Abstract
Atrial-selective inhibition of cardiac Na+ channel current (INa) and INa-dependent parameters has been shown to contribute to the safe and effective management of atrial fibrillation. The present study examined the basis for the atrial-selective actions of ranolazine. Whole cell INa was recorded at 15°C in canine atrial and ventricular myocytes and in human embryonic kidney (HEK)-293 cells expressing SCN5A. Tonic block was negligible at holding potentials from −140 to −100 mV, suggesting minimal drug interactions with the closed state. Trains of 40 pulses were elicited over a range of holding potentials to determine use-dependent block. Guarded receptor formalism was used to analyze the development of block during pulse trains. Use-dependent block by ranolazine increased at more depolarized holding potentials, consistent with an interaction of the drug with either preopen or inactivated states, but was unaffected by longer pulse durations between 5 and 200 ms, suggesting a weak interaction with the inactivated state. Block was significantly increased at shorter diastolic intervals between 20 and 200 ms. Responses in atrial and ventricular myocytes and in HEK-293 cells displayed a similar pattern. Ranolazine is an open state blocker that unbinds from closed Na+ channels unusually fast but is trapped in the inactivated state. Kinetic rates of ranolazine interactions with different states of atrial and ventricular Na+ channels were similar. Our data suggest that the atrial selectivity of ranolazine is due to a more negative steady-state inactivation curve, less negative resting membrane potential, and shorter diastolic intervals in atrial cells compared with ventricular cells at rapid rates.
Keywords: ion channels, pharmacology, arrhythmia, antiarrhythmic drugs
the antianginal drug ranolazine has been shown to exert potent atrial-selective use-dependent inhibition of Na+ channel current (INa)-dependent parameters and thus to suppress atrial fibrillation (AF) in experimental models of the disease (1, 2, 4–6). Ranolazine blocks both peak and late cardiac INa, and this inhibition is significantly reduced by site-directed mutagenesis of a single residue (F1760A) known to contribute critically to the binding of local anesthetics to the Na+ channel (10). Thus, ranolazine appears to bind to human cardiac Na+ channels at the local anesthetic receptor within the pore of the channel (10). The drug shows greater potency for inhibition of the slowly inactivating component of cardiac INa (late INa) in canine ventricular myocytes (28) and in human embryonic kidney (HEK)-293 cells expressing the ΔKPQ mutant channel (10), R1623Q LQTS mutant channel (22), or inactivation-deficient mutants of neuronal and skeletal muscle Na+ channels (29). The mechanism underlying the atrial selectivity of ranolazine to inhibit INa remains unknown.
The following are among the mechanisms proposed to explain the atrial-selective depression of INa and INa-dependent parameters by ranolazine: 1) differences in the affinity and/or kinetics of binding/unbinding of ranolazine between atrial and ventricular Na+ channels, 2) differences in the voltage dependence of the steady-state activation and inactivation of atrial compared with ventricular Na+ channels, and 3) differences in the resting membrane potential and action potential shape in atrial compared with ventricular cells. Considerable evidence has been advanced in support of the last two mechanisms. A more negative position of the steady-state inactivation curve of atrial compared with ventricular Na+ channels is known to give rise to a reduced availability of Na+ channels in atrial versus ventricular cells (6). A reduced availability of Na+ channels in atrial cells compared with ventricular cells also results from the more positive resting membrane potential of atrial cells. Atrial cells also exhibit a much slower phase 3 repolarization, resulting in marked abbreviation or elimination of the diastolic interval at rapid rates of activation. At very fast rates, the repolarization in atrial tissue, but not ventricular tissue, is incomplete, resulting in a more depolarized takeoff potential.
Relatively little is known about the interaction of ranolazine with different states of the Na+ channel, and what is known has been the subject of debate. Based on its effect to shift the steady-state inactivation curve of late INa, ranolazine was initially classified as an inactivated state blocker (22, 28) but was later shown to preferentially block the open state of the Na+ channel and to moderately interact with the inactivated state (29). Block of the open state of the Na+ channel is thought to be required for a drug to preferentially block the late component of INa (8), which argues against the possibility that ranolazine is an inactivated state blocker.
The present study was designed to test the hypothesis that differences in the interactions of ranolazine with atrial and ventricular Na+ channels contribute to its atrial selectivity. Interactions of ranolazine with the closed, inactivated, and open states of cardiac Na+ channels were examined in atrial and ventricular canine myocytes, and the corresponding binding and unbinding rates were calculated.
Our results indicate that ranolazine is an open state blocker that unbinds from Na+ channels at resting potentials unusually fast but is trapped in the inactivated state. Ranolazine binding and unbinding rates for open, closed, and inactivated states were found to be similar in atrial and ventricular myocytes. These findings rule out the possibility that differences in the binding site properties contribute prominently to the atrial-selective actions of ranolazine.
MATERIALS AND METHODS
Cell dissociation.
Adult mongrel dogs (21–30 kg) of either sex were given heparin sodium (200 IU/kg) and anesthetized with pentobarbital sodium (35mg/kg iv), and hearts quickly placed in cardioplegic solution. Single myocytes were obtained by enzymatic dissociation from a section of the ventricular free wall supplied by the left circumflex coronary artery (31) or the atrium perfused through the ostium of the right coronary artery. This investigation conformed with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee.
Ruptured-patch voltage clamp.
Whole cell cardiac INa was recorded in low external Na+ at 15°C in canine atrial and ventricular myocytes and in a cell line expressing the α-subunit of SCN5A. Internal Na+ was varied such that the Na+ reversal potential was either 35 or −60 mV. Ranolazine use dependence was measured during trains of 40 pulses to −30 mV after a rest of 15 s at a holding potential of −140 mV. To evaluate the effects of rate, the pulse duration was kept constant, and the diastolic interval was either 150, 70, 50, or 20 ms. Conversely, the influence of pulse duration was evaluated at constant diastolic interval and pulse durations of 5, 20, or 200 ms. Tonic block was determined during a single variable pulse or as the block that develops with the first pulse of a train after 15 s at holding potential. Currents were normalized to control currents. The influence of holding potential on tonic and use-dependent block was determined at −100, −110, or −120 mV in the presence of 10 μM ranolazine using trains of 50 ms steps to −30 mV separated by 250 ms. Recovery from tonic block was measured using standard dual-pulse protocols. Recovery from steady-state use-dependent block induced by 40 μM ranolazine was measured after a train of 20 ms steps to −30 mV separated by 30 ms at a holding voltage of −140 mV.
INa recording in HEK-293 cells.
Recovery of INa after a single step to −20 mV for 1,000 ms was measured in HEK-293 cells stably expressing the α-subunit of SCN5A at 16°C and at holding potential of −140 mV. Recovery intervals ranged between 1 and 10,000 ms (1, 2, 3, 4, 5, 10, 20, 50, 100, 200, 500, 1,000, 2,000, 5,000, and 10,000 ms).
Voltage-clamp solutions.
The external solution used in whole cell ruptured-patch voltage-clamp experiments in myocytes and HEK-293 cells contained (in mM) 2 CaCl2, 10 glucose, 1 MgCl2, 40 NaCl, 120 N-methyl-d-glucamine, and 10 HEPES, with pH adjusted to 7.4 with HCl. CdCl2 (300 μM) was added to the external solution to block Ca2+ current and to partially reduce INa. The pipette solution contained (in mM) 1 MgCl2, 5 NaCl, 145 Cs-aspartate, 10 HEPES, 5 EGTA, and 5 MgATP, with pH adjusted to 7.1 with CsOH.
The external solution for voltage-clamp experiments in HEK-293 cells used to produce outward currents at −30 mV contained (in mM) 2 CaCl2, 10 glucose, 1 MgCl2, 5 NaCl, 120 N-methyl-d-glucamine, 20 tetraethylammonium chloride, and 10 HEPES, with pH adjusted to 7.4 with HCl. CdCl2 (300 μM) was added to the external solution to block Ca2+ current and to partially reduce INa. The pipette solution contained (in mM) 1 MgCl2, 100 NaF, 30 NaCl, 10 HEPES, 5 EGTA, and 5 MgATP, with pH adjusted to 7.1 with CsOH.
Statistical analysis of experimental data.
Statistical analysis was performed using a paired Student's t-test and one-way repeated-measures or multiple-comparison ANOVA followed by Bonferroni's test, as appropriate. All data are expressed as means ± SD. Statistical significance was assumed at P < 0.05.
Theoretical data analysis.
Each train of decreasing INa peaks was normalized to the first pulse and fit with a single exponential function of the following form: INa(n) = bss + (1 − bss) × e(−λ × n), where n is the pulse number, bss is the steady-state block, and λ is the uptake rate (in pulses−1). The quality of the exponential fit is shown in the Supplemental Material in Supplemental Figs. S1 and S2.1 The dependence of λ on pulse duration, diastolic interval, and ranolazine concentration was analyzed using guarded receptor formalism (see below) and λ and bss, obtained using single-exponential fits to experimental data.
Kinetic binding and unbinding rates of ranolazine with closed, open, and inactivated states of the Na+ channel were calculated using the guarded receptor model (27). We chose a holding potential of −140 mV and a test potential of −30 mV to assure that rates of channel transitions among states were much faster than drug binding/unbinding, in accordance with guarded receptor requirements (24). The durations of voltage steps (Ts) and diastolic intervals (Tr) during these trains, as well as the ranolazine concentration (D), were selected to attain considerable bss at the end of each train while keeping λ at least 0.3 or smaller to ensure robust parameter estimation. According to guarded receptor formalism, λ linearly depends on Ts, Tr, and D (11), as follows:
where the subscripts c, i, and o indicate the kinetic binding rate (k) and unbinding rate (u) for ranolazine interactions with the closed, inactivated, and open states, respectively. The time that the channel spends in the open state (to) corresponds to the mean open time at the step potential, and it cannot be varied in our protocol. Supplemental Figures S3 and S4 show visual illustrations of the linear dependence of λ values on Tr and Ts (consistency check).
This expression was rearranged to obtain the dependence of λ on five variables: (Tr × D), Tr, (Ts × D), Ts, and D, as follows:
The multivariate linear regression procedure was implemented in MathCad using a matrix algorhythm (9). Results were verified with SigmaStat 3.5. Using this regression, we obtained five slope factors and a constant together with corresponding SDs and coefficients of linear regression for each parameter. Slope factors corresponded to the values of kc, uc, ki, ui, and ko × to, whereas the constant gave values of uo × to. If we assume that both atrial and ventricular Na+ currents have the same mean open time (inactivation time constant) of to = 1 ms, all six kinetic rate constants can be calculated. The results of this analysis are shown in Table 1.
Table 1.
Kinetic rates of ranolazine interactions with three states of the fast Na+ channel obtained with multivariate regression analysis of the nonaveraged data set
| Atrial Channels | Ventricular Channels | P | |
|---|---|---|---|
| Open channel state | |||
| ko, μM−1 · s−1 | 2.97 ± 0.15 | 3.22 ± 0.22 | 0.349 |
| uo, s−1 | 5.35 ± 2.69 | 5.93 ± 4.26 | 0.908 |
| Kd, μM | 1.80 ± 0.91 | 1.84 ± 1.33 | 0.980 |
| Closed channel state | |||
| kc, μM−1 · s−1 | 0.0030 ± 0.0015 | 0.0038 ± 0.0023 | 0.771 |
| uc, s−1 | 0.86 ± 0.09 | 1.07 ± 0.14 | 0.208 |
| Kd, μM | 286 ± 147 | 285 ± 172 | 0.997 |
| Inactivated channel state | |||
| ki, μM−1 · s−1 | −0.005 ± 0.008 | −0.004 ± 0.012 | |
| ui, s−1 | 0.10 ± 0.15 | 0.06 ± 0.24 |
Values are means ± SD; n ≈ 120. Shown are the binding rates (k) and unbinding rates (u) of ranolazine with closed (subscript c), open (subscript o), and inactivated (subscript i) states of the Na+ channel. Kd is the dissociation constant. P is the probability that the difference between corresponding parameters for atrial and ventricular channels is not significant.
RESULTS
Ranolazine interactions with the inactivated state: effect of step duration.
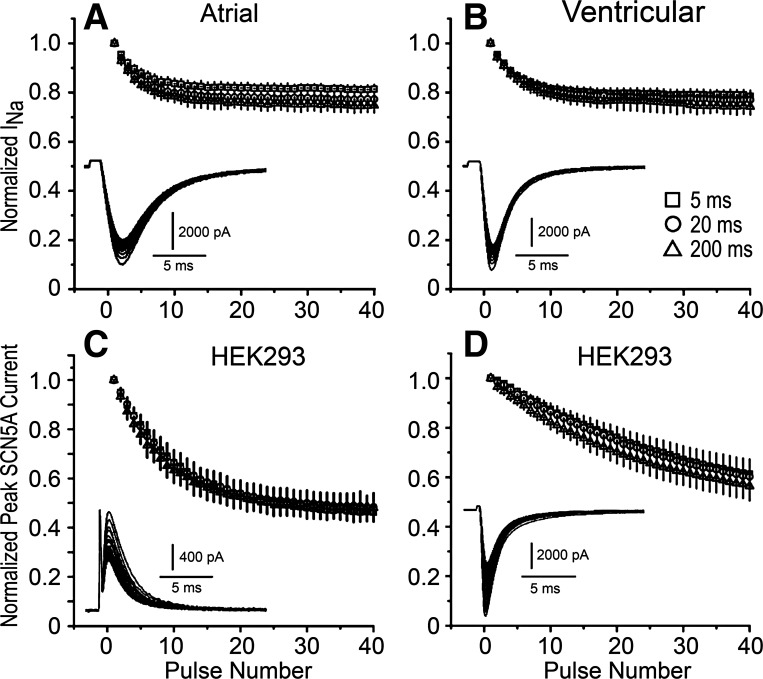
We initially examined whether ranolazine interacts strongly with inactivated states of the Na+ channel. The opening of single Na+ channels typically occurs in the initial 20 ms of a depolarizing pulse, after which channels enter an inactivated state (12). If ranolazine has significant interactions with the inactivated state, prolonging the test step during a train of pulses of constant diastolic interval should result in a greater steady-state inhibition of INa. Figure 1 shows the results of an experiment in which INa was recorded from atrial and ventricular myocytes over a range of test pulse durations in the absence and presence of 25 μM ranolazine. The holding potential was −140 mV to assure that both atrial and ventricular Na+ channels were in the resting state between pulses, and test pulses were to −30 mV. The use-dependent block induced by ranolazine was similar in atrial and ventricular myocytes and was unaffected by pulse duration (P > 0.05; Fig. 1, top). We further investigated the use-dependent block of INa by ranolazine in HEK-293 cells expressing the α-subunit of SCN5A. Na+ channels have been associated with a number of regulatory subunits in native myocytes (15), which can affect the kinetics of transition between states, but should not affect ranolazine binding within the pore of the channel (α-subunit). Na+ concentrations were adjusted such that the Na+ reversal potential was either −60 or 35 mV to record outward or inward INa (Fig. 1, C and D, respectively), and the same pulse protocols were used as above. Once again, the use-dependent block of INa by ranolazine was unaffected by pulse duration (Fig. 1, bottom). When INa was inward (Fig. 1, A, B, and D), there was a tendency for a larger decrease of INa during pulse trains when the step duration was increased from 5 to 200 ms, but it failed to reach statistical significance. On the other hand, when the Na+ reversal potential was adjusted to produce an outward INa at −30 mV (Fig. 1C), this tendency disappeared, suggesting that the somewhat larger decrease of INa during the 200-ms pulse train may be due to an intracellular accumulation of Na+ in these relatively small cells. Regardless of the source of myocytes, presence of regulatory subunits, or direction of ion flux, use dependence was not significantly affected by pulse duration. These results are inconsistent with a strong interaction of ranolazine with the inactivated state of the Na+ channel.
Fig. 1.
Use-dependent block of the Na+ channel by ranolazine (25 μM) is unaffected by test pulse duration. A train of 40 pulses was applied after a 15-s rest. The diastolic interval was kept constant at 150 ms, and pulse durations were 5, 20, or 200 ms. A: atrial myocytes (n = 6). B: ventricular myocytes (n = 5 cells) C: human embryonic kidney (HEK)-293 cells expressing SCN5A outward Na+ current (INa; n = 6). D: HEK-293 cells expressing SCN5A inward INa (n = 6). Insets in A–D show typical current traces during 200-ms test pulses. Data are means ± SD.
Ranolazine unbinding at holding potential: effect of diastolic interval.
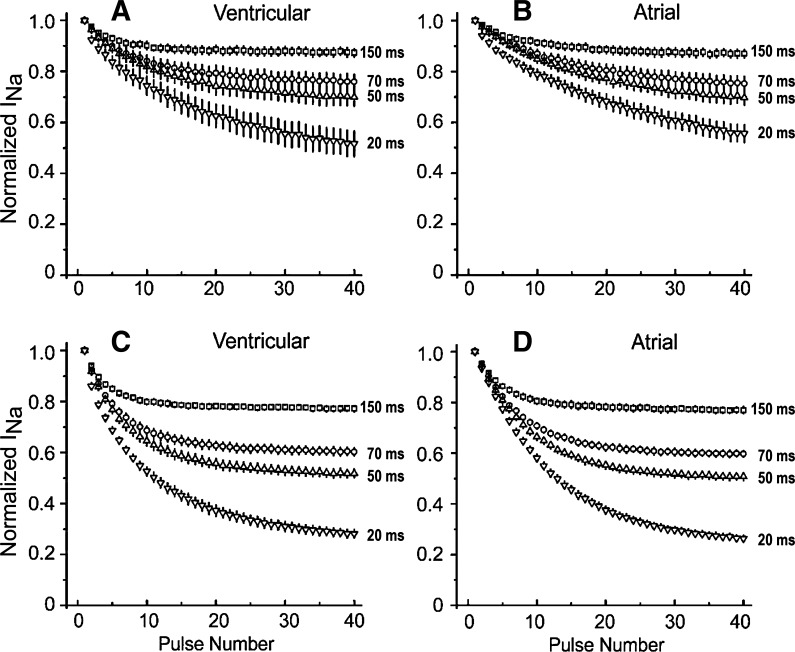
Use-dependent interactions of ranolazine with open and closed states were investigated by reducing the diastolic interval during a train of test pulses of fixed duration. If the drug interaction with the closed state is weak, ranolazine will primarily unbind between test pulses when the membrane is clamped to −140 mV. Abbreviation of the time for unbinding is expected to result in the accumulation of block and stronger steady-state inhibition as the diastolic interval is reduced. Figure 2 shows the effects of abbreviating the diastolic interval during a train of 40 pulses (20-ms duration) in ventricular (A and C) and atrial (B and D) myocytes exposed to 10 μM (Fig. A and B) or 25 μM (C and D) ranolazine. Shortening of the diastolic interval led to an increase in the magnitude of bss, but more pulses were required to reach this level of block. These results suggest weak interactions with the closed state and strong block of the open state of the Na+ channel. The sensitivity of bss during trains of 40 pulses at various diastolic intervals from 20 to 150 ms suggests that the unbinding of ranolazine at negative potentials is unusually fast and is comparable with that of inactivated state blockers but not to that of open state blockers.
Fig. 2.
Abbreviation of the diastolic interval accentuates the development of use-dependent block of INa activated in atrial and ventricular cardiac myocytes by test pulses of constant duration in the presence of 10 μM (A and B) and 25 μM (C and D) ranolazine. A and C: inward INa recorded from ventricular myocytes during a train of pulses of 20-ms duration and diastolic intervals of 150, 70, 50, or 20 ms as a fraction of INa of the first pulse of the train (n = 6). B and D: INa recorded from atrial myocytes during a train of pulses of 20-ms duration and diastolic intervals of 150, 70, 50, or 20 ms as a fraction of INa of the first pulse of the train (n = 6). Data are means ± SD.
Calculation of kinetic rates of ranolazine interactions with the Na+ channel.
The analysis of the magnitude of the peak INa decline during pulse train protocols with step durations (Ts) of 5, 20, and 200 ms and interpulse intervals (Tr) of 20, 50, 70, and 150 ms were performed using guarded receptor formalism. A single exponential function was fit to relative INa during pulse trains to obtain λ (in pulses−1) and bss for each combination of Ts, Tr, and D. The set of λ as a function of Ts, Tr, and D was analyzed using multivariate linear regression to calculate values for k and u for ranolazine interactions with closed, open, and inactivated states of the Na+ channel (Table 1). There were no statistically significant differences between the ventricle and atrium for any kinetic parameter for any of the three channel states (P > 0.2 for all rates; Table 1).
Our analysis indicated that the kinetic rates of ranolazine binding and unbinding from the inactivated state of the channel (ki and ui, respectively) for both atrial and ventricular cells were not significantly different from zero. A ki value equal to zero indicates that the inactivation gate blocks ranolazine access to the binding site. A ui value equal to zero coupled with strong interactions with the open state suggests that ranolazine is trapped by the inactivation gate as it binds to the open channel.
On the other hand, ranolazine displays fast binding to and unbinding from the open state of the channel (ko and uo, respectively) with a calculated dissociation constant (Kd) of ∼1.8 μM (Table 1). Ranolazine interactions with the Na+ channel at a very negative holding potential, when all channels are presumably in the closed state, proceeded with much slower rates (kc and uc), and the calculated Kd was ∼285 μM.
Comparison of the calculated recovery rate, open state block, and tonic block with those recorded experimentally.
These series of experiments were designed to measure additional parameters that can be directly compared with corresponding values calculated using k values obtained using the pulse protocols shown in Table 1. They serve to independently verify the calculated kinetic rates.
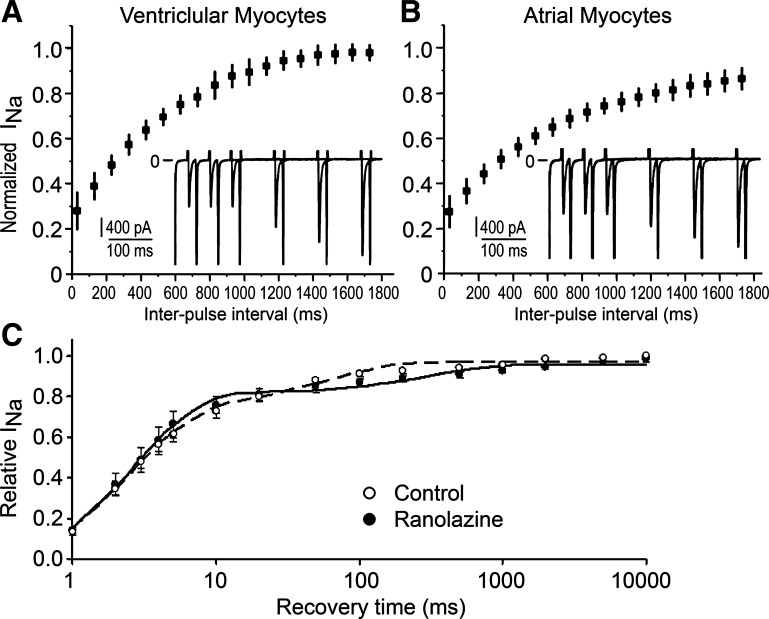
Recovery from the use-dependent block was measured directly, after a train of 20-ms pulses to −30 mV with a diastolic interval of 30 ms. Figure 3, A and B, shows similar use dependence and recovery from block in atrial and ventricular myocytes in the presence of 40 μM ranolazine at a holding potential of −140 mV. The shortest recovery interval was 30 ms, which essentially excludes channel recovery from normal inactivation. Recovery time constants obtained using single-exponential fits to individual recovery curves ranged between 650 and 700 ms and were not statistically different between cell types (Table 2). Additionally, recovery from the use-dependent block at −140 mV was studied in a HEK-293 cell line expressing SCN5A after a single 1,000-ms “loading” pulse to −20 mV. This protocol was selected based on the assumption that ranolazine preferentially blocks the inactivated state of the Na+ channel. Recovery intervals were chosen to probe both fast and slow recovery starting from 1 ms, so that recovery followed a double-exponential course both in control and in the presence of ranolazine (Fig. 3C). Fast recovery from normal inactivation had a time constant of 2.6 ± 0.8 ms and an amplitude of 78 ± 7%. Slow recovery from normal inactivation in the control had a time constant of 55 ± 17 ms and an amplitude 22 ± 7%. In the presence of 30 μM ranolazine, the second component of recovery, which represents the unbinding of the drug rather than recovery from normal inactivation, became much slower, with a time constant of 660 ± 140 ms and an amplitude equal to 12.5 ± 2.5%. Thus, Na+ channel recovery from ranolazine block was almost identical to the values obtained in canine myocytes. Recovery time constants obtained experimentally in this and other studies (Table 2) were in good agreement with those predicted from calculated kinetic rates using following formula: recovery time constant = 1/(kc × D + uc) and served as an independent confirmation for the accuracy of kinetic rates calculated from pulse train data using guarded receptor formalism.
Fig. 3.
Rate of recovery of inward INa from use-dependent block by ranolazine. A and B: recovery of INa from block induced by a train of 40 pulses in ventricular (A) and atrial (B) myocytes in the presence of 40 μM ranolazine. Insets show the last two pulses of the train with four subsequent pulses overlaid as the interpulse interval was increased. n = 6 for each cell type. C: recovery in HEK-293 cells expressing SCN5A (n = 6) after a single 1,000-ms pulse to −20 mV in the control and in the presence of 30 μM ranolazine. Dashed and solid lines are the best double-exponential fits to averaged recovery data in the control and in the presence of ranolazine, respectively. The time scale is logarithmic.
Table 2.
Time constants for recovery from block calculated from the kinetic rates of binding and unbinding at rest compared with experimentally obtained values at −140 mV and 15°C
| Experimental Values | Calculated Values | Reference | |
|---|---|---|---|
| Ventricular cells, 40 μM | 650 ± 170 | 820 ± 110 | |
| Atrial cells, 40 μM | 707 ± 160 | 1,020 ± 110 | |
| HEK-293 stable cell line expressing SCN5A, 30 μM | 660 ± 140 | 845 ± 110 | |
| HEK-293 cells expressing rNav1.4-WCW, 100 μM | 560 ± 40 | 690 ± 90 | 29 |
Values are means ± SD (in ms). Rat (r)Nav1.4-WCW is the L435W/L437C/A438W mutant Na+ channel. See Table 1 for the kinetic rates of binding and unbinding at rest. HEK-293 cells, human embryonic kidney-293 cells.
Additionally, the calculated kinetic rates of the ranolazine interaction with the open state of the Na+ channel predicted that the amount of block attained during a single pulse assuming an open time of 1.0 ms should be 9.7% and 9.0% for ventricular and atrial cells, respectively, when block was estimated as 1 − e(−1.0/open time constant). These values were in good agreement with the amplitude of block during a single pulse (12.5 ± 2.5%) obtained in the HEK-293 cell line expressing SCN5A.
Another independent verification of the validity of the calculated kinetic rates of ranolazine interactions with closed states of the Na+ channel was obtained by comparing the predicted Kd values for tonic block with those found experimentally. In a HEK-293 cell line expressing SCN5A, ranolazine blocked the peak INa recorded under voltage-clamp conditions (holding at −140 mV, steps to −20 mV for 20 ms every 1,000 ms), with an IC50 of 260 ± 2 μM. This value was in good agreement with a calculated Kd = uc/kc ≈ 285 μM for the tonic block due to ranolazine interactions with the closed state of the channel at −140 mV. Table 3 shows a comparison of Kd values for ventricular and atrial cells calculated using kinetic rates with IC50 values for tonic block obtained in our and other similar studies (10, 28, 29).
Table 3.
Comparison of calculated Kd values for the block of peak Na+ current at slow stimulation rates (tonic or resting block) with IC50 values for the block of slow inactivating/late Na+ current
| Conditions | Kd or IC50, μM | Reference | |
|---|---|---|---|
| Calculated values | |||
| Ventricular cells | 15°C, −140 mV | 285 ± 170 | |
| Atrial cells | 15°C, −140 mV | 286 ± 150 | |
| Experimental values | |||
| HEK-293 cells expressing SCN5A | 22°C, −140 mV | 259 ± 2 | |
| Canine ventricular cells | 22°C, −140 mV | 294 | 28 |
| Transgenic ΔKPQ murine cardiomyocytes | 22°C, −100 mV | 135 | 10 |
| HEK-293 cells expressing rNav1.4-WCW | 22°C, −140 mV | 225 ± 16 | 29 |
| HEK-293 cells expressing rNav1.5-WCW | 22°C, −140 mV | 137 ± 196 | 29 |
Values are means ± SD.
Taken together, these results suggest that differences in the affinity and/or kinetics of binding/unbinding of ranolazine between atrial and ventricular Na+ channels do not underlie the atrial selectivity of the drug to block INa. We did not find any statistically significant differences in ranolazine interactions with ventricular and atrial Na+ channels at a very negative holding potential, when complete recovery from inactivation in both cell types is expected. This result is consistent with the assumption that the ranolazine binding site(s) is identical in atrial and ventricular Na+ channels.
Effect of holding potential on the block development in atrial and ventricular myocytes.
We evaluated to what extent the more negative steady-state inactivation relationship in the atrium contributes to the atrial selectivity of ranolazine. Moderately depolarized holding potentials (−120, −110, and −100 mV) were used to unmask the effects of differences in channel availability.
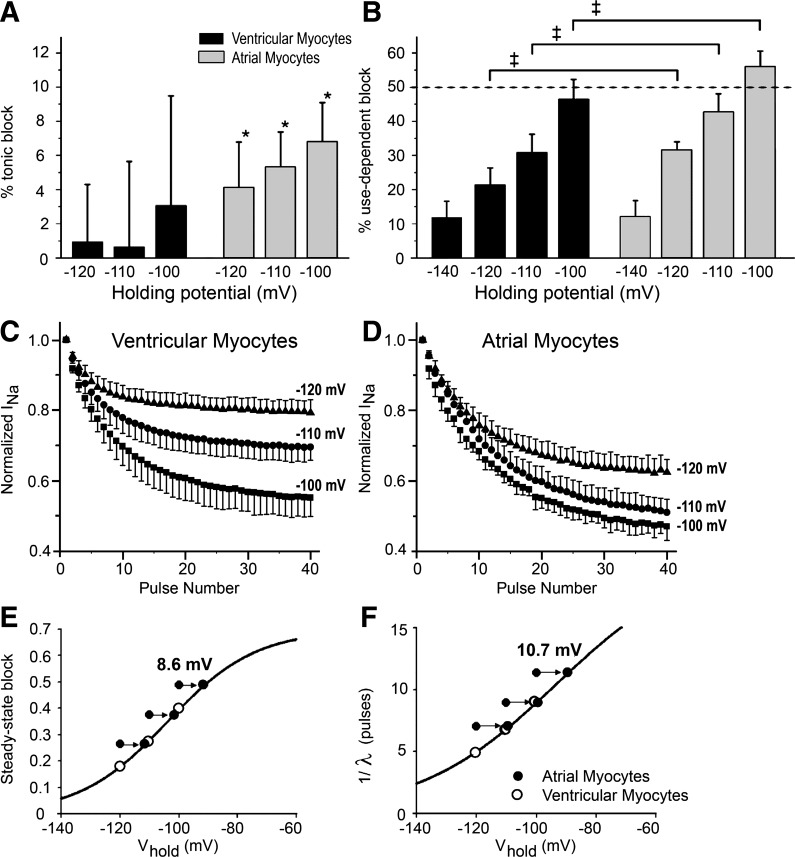
Tonic block was measured in atrial and ventricular myocytes during the first pulse of a train after a 15-s rest. Currents recorded in the presence of 10 μM ranolazine were divided by control currents without drug. Trains were composed of 50-ms pulses to −30 mV separated by diastolic intervals of 250 ms. Figure 4A shows average tonic block in ventricular and atrial myocytes at the different holding voltages. Average ranolazine-induced tonic block did not reach statistical significance from zero in ventricular myocytes but did so in atrial myocytes (<6%, P < 0.01). The degree of tonic block was not significantly affected by holding potential (Table 4) in the range of −100 to −120 mV. In contrast, use-dependent block was a sensitive function of holding potential (Fig. 4B and Table 4). Use-dependent block increased significantly at more depolarized holding potential (P < 0.01 by ANOVA) and was significantly larger in atrial compared with ventricular myocytes. The larger block implies a slower unbinding of ranolazine at the more depolarized holding potential, which is expected based on the fact that the fraction of noninactivated Na+ channels from which the drug can readily unbind is smaller, effectively trapping more of the drug in the inactivated state.
Fig. 4.
Effects of holding potential (Vhold) on tonic and use-dependent block in cardiac myocytes by 10 μM ranolazine. Tonic block was defined as the magnitude of block during the 1st pulse, and use-dependent block was defined as the magnitude of block during the 40th pulse of a train relative to the 1st pulse. Trains were 40 pulses (50 ms) to −30 mV. A: percent tonic block as a function of Vhold in 10 ventricular cells and 5 atrial cells. Values are means ± SD. Tonic block in ventricular myocytes was not statistically significant, whereas tonic block in atrial cells was statistically different from zero (*P < 0.01). B: use-dependent block as a function of Vhold in 10 ventricular cells and 5 atrial cells. Values are means ± SD. The diastolic interval was 250 ms when holding at −100, −110, and −120 mV. The diastolic interval was 150 ms when holding at −140 mV. Block in atrial cells was significantly greater than ventricular cells at −100, −110, and −120 mV (‡P < 0.01). C and D: development of use-dependent block in ventricular (C) and atrial (D) myocytes during pulse trains delivered using different Vhold in the presence of 10 μM ranolazine at a diastolic interval of 250 ms. INa during each successive pulse was normalized to the current recorded during the first pulse to eliminate the contribution of tonic block. A small degree of accumulation of inactivation (<5%) was observed in the absence of drug (not shown). This effect was eliminated by dividing the relative current in the presence of ranolazine by the relative current in the control for each pulse in the train. These data were fit with monoexponential functions to obtain steady-state block and uptake rates as a function of Vhold in ventricular and atrial myocytes, which were analyzed as shown in E (steady-state block) and F (λ−1). Arrows indicate the shift of values obtained in atrial cells by 8.6 mV (steady-state block) and by 10.7 mV (λ−1), which was required to obtain the best fit (minimal sum of squared errors) of all six data points by the following single Boltzmann function: A/{1 + e[(V1/2 − V)/s]} (solid lines in E and F).
Table 4.
Tonic and use-dependent block with 10 μM ranolazine obtained at different holding potentials in ventricular and atrial myocytes
| Tonic block, % |
Use-Dependent Block, % |
|||
|---|---|---|---|---|
| Ventricular myocytes | Atrial myocytes | Ventricular myocytes | Atrial myocytes | |
| Holding potential | ||||
| −120 mV | 0.9 ± 3.3* | 4.1 ± 2.7† | 21 ± 5 | 32 ± 2‡ |
| −110 mV | 0.6 ± 5.0* | 5.3 ± 2.1† | 31 ± 5 | 43 ± 5‡ |
| −100 mV | 3.1 ± 6.4* | 6.8 ± 2.3† | 47 ± 6 | 56 ± 5‡ |
Values are means ± SD; n = 10 ventricular myocytes and 5 atrial myocytes. Trains of 50-ms pulses to −30 mV with 250 ms at holding potentials between pulses were used.
Not significantly different from zero (P > 0.3).
Significantly different from zero (P < 0.05). In both cell types, tonic block was not significantly dependent on holding potential (P > 0.3 by ANOVA).
Significantly different from the use-dependent block in ventricular cells at all holding potentials (P < 0.05). In both cell types, use-dependent steady-state block was significantly dependent on holding potential (P < 0.01 by single-factor ANOVA).
To explain the atrial selectivity of ranolazine to produce use-dependent depression of INa, we hypothesized that the voltage dependence of the block in atrial and ventricular myocytes followed the same Boltzmann function but was shifted to more negative potentials in atrial cells due to the more negative position of the steady-state activation and inactivation curves. To test this hypothesis, the development of use-dependent block at different holding potentials was analyzed using the same approach as that used initially to analyze use-dependent block at a holding potential of −140 mV. We compared two measures of this block: steady-state inhibition and the rate λ−1 of block development (expressed as the number of pulses; Fig. 4, E and F). bss (Fig. 4E) and pulse number (Fig. 4F) progressively increased with more positive holding potentials, and data points for atrial myocytes were shifted to the left of the ventricular data. Using a nonlinear fitting procedure, we found the best values to describe this atrial shift that minimized the sum of square errors for a single Boltzmann function. As shown in Fig. 4E, the values of bss obtained in both atrial and ventricular myocytes fell on the same Boltzmann function when voltage dependence for atrial cells was shifted by 8.6 mV. A similar analysis for the voltage dependence of the “time constant” [expressed as the number of pulses (λ−1)] is shown in Fig. 4F and yielded an optimal shift of atrial values equal to 10.7 mV. We also analyzed data obtained using similar trains of pulses with 150-ms diastolic potential (results not shown). These data yielded optimal shifts between ventricular and atrial data points equal to 11.0 mV (bss) and 12.1 mV (λ−1). Values of the optimal voltage shift between atrial and ventricular data were in good quantitative agreement with known differences in the voltage dependence of steady-state inactivation between atrial and ventricular myocytes (6).
DISCUSSION
In contrast to previous reports (22, 28) showing that ranolazine primarily binds to inactivated Na+ channels, we found ranolazine to be an open state blocker that recovers from block at a resting potential that is unusually fast and is trapped in the inactivated state. Kinetic rates of ranolazine interactions with different states of atrial and ventricular Na+ channels were not statistically different, indicating that atrial and ventricular ranolazine-binding sites are similar and cannot contribute to the atrial selectivity of ranolazine's actions. Other mechanisms, including a more negative position of the steady-state inactivation curve (6), less negative resting membrane potential, and slower phase 3 repolarization giving rise to briefer diastolic intervals in atrial cells than in ventricular cells, particularly at fast rates (6), are likely responsible for the atrial-selective action of ranolazine to depress INa and decrease Vmax, slow conduction time, and increase postrepolarization refractoriness and the diastolic threshold of excitation in tissues. Moreover, this shifted position of the atrial steady-state inactivation curve will affect the composition of the mixture of different states of the Na+ channel, thus affecting apparent rates of interaction and equilibrium block.
Undrovinas and co-workers (28) arrived at their conclusion that ranolazine strongly blocks the inactivated state of the Na+ channel from their analysis of the shift of the steady-state inactivation curve by ranolazine using Bean's equation (3). However, this equation, developed to estimate lidocaine block of the Na+ channel, explicitly excludes the possibility that a drug can interact with the open (transient) state of the channel. It is applicable only for steady-state conditions and can be considered as a simplified version of dynamic guarded receptor formalism. Despite these limitations, the calculated value of Kd for the ranolazine interaction with the high-affinity site [1.17 μM (28)] was in the same range as the value of Kd for the open state calculated in our study (1.8 μM). Similarly, Rajamani et al. (22) interpreted the ranolazine-induced shift in the steady-state inactivation curve to mean that ranolazine blocks the inactivated state of the Na+ channel. On the other hand, the shift of the steady-state inactivation curve by lidocaine has been shown to be more accurately explained if its interaction with the preopen state (the state when one or several but not all activation gates are open) is taken into consideration (26). The interaction of a traditional Na+ channel blocker, saxitoxin, with a transient closed state, which is different from the “ultimate rest state” and is equivalent to the preopen (nonconductive) state, has been shown to better explain the use-dependent block observed in rat ventricular myocytes (16). The findings presented in our accompanying model companion paper (21a) show that ranolazine binding to the preopen state of the channel will also result in a hyperpolarizing shift of the steady-state inactivation curve.
Our results can be favorably compared with some other characteristics of ranolazine interactions with Na+ channels obtained in independent experiments. For example, the calculated time constant for recovery from block at a holding potential of −140 mV and 15°C (≈800 ms) was close to experimentally obtained values (Table 2). Similarly, a good correspondence was found between Kd values for tonic block of fast INa (∼285 μM) and IC50 values for the inhibition of peak INa at slow pacing rates (Table 3). The calculated tonic block of peak INa at −140 mV and at a clinically relevant concentration (10 μM) in atrial cells was 3.4 ± 5.4% and in ventricular cells was 3.4 ± 6.2%, i.e., not significantly different from zero, in agreement with experimental data.
Interestingly, the calculated value of Kd for the open state of the Na+ channel of 1.8 μM was very close to the values of Kd for the open state of the inactivation-deficient WCW mutant Na+ channel [2.68 μM for rat (r)Nav1.4-WCW and 1.99 μM for human (h)Nav1.7-WCW], as reported by Wang et al. (29). Unfortunately, the experimental data did not permit the authors to estimate the Kd value for hNav1.5-CW using the same method. However, these authors also determined the IC50 value for open state block as the percent inhibition of slow INa at the end of a 50-ms pulse and obtained very similar values for rNav1.4-WCW (2.4 ± 0.2 μM) and hNav1.7-WCW (1.7 ± 0.1 μM). A much higher value of IC50 was obtained for hNav1.5-CW: 6.2 ± 0.7 μM. This difference between Na+ channel isoforms may be due to an artifact of data analysis, because the authors reported an increase of hNav1.5-CW current in 50% of the cells at low concentrations (1 μM) of ranolazine. Nevertheless, this value of IC50 for the cardiac version of the Na+ channel was in agreement with other published data obtained for late INa in canine cardiomyocytes: 6.46 μM (28) and 5.9 μM (1). Further analysis of this discrepancy is presented in the accompanying model paper (21a). Kinetic rates of ranolazine interactions with the open state of rNav1.4-WCW and hNav1.7-WCW, obtained by Wang et al. (29), were 4 and 2.5 times larger than those obtained in the present study, likely due to reduced interference of the deficient inactivation gate in these WCW mutants with ranolazine access to the channel binding site.
Ranolazine is unique among open state blockers in that it displays much faster recovery from block than pure open state blockers (25). This property prevents the accumulation of ranolazine block at normal heart rates but permits potent block of the Na+ channel at the fast rates encountered during tachyarrhythmia. In this respect, ranolazine is more similar to lidocaine (a typical class 1B drug) but is more potent and selective in its block of late INa compared with peak INa.
According to our use-dependent data analysis, ranolazine binds exclusively to preopen/open state(s) of the Na+ channel, and its interactions with the binding site on the α-subunit of atrial and ventricular Na+ channel are similar. Therefore, the atrial selectivity of ranolazine cannot be explained by differences in binding affinities or kinetic rates.
Considerable evidence has been advanced in support of the other mechanisms thought to underlie the atrial-selective action of ranolazine to inhibit Na+ channel-dependent parameters. A more negative position of the steady-state inactivation curve of atrial compared with ventricular Na+ channels gives rise to a larger fraction of inactivated Na+ channels in atrial versus ventricular cells (6) at any given voltage, leading to a slower recovery from block because ranolazine cannot unbind from inactivated channels, according to the calculated rate constants. A further increase in the fraction of inactivated Na+ channels in atria results from the more positive resting membrane potential of atria. Atrial cells also exhibit a much slower phase 3 repolarization, resulting in the abbreviation or elimination of the diastolic interval, a more positive takeoff potential at rapid rates of activation, and longer time spent at potentials at which the preopen state dominates. Ranolazine exacerbates these differences because it prolongs atrial action potentials without affecting the ventricle. As a consequence of these differences, the fraction of noninactivated Na+ channels in atria is much smaller, particularly at rapid rates. Because much of the recovery from Na+ channel block occurs from noninactivated states of the channel, atrial cells show a greater accumulation of use-dependent Na+ channel block (7, 13). These conclusions are supported by our data showing that atrial cells displayed significantly larger use-dependent block at moderately depolarized holding potentials than ventricular cells.
The relative contribution of differences in channel gating properties, such as the more negative position of the steady-state inactivation curve in atrial versus ventricular cells compared with the contribution from the electrophysiological properties of the atrial action potential (higher diastolic potential and slower slope of the repolarization), cannot be readily assessed by our experiments. We address this question and others regarding the unique behavior of ranolazine in a computational model of ranolazine interaction with different states of the Na+ channel in the accompanying paper (21a). We acknowledge that ranolazine binding constants, Na+ channel gating kinetics, and the positions of activation and inactivation curves are temperature dependent. In the accompanying paper (21a), we modeled these effects using a Q10 of 1.65 and included comparisons to data obtained at 37°C and reduced external Na+.
The atrial selectivity explored by this study applies to the effects of ranolazine on peak INa and its corresponding electrophysiological parameters. It is critical to appreciate that this atrial sensitivity does not preclude an effect in the ventricle, especially at depolarized potentials and fast rates. Our results provide a basis to test the potential of ranolazine to reduce atrial tachycardia while showing ventricular safety in patients. Although there are no placebo-controlled randomized clinical trials that have specifically tested the atrial-selective antiarrhythmic activity of ranolazine, MERLIN TIMI-36 evaluated the effectiveness and safety of ranolazine during acute and long-term treatment in patients with acute coronary syndromes. In this trial, ranolazine treatment was associated with significant reductions of supraventricular tachyarrhythmia (23). Additionally, anti-AF efficacy has also been shown by a number of small exploratory clinical studies (14, 19, 20) in which ranolazine either terminated paroxysmal AF or prevented postoperative AF. Importantly, these reductions occurred without causing ventricular tachycardia or ventricular proarrhythmia, indirectly demonstrating a greater atrial sensitivity. A single dose of 2,000 mg ranolazine converted 77% of AF patients, including patients with structural cardiac abnormalities, with no significant adverse reactions (19, 21).
In the ventricle, Moss et al. (18) showed that ranolazine reduced the QTc prolongation present in SCN5A-ΔKPQ mutation patients without prolonging the QRS interval, whereas the Na+ channel blocker flecainide prolonged the QRS in these patients (17, 30). If we take QRS prolongation as a surrogate of peak INa inhibition, these studies suggest an insensitivity of ventricular peak INa to ranolazine. The effect of both agents to abbreviate QTc is attributable to their action to inhibit late INa.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-47678 (to C. Antzelevitch) and by the Masons of New York State and Florida.
DISCLOSURES
C. Antzelevitch received research support and is a consultant to Gilead Sciences, Inc. L. Belardinelli and S. Rajamani are employees of Gilead Sciences, Inc.
Supplementary Material
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation 110: 904–910, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J Electrocardiol 42: 543–548, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol 81: 613–642, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burashnikov A, Antzelevitch C. New pharmacological strategies for the treatment of atrial fibrillation. Ann Noninvasive Electrocardiol 14: 290–300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm 5: 1735–1742, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 116: 1449–1457, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmeliet E, Mubagwa K. Antiarrhythmic drugs and cardiac ion channels: mechanisms of action. Prog Biophys Mol Biol 70: 1–72, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol 292: H66–H75, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Draper NR, Smith H. Applied Regression Analysis. New York: Wiley-Interscience, 1998. [Google Scholar]
- 10. Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 148: 16–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilliam FR, Starmer CF, Grant AO. Blockade of rabbit atrial sodium channels by lidocaine. Characterization of continuous and frequency-dependent blocking. Circ Res 65: 723–739, 1989. [DOI] [PubMed] [Google Scholar]
- 12. Grant AO, Dietz MA, Gilliam FR, 3rd, Starmer CF. Blockade of cardiac sodium channels by lidocaine. Single-channel analysis. Circ Res 65: 1247–1262, 1989. [DOI] [PubMed] [Google Scholar]
- 13. Hondeghem LM, Katzung BG. Mechanism of action of antiarrhythmic drugs. In: Physiology and Pathophysiology of the Heart, edited by Sperelakis N. Dordrecht, The Netherlands: Kluwer Academic, 1995, p. 589–603. [Google Scholar]
- 14. Kaliebe JW, Murdock DK. Suppression of non-sustained ventricular tachycardia with ranolazine: a case report. WMJ 108: 373–375, 2009. [PubMed] [Google Scholar]
- 15. Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel a and b subunits in single ventricular myocytes from mouse heart. Circulation 109: 1421–1427, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Makielski JC, Satin J, Fan Z. Post-repolarization block of cardiac sodium channels by saxitoxin. Biophys J 65: 790–798, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, Severski P, Rosero S, Daubert JP, Qi M, Cieciorka M, Manalan AS. Safety and efficacy of flecainide in subjects with long QT-3 syndrome (DeltaKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol 10: 59–66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol 19: 1289–1293, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murdock DK, Kersten M, Kaliebe J, Larrian G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a reveiw of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J 9: 260–267, 2009. [PMC free article] [PubMed] [Google Scholar]
- 20. Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J 8: 175–181, 2008. [PMC free article] [PubMed] [Google Scholar]
- 21. Murdock DK, Reiffel JA, Kaliebe J, Larrian G. The conversion of paroxysmal or initial onset atrial fibrillation with oral ranolazine: implications for a new “pill-in-pocket” approach in structural heart disease. J Atr Fibrillation 2: 705–710, 2010. [Google Scholar]
- 21a. (a) Nesterenko VV, Zygmunt AC, Rajamani S, Belardinelli, Antzelevitch C. Mechanisms of atrial-selective block of Na+ channels by ranolazine: II. Insights from a mathematical model. Am J Physiol Heart Circ Physiol. First published August 5, 2001; doi:10.1152/ajpheart.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajamani S, El-Bizri N, Shryock JC, Makielski JC, Belardinelli L. Use-dependent block of cardiac late Na+ current by ranolazine. Heart Rhythm 6: 1625–1631, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 116: 1647–1652, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Starmer CF. Characterizing activity-dependent processes with a piecewise exponential model. Biometrics 44: 549–559, 1988. [PubMed] [Google Scholar]
- 25. Starmer CF, Lastra AA, Nesterenko VV, Grant AO. Proarrhythmic response to sodium channel blockade. Theoretical model and numerical experiments. Circulation 84: 1364–1377, 1991. [DOI] [PubMed] [Google Scholar]
- 26. Starmer CF, Nesterenko VV, Undrovinas AI, Grant AO, Rosenshtraukh LV. Lidocaine blockade of continuously and transiently accessible sites in cardiac sodium channels. J Mol Cell Cardiol 23, Suppl I: 73–83, 1991. [DOI] [PubMed] [Google Scholar]
- 27. Starmer CF, Nesterenko VV, Undrovinas AI, Packer DL, Gilliam FR, Grant AO, Rosenshtraukh LV, Strauss HC. Characterizing ion channel blockade with the guarded receptor hypothesis. In: Molecular and Cellular Mechanisms of Antiarrhythmic Agents, edited by Hondeghem LM. Mount Kisco, NY: Futura Publishing, 1989, p. 179–200. [Google Scholar]
- 28. Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 17: S161–S177, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 noltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol 73: 940–948, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Windle JR, Geletka RC, Moss AJ, Zareba W, Atkins DL. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A:DeltaKPQ mutation. Ann Noninvasive Electrocardiol 6: 153–158, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zygmunt AC. Intracellular calcium activates chloride current in canine ventricular myocytes. Am J Physiol Heart Circ Physiol 267: H1984–H1995, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.