology approaches. As we have recently reported, NaCh expression underlying both INaT and INaL remains robust over 5 days in culture in VCMs of the normal dog (18). This time frame is sufficient to silence genes of interest causing a significant NaCh protein level reduction (18). In the present study, we show that HF-related differences in INaL remained during the primary VCMs culture. Indeed INaL density was larger (Fig. 3B) and inactivation kinetics was slower (Fig. 3, C and D) in VCMs from failing compared with normal heart. Therefore, the primary culture of adult dog cardiomyocytes is a suitable model to study molecular mechanisms of INaL regulation in normal and failing VCMs.
Molecular Mechanisms of INaL Modulation by Navβ1 and Navβ2 Subunits
The effects of the β-subunits on Nav1.5 and other Navs were extensively studied in the past both in the heterologous expression systems and, recently, in the native heart tissues although with contrasting results (7, 14, 19, 27). Our study was not aimed to solve these controversies but rather to assess whether Navβ1 or Navβ2 modulates INaL in the native NaCh environment of VCMs isolated from both normal and failing hearts. Below we discuss three important questions of the molecular mechanisms of INaL modulation by the β-subunits.
Which Nav underlies INaL in the heart and, therefore, is targeted by the SCN1B and SCN2B silencing?
Using antisense morpholino oligonucleotides we have recently reported that Nav1.5 is the major (at least 60%) contributor to the INaL in adult cardiac myocytes from normal dogs (18). Although the major target in our study is likely Nav1.5, the neuronal NaCh isoforms (Nav1.1, Nav1.2, Nav1.3) reported in the heart (11, 15, 16) may also contribute to INaL. Indeed it has been shown that low doses of TTX, which preferentially blocks neuronal NaCh, reduce AP duration in cardiac Purkinje fibers (6) and block the persistent non-inactivating INaP current in the rat (13, 37). Single channel studies revealed multiple conductance levels of NaCh also pointing to a possible contribution of the different (neuronal) NaCh isoforms to the INaL in the rat (29) and in humans (our unpublished data). Recently it has been reported that the INaP, produced by Nav1.1, can be reduced (2) or increased by the Navβ1 (39).
2) What are manifestations of INaL modulation by SCN1B and SCN2B?
A recent study has shown that SCN1B gene silencing caused increased INaP as a result of increased expression of SCN5A in the SCN1B-null mice (14). The biophysical properties of INaP recorded in the SCB1-null mice, however, have not been fully examined, which makes it difficult to compare them with well-known properties of the slowly inactivating INaL reported in a variety of species including humans, dogs, guinea pig, rabbit, and rat (26). To explain the augmented INaP in SCN1B-null mice, Lopez-Santiago et al. (14) suggest a transcriptional regulation mechanism. Their suggestion is based on the fact that in the absence of Navβ1 the transcription/translation of SCN5A in these hearts appears to be deregulated leading to the Nav1.5 protein upregulation. Contrary to these results, another recent study has shown that the post-transcriptional silencing of SCN1B by siRNA reduces mRNA and protein levels of Nav1.5 in neonatal rat VCMs (7). Accordingly, the INaL component is expected to be decreased but has not been assessed by Deschenes et al. (7). Different results could reflect acute (siRNA) versus long-term (SCN1B-null mice) effects, compensatory effects, or, possibly, age effects (newborn vs. older animals), as well as species-dependent effects. Furthermore, regulation of NaCh can also depend on the state of disease. Indeed, recently it has been shown that Nav1.5 expression and inactivation modulation by the Navβ1 is dependent on the state of tubulin cytoskeleton polymerization (4), which is reportedly increased in failing human hearts and in HF animal models (12).
3) How do Navβ1 or Navβ2 subunit interactions with NaCh produce observed INaL changes?
Direct interaction between cytoplasmic COOH terminus (CT) domain of Nav1.1 with Navβ1 and Navβ3 has recently been demonstrated (39) and thus offers possible molecular mechanisms for INaL modulation by Navβ1 found here (if similar interactions exist for Nav1.5). The role of the CT to regulate Nav1.5 inactivation has been recently suggested via the Ca2+-calmodulin-dependent interaction with the III-IV linker, responsible for the initial fast inactivation (1, 33), and has been elucidated for INaL regulation (20). It has been also suggested that the Navβ1 subunit modulates Nav1.5 inactivation via interaction with the microtubule cytoskeleton (4).
Although the role of Navβ2 for the function of Nav still is not clear, the present study is the first to our knowledge to demonstrate Navβ2 effect on INaL in the native cardiac cells. Initially, Navβ2 has been implemented in intercellular adhesion and recruitment of a cytoskeleton protein ankyrin to the plasma membrane at sites to cell-to-cell contact (17). Accordingly, a direct role of Navβ2 subunit on the gating of Nav was not suggested. NaCh protein has direct attachments to the submembrane cytoskeleton via ankyrin (1) and can be related to the cytoskeleton-dependent effects on its gating (5, 43). Recently, we reported that heterologously expressed Navβ2 did not reveal any effect on INaL produced by human Nav1.5 (19) that can be explained by a lack of coassembly of the Nav1.5 α-subunit with the Navβ2 subunit (49, 50). Accordingly, for the first time here we report evidence of Navβ2 to modulate INaL in adult dog VCMs.
The NaCh protein consists of the main pore forming α-subunit surrounded by the covalently bound Navβ1 and Navβ3 as well as disulfide-linked Navβ2 and Navβ4 subunits (27). The Navβ3 and Navβ4 subunits are highly homologous with their counterparts Navβ1 and Navβ2, respectively, but may have a distinct and opposite function for NaCh gating (2, 10, 28, 32, 47). Therefore, besides the aforementioned direct effects on NaCh, the deprivation of Navβ1 or Navβ2 subunits from this multiprotein complex may enhance effects of their counterparts. Indeed, heterologous coexpression of Navβ4 with neuronal Nav1.1 can induce INaP (2) or can modulate Nav1.5-related INaL (28). The same idea could be extended for Navβ3 subunit, which may lead to faster inactivation of NaCh (10, 32), and contribute to Navβ1 silencing effects on INaL in myocytes from HF reported here. Therefore, elucidation of interactive effects of Navβ3 and Navβ4 on INaL in failing heart merits consideration in future studies.
Physiological Significance
As mentioned above, INaL contribution into HF mechanisms has been demonstrated in experiments where correction of INaL in failing cardiomyocytes resulted in 1) rescue of normal repolarization (Figs. 5 and 6), 2) decrease beat-to-beat APD variability, and 3) improvement of Ca2+ handling and contractility (22, 25, 41, 42, 44). Accordingly, INaL has emerged as a novel target for cardioprotection to treat the failing heart (24, 26, 34). The fact that INaT is decreased but INaL is increased in HF (21, 23, 25, 42, 45, 48) suggests that not all NaCh must be equally targeted. Class I antiarrhythmic drugs that block INaT are proarrhythmic in HF because they slow conduction, thus worsening conduction problems (38), and facilitate the development of re-entry. Accordingly, new strategies for treatment must be taken into account considering the aforementioned constraint: the new type of smart drug or modulator should preferentially reduce INaL but not affect INaT (40). Therefore, the results of the present study suggest that silencing SCN1B but not SCN2B could be a plausible mechanism to modulate INaL in HF with the aim to improve both contractility and rhythm.
Study Limitations
Besides being a suitable model for the patch-clamp and molecular biology studies discussed above, the primary culture of adult VCMs has its limitations. A major limitation arises from the notion that over the culturing period VCMs undergo changes in their morphology and protein expression levels including those of ion channels (31) that, in turn, change their AP shape and duration. Another possibility to affect AP determinants is that post-transcriptional silencing of Navβ may affect other off-target ion channel expression. We found L-type Ca2+ channels remained unaffected (Supplemental Fig. S3). On the other hand, recently it has been shown that siRNA induced silencing of Navβ1 caused reduction in expression of some potassium channels, which may affect repolarization process, besides INaL modulation (7). Accordingly, although cell culture well suits the purpose to specifically test modulation of INaL by Navβ1 and Navβ2 subunits (luckily the density of INaL does not change in control cell cultures), our results in cultured VCMs with regard to AP changes (Fig. 5) should be treated with caution. For example, although the direction of change of AP duration in the cultured cells is the same as expected from the physiological role of INaL to support the AP plateau given unaltered ICaL (Supplemental Fig. S3), the specific values of the change may not necessarily report those in intact myocardium. Therefore, our respective experimental data with APs in cell culture play only a supportive role in the present study to illustrate that cultured cells maintain electrical excitability due to the robust INaT and still generate cardiac-like (yet changed) APs. To overcome this limitation and to illustrate physiological relevance of INaL modulation by Navβ1 and Navβ2, we used an E-C coupling model (Fig. 6) to simulate respective AP changes in both normal and failing VCMs. Another limitation of the study is that roles of Navβ3 and Navβ4 subunits in INaL modulation were not evaluated. Elucidation of interactive effects of Navβ3 and Navβ4 on INaL awaits future studies.
Conclusion
Based on our results with a highly effective RNA interference-based method for post-transcriptional silencing SNCB1 and/or SNCB2 genes in intact ventricular cardiac myocytes, we conclude that Navβ1 and Navβ2 exhibit oppositely directed modulation of INaL in ventricular myocytes of normal and failing dog hearts. We illustrate importance of this modulation to affect AP duration and shape both experimentally (Fig. 5) and in silico (Fig. 6).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-53819 and HL-074238, by a grant-in-aid from the American Heart Association (0350472Z; to A. Undrovinas), and, in part, by the Intramural Research Program of the National Institute on Aging (to V. A. Maltsev; the numerical modeling part).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc Med 15: 35–40, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci 29: 2027–2042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110: 904–910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casini S, Tan HL, Demirayak I, Remme CA, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken AC, Veldkamp MW. Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 85: 691–700, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Abnormal cardiac Na+ channel properties and QT heart rate adaptation in neonatal ankyrinB knockout mice. Circ Res 86: 441–447, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol Heart Circ Physiol 236: H561–H567, 1979 [DOI] [PubMed] [Google Scholar]
- 7. Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol 45: 336–346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15: 188–200, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol Heart Circ Physiol 265: H747–H754, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Fahmi AI, Patel M, Stevens EB, Fowden AL, John JE, 3rd, Lee K, Pinnock R, Morgan K, Jackson AP, Vandenberg JI. The sodium channel beta-subunit SCN3b modulates the kinetics of SCN5a and is expressed heterogeneously in sheep heart. J Physiol 537: 693–700, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haufe V, Cordeiro JM, Zimmer T, Wu YS, Schiccitano S, Benndorf K, Dumaine R. Contribution of neuronal sodium channels to the cardiac fast sodium current INa is greater in dog heart Purkinje fibers than in ventricles. Cardiovasc Res 65: 117–127, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res 45: 273–278, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Ju YK, Saint DA, Gage PW. Inactivation-resistant channels underlying the persistent sodium current in rat ventricular myocytes. Proc Biol Sci 256: 163–168, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SK, Lopatin AN, Isom LL. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol 43: 636–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maier S, Westenbroek R, Yu FH, Vien T, Scheuer T, Catterall WA. Functional expression and localization of brain Nav1.1 sodium channel α-subunits in single cardiac myocytes (Abstract). Circulation 104: II-309, 2001 [Google Scholar]
- 16. Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci USA 99: 4073–4078, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem 275: 11383–11388, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5-antisense inhibition. Am J Physiol Heart Circ Physiol 295: H667–H676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its β1 subunit. J Physiol Sci 59: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of the late sodium current by Ca2+, calmodulin, and CaMKI I in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol 294: H1597–H1608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maltsev VA, Sabbah HN, Higgins RSD, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 98: 2545–2552, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Maltsev VA, Sabbah HN, Tanimura M, Lesch M, Goldstein S, Undrovinas AI. Relationship between action potential, contraction-relaxation pattern, and intracellular Ca2+ transient in cardiomyocytes of dogs with chronic heart failure. Cell Mol Life Sci 54: 597–605, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maltsev VA, Sabbah HN, Undrovinas AI. Down-regulation of sodium current in chronic heart failure: effects of long-term therapy with carvedilol. Cell Mol Life Sci 59: 1561–1568, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maltsev VA, Sabbah HN, Undrovinas AI. Late sodium current is a novel target for amiodarone: studies in failing human myocardium. J Mol Cell Cardiol 33: 923–932, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur J Heart Fail 9: 219–227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maltsev VA, Undrovinas A. Late sodium current in failing heart: Friend or foe? Prog Biophys Mol Biol 96: 421–451, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res 67: 448–458, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation 116: 134–142, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milburn T, Saint DA, Chung SH. The temperature dependence of conductance of the sodium channel: implications for mechanisms of ion permeation. Receptors Channels 3: 201–211, 1995 [PubMed] [Google Scholar]
- 30. Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflügers Arch 431: 814–827, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res 39: 280–300, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. β3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci USA 97: 2308–2313, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Motoike HK, Liu H, Glaaser IW, Yang AS, Tateyama M, Kass RS. The Na+ channel inactivation gate is a molecular complex: a novel role of the COOH-terminal domain. J Gen Physiol 123: 155–165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart 92: iv1–iv5, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabbah HN, Goldberg AD, Schoels W, Kono T, Webb C, Brachmann J, Goldstein S. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: relation to haemodynamics and sympathoadrenergic activation. Eur Heart J 13: 1562–1572, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. J Physiol 453: 219–231, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation 112: 2517–2529, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci 24: 10022–10034, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Undrovinas A, Maltsev VA. Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart. Cardiovasc Hematol Agents Med Chem 6: 348–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 17: S169–S177, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci 55: 494–505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Undrovinas AI, Shander GS, Makielski JC. Cytoskeleton modulates gating of voltage-dependent sodium channel in heart. Am J Physiol Heart Circ Physiol 269: H203–H214, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci 60: 245–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38: 475–483, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Winslow RL, Rice J, Jafri S, Marban E, O′Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure. II: Model studies. Circ Res 84: 571–586, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. J Neurosci 23: 7577–7585, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zicha S, Maltsev VA, Nattel S, Sabbah HN, Undrovinas AI. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure. J Mol Cell Cardiol 37: 91–100, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zimmer T, Benndorf K. The intracellular domain of the β2 subunit modulates the gating of cardiac Nav1.5 channels. Biophys J 92: 3885–3892, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zimmer T, Biskup C, Bollensdorff C, Benndorf K. The β1 subunit but not the β2 subunit colocalizes with the human heart Na+ channel (hH1) already within the endoplasmic reticulum. J Membr Biol 186: 13–21, 2002 [DOI] [PubMed] [Google Scholar]