Abstract
Type 2 diabetes (T2D) is rapidly rising in prevalence. Characterized by either inadequate insulin production or the inability to utilize insulin produced, T2D results in elevated blood glucose levels. The "gold-standard" in assessing insulin sensitivity is a hyperinsulinemic-euglycemic clamp or insulin clamp. In this procedure, insulin is infused at a constant rate resulting in a drop in blood glucose. To maintain blood glucose at a constant level, exogenous glucose (D50) is infused into the venous circulation. The amount of glucose infused to maintain homeostasis is indicative of insulin sensitivity. Here, we show the basic clamp procedure in the chronically catheterized, unrestrained, conscious rat. This model allows blood to be collected with minimal stress to the animal. Following the induction of anesthesia, a midline incision is made and the left common carotid artery and right jugular vein are catheterized. Inserted catheters are flushed with heparinized saline, then exteriorized and secured. Animals are allowed to recover for 4-5 days prior to experiments, with weight gain monitored daily. Only those animals who regain weight to pre-surgery levels are used for experiments. On the day of the experiment, rats are fasted and connected to pumps containing insulin and D50. Baseline glucose is assessed from the arterial line and used a benchmark throughout the experiment (euglycemia). Following this, insulin is infused at a constant rate into the venous circulation. To match the drop in blood glucose, D50 is infused. If the rate of D50 infusion is greater than the rate of uptake, a rise in glucose will occur. Similarly, if the rate is insufficient to match whole body glucose uptake, a drop will occur. Titration of glucose continues until stable glucose readings are achieved. Glucose levels and glucose infusion rates during this stable period are recorded and reported. Results provide an index of whole body insulin sensitivity. The technique can be refined to meet specific experimental requirements. It is further enhanced by the use of radioactive tracers that can determine tissue specific insulin-stimulated glucose uptake as well as whole body glucose turnover.
Protocol
A: Surgical Catheterization of Arterial and Venous Circulation
Part 1: Arterial and Venous Catheter Preparation
Cut 15 cm of PE-50 (internal diameter of .58mm (.023") x an outer diameter of .965mm (.038"). Cut a 1mm section of silastic tubing (0.76mm (0.030") internal diameter x 1.65mm (0.065") outer diameter) for use as restraining bead. The restraining bead prevents the rat from pulling out the catheter once it is in place.
Insert the tips of micro dissecting forceps into the lumen of the restraining bead and gently hold the tips of the forceps apart to stretch the opening wider. Using another pair of micro dissecting forceps, slide the silastic tubing into the lumen of the restraining bead. Secure with strong adhesive glue (Loctite Super Glue). The bead must lie flat around the catheter. Allow catheter to dry (24h).
For a 300 gram rat, the artery line is ~2.7cm from the restraining bead while the jugular line is ~3.2cm. Catheter lengths from the restraining are adjusted by 0.5cm for every 100g increase in body weight. Do not bevel the edges of the catheter as this may puncture the vessel during insertion.
Immediately prior to surgery, fill the lines with heparinized saline (10U/ml), seal both ends with a stainless steel tubing plug and place in ethanol (70%) to sterilize. Air dry prior to insertion.
Part 2: Surgical Preparation
Procedures were approved by the University of Calgary Animal Care and Use Committee and abide by theCanadian Association for Laboratory Animal Science guidelines for experimentation. The procedures outlined below were performed on adult, male Sprague-Dawley rats (~300g). All procedures are performed under isoflurane, although it is possible to use injectable anesthetics. All surgical procedures are performed ensuring aseptic techniques. Surgical equipment, beakers and heparinized saline are autoclave sterilized. Surgical gloves, syringes and cotton tipped applicators are purchased sterilized from the supplier.
Weigh the rat and record the result. The weight will be important in the post-surgical monitoring of animals. Only animals that regain weight to pre-surgery levels should be used for experimentation. Anesthetize rat (3% isoflurane) in an anesthetic box. Keep the rat at ~2% isoflurane during surgery using a nose cone. Surgery must be conducted in a disinfected area that promotes asepsis.
Prepare the animal by removing hair from the surgical site (neck and shoulder blades). A small hair clipper or chemical depilatory can be used. Perform this procedure in an area separate from where the surgery is to be conducted.
Secure animal to surgical table. Ensure animal is fully anesthetized by checking for the presence of foot/eye reflexes. Prepare the surgical sites with an appropriate skin disinfectant (70% ethanol followed by betadine scrub followed by 70% ethanol).
Part 3: Surgery
Make small vertical midline incision 1cm superior to the sternum (Pick up skin longitudinally along medial axis with forceps and cut with scissor or scalpel).
Blunt dissect using micro dissecting forceps to expose the left sternomastoid muscle. Reflect this muscle to expose approximately 1cm of the left carotid artery. Use micro dissecting forceps under carotid artery to hold the artery in place. Gently tease off connective tissue from the carotid artery. It is important to isolate the vagus nerve from the artery without damaging either the artery or the nerve. Isolate the artery then ligate the cephalad end with 4-0 silk suture (this will be used to manipulate the carotid artery during surgery). Note: 4-0 sutures should be sterilized with 70% ethanol prior to use.
Clamp the vessel with serrated, micro dissecting forceps. Puncture the ligated end with a 21 gauge VENOJECT Multi-Sample Luer Adapter. Remove the stainless steel tubing plug and carefully insert catheter with the aid of a sterile catheter introducer to admit it into the artery. Ensuring the catheter is secure with forceps, partially release micro dissecting forceps clamping the artery and continue inserting the catheter to the bead. At this point, the tip of the catheter should be in the aortic arch. Take care not to release catheter, pressure of the vessel will force it out.
Tie two 4-0 sutures securely below bead and one above and confirm that the catheter will sample. Flush the line with 10U/ml heparinized saline. Re-plug the external end of the catheter with a stainless steel tubing plug.
Using the same incision, blunt dissect to expose right external jugular vein. Isolate carefully and ligate the cephalad end with silk suture. Puncture the vein with a 21 gauge VENOJECT Multi-Sample Luer Adapter. Remove the stainless steel tubing plug and insert catheter with sterile catheter introducer to the bead and tie two sutures below the bead. Tie a third suture above bead and confirm that it samples. Flush with the venous line 10U/mL heparinized saline. Re-plug the external catheter end with stainless steel tubing plug.
Tunnel 14-gauge blunt needle under skin and make incision in the back between the shoulder blades. Thread the catheters through the needle to exteriorize them at the back of the rat. Approximately 4cm of line is visible. Cut 0.5cm of Tygon S-50-HL Medical Tubing, place it around both the exteriorized catheters and secure with a tape as required (blue for vein, red for artery). Retest catheters to ensure patency, flush and fill with 150U/ml heparinized saline to prevent clotting.
Close all incisions with 3-0 silk suture. Place rat prone, in pre-warmed, clean cage with food in the bottom of the cage.
B: Postoperative Care
Part 1: Immediate Postoperative Care and Monitoring
Once the rat regains full ambulatory ability and alertness return the rat to animal housing.
Allow the rat to recover for 3-5 days.
Monitor daily for infection, pain and changes in weight. Infection may be of concern if discharge from incision sites, general lethargy and/or pain is observed. Pain is indicated by hunched posture, ruffled back fur and absence of ambulatory and/or eating behavior. A loss in weight may be experienced immediately following surgery up to three days post-surgery, however, weight should stabilize and/or increase to within 10% of the pre-surgical weight within 5 days. Severe weight loss may indicate infection, stroke and/or pain.
Retest catheters daily to ensure patency.
Part 2: Maintaining Catheter Patency
Each day during post-op recovery, fill 1ml Slip Tip Syringes with 150U/ml heparinized saline and cap it with a 22 gauge blunt needle. The blunt needle is inserted into 20cm of PE-50 tubing with 23 gauge stainless steel tubing coupler at the other end.
Remove air bubbles by placing the end with the tubing coupler higher than the rest of the 1ml syringe and pushing the bubbles out of the line.
Clamp off the arterial line externalized from the rat with hemostats just below the plug.
Remove the steel catheter plug using the second pair of hemostats.
Insert the tubing coupler connected to the syringe into the arterial line and release the hemostats occluding the externalized arterial catheter.
Aspirate the arterial blood of the catheter into syringe. If the catheter does not draw easily it may be necessary to very gently push in a small amount of flush solution through the catheter to dislodge the tip of the catheter in case it is wedged against the vessel wall. The catheter has been completely cleared when the blood reaches the syringe.
Clamp the PE-50 tubing close to the 1ml syringe and dispose of the syringe. Replace with a new syringe filled with fresh 150U/ml heparinized saline. Unclamp the tubing and holding the new syringe upright, flick the new syringe with a finger to dislodge any possible new air bubbles upwards and away from the line. Note: Care must be ensured as air bubbles can cause a stroke.
Inject the 150U/ml heparinzed saline until the catheter line is clear and free of blood.
Clamp off the arterial line externalized from the rat with hemostats just below the tubing coupler. Remove the syringe connector from the arterial catheter line and replace it with the stainless steel tubing plug.
Release the hemostats occluding the externalized arterial catheter.
Repeat for venous line. However, because of the low venous pressure sampling is often not possible. If the 150U/ml heparinzed saline can be infused with minimal resistance it is probable that the catheter is well positioned in the vein.
C: Hyperinsulinemic-Euglycemic Clamp
Part 1: Clamp Set-up and Preparation
Weigh the rat and record the weight. This will be required to determine insulin and glucose infusion rates. Fast the rat for at least 5h prior to experiment. This ensures the animal is in a postprandial state and the contribution of glucose from dietary sources is minimized. Place the rat in a small container/cage with bedding that limits excessive movement. The animal should be able to turn around and groom freely.
Set up lines and infusion pumps as seen in Figure 1. P50 connection tubing lengths are as follows: syringe to Y connector is 8cm, Y connector to rat is 15cm, arterial line is 20cm, and venous line is 10cm in length. Remove stainless steel tubing plugs and flush jugular and arterial lines to ensure infusion and sampling respectively with 10U/ml heparinized saline.
Determine insulin volume required. This will vary depending on the insulin level and weight of the rat. Here, 4mU/kg/min is administered at a rate of 2uL/min. This is a high physiological dose. Where possible, attempts should be made to limit infusion volumes. Insulin should be appropriately diluted and be administered in the presence of plasma. A 3% rat plasma solution in saline is used to dilute the insulin in this laboratory. Insulin is HumulinR 100U/ml (Eli Lily); although other fast acting insulin can be employed. Ensure diluted insulin is well mixed prior to putting in infusion syringe.
Prepare 50% dextrose. This can be placed directly into syringe on the infusion pump. Mark pump with 'glucose' to avoid future confusion. Remember to ensure sufficient volumes of insulin and glucose for the study. Clamps generally last ~2h. In the present protocol, 3ml syringes are used.
Place glucose and insulin syringes onto Harvard Apparatus Model 11 Plus Syringe Pumps. Advance glucose to Y-Connector and clamp line with a hemostat. Advance insulin to animal, clamp line with hemostat. Clamp lines and allow rat to relax for 30min prior to starting the experiment. NOTE: Hemostat tip guards are suggested in order to avoid permanent crimping of the lines.
Prepare centrifuge and EDTA-coated tubes for plasma collection. In the present study, additional blood samples will be obtained once the animal is clamped.
Part 2: Experimental Protocol
A baseline insulin sample and hematocrit sample should be acquired. Hematocrit sampling ensures blood volume is maintained throughout the experiment. Generally, hematocrit levels should not fall more than 10% of the initial value.
Obtain a baseline glucose sample (One Touch Ultra, LifeScan, Inc.) and determine the whole blood glucose level to be clamped. Here, we clamp at euglycemia, or 5.0-5.5mM (Figure 2). After each blood sample, flush arterial line with a small volume of 10U/ml heparinized saline to prevent clotting. NOTE: Ensure no clots or air bubbles are in the line prior to infusing. These can give the animal a stroke.
Start insulin infusion. Take blood glucose at 5-10min intervals, monitoring glucose at each time point. Adjust glucose infusion rate as required until a steady state is achieved. This process is generally a trial and error process and can take 30min to >2h. The levels of glucose required to maintain glycemia are dependent upon the experimental protocol, species and conditions present (Figure 2).
Steady state glucose levels are three consecutive readings within a defined range. Here, three readings within ~1mM is considered clamped (e.g. 4.8, 5.2, 5.6mM). Once steady state is achieved, record the glucose infusion rate required to maintain glycemia for a 30min period. During this time additional blood samples can be obtained. At minimum, a second plasma insulin sample and hematocrit sample should be obtained.
Once clamp is completed, animals can be used for further testing or euthanized and tissues collected for further analysis. Depending on the animal, catheter lines can stay patent for 5-7 days.
D: Hyperinsulinemic-Euglycemic (Insulin) Clamp Results
When performed correctly, the clamp procedure assesses the steady state insulin sensitivity of the rat. In presenting data obtained from the clamp, it is essential to document glucose levels and glucose infusion rates. Stable glucose levels over a time course of a minimum of 30min (Figure 3) are indicative of a steady state. Glucose is considered stable only if whole blood glucose is maintained within ~1mM. Glucose infusion rates show the levels of exogenous glucose required to maintain glycemia. Where possible, these figures should be shown as a time course rather than single, averaged value (Figure 4).
Other recommended measures to be reported are plasma insulin and hematocrit. The determination of both fasting and clamped insulin levels confirm that insulin has been successfully administered and will detect any differences in levels between treatment groups (Figure 5). Obtaining hematocrit measures at baseline and at the conclusion of the insulin clamp are suggested (Figure 6). This is to ensure hematocrit levels do not fall more than 5% during the experiment and the accompanying alterations in blood volume and flow do not influence glucose disposal.
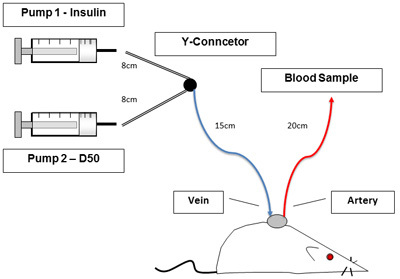 Figure 1. Experimental set-up. Figure 1 shows unrestrained, conscious rat during the clamp procedure. Catheters allow blood sampling and infusions without handling the animal. Pumps to the left contain insulin and glucose.
Figure 1. Experimental set-up. Figure 1 shows unrestrained, conscious rat during the clamp procedure. Catheters allow blood sampling and infusions without handling the animal. Pumps to the left contain insulin and glucose.
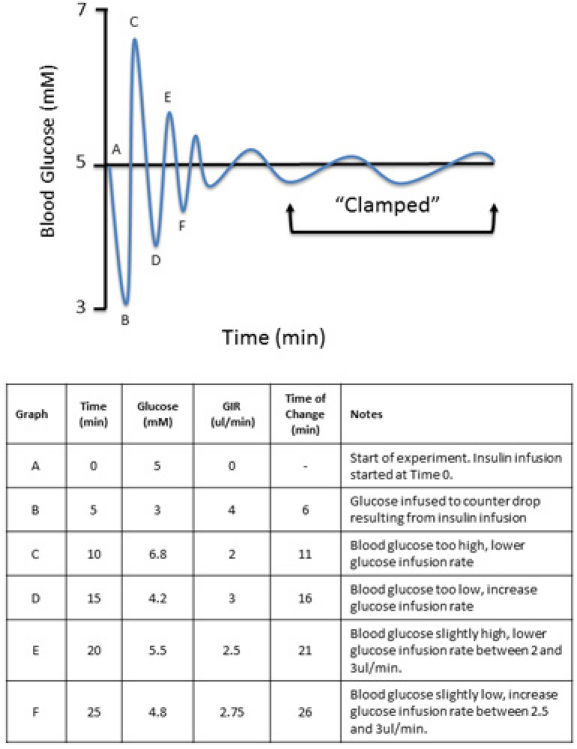 Figure 2. Expected data during the clamp procedure. To obtain a clamp at baseline levels (euglycemia, 5mM), the levels of exogenous glucose (D50) are manipulated until the baseline or 'clamp' is achieved.
Figure 2. Expected data during the clamp procedure. To obtain a clamp at baseline levels (euglycemia, 5mM), the levels of exogenous glucose (D50) are manipulated until the baseline or 'clamp' is achieved.
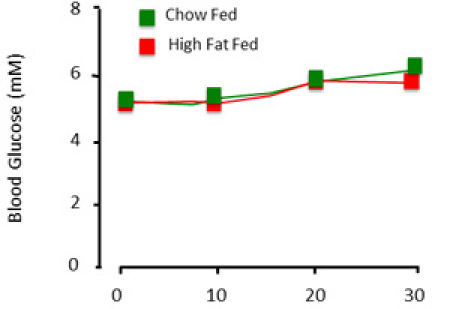 Figure 3. Expected plasma glucose results of the clamp procedure. When the animal is 'clamped', blood glucose is relatively stable across time and experimental groups.
Figure 3. Expected plasma glucose results of the clamp procedure. When the animal is 'clamped', blood glucose is relatively stable across time and experimental groups.
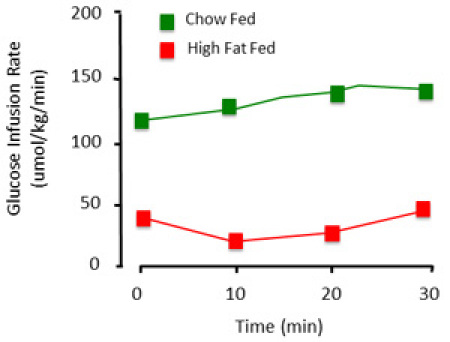 Figure 4. Representative glucose infusion rates of the clamp procedure. The amount of exogenous glucose required to maintain euglycemia differs. This is illustrated with a control (chow fed) and high fat fed (insulin resistant) animals. The high fat fed animal requires less glucose infused to maintain glycemia, primarily because it is insensitive to the insulin infused.
Figure 4. Representative glucose infusion rates of the clamp procedure. The amount of exogenous glucose required to maintain euglycemia differs. This is illustrated with a control (chow fed) and high fat fed (insulin resistant) animals. The high fat fed animal requires less glucose infused to maintain glycemia, primarily because it is insensitive to the insulin infused.
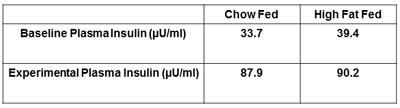 Figure 5. Representative plasma insulin of the rat. The plasma insulin during the insulin clamp should be higher than the fasted, baseline plasma insulin. This ensures insulin was properly administered to the animal during the insulin clamp.
Figure 5. Representative plasma insulin of the rat. The plasma insulin during the insulin clamp should be higher than the fasted, baseline plasma insulin. This ensures insulin was properly administered to the animal during the insulin clamp.
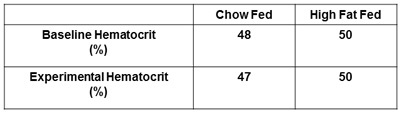 Figure 6. Reporting hematocrit. The baseline hematocrit and hematocrit following the experiment must be obtained and reported. This ensures hematocrit levels do not fall more than 5% of baseline levels resulting from excessive arterial blood sampling.
Figure 6. Reporting hematocrit. The baseline hematocrit and hematocrit following the experiment must be obtained and reported. This ensures hematocrit levels do not fall more than 5% of baseline levels resulting from excessive arterial blood sampling.
Discussion
Initially developed for investigation of insulin sensitivity in humans, the clamp procedure has now been adapted to other species including laboratory rats and mice. Investigating animal models of insulin resistance provides a significant aid in understanding the pathophysiology of insulin sensitivity and associated pathologies as well as identifying therapeutic interventions that have clinical value1,2. Several methods to evaluate insulin sensitivity in animals have been employed3. Such techniques include variations of glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs)4-6 as well as indexes of insulin sensitivity/resistance derived from fasting steady-state conditions7-9. However, when insulin sensitivity is the primary experimental objective and technical feasibility is not limiting the insulin clamp remains the standard. Here we present the most basic clamp procedure in the conscious, unrestrained rat. Insulin is administered at a constant rate while variable exogenous glucose is infused to maintain glycemia. Access to the circulation of the animal is obtained through implantation of arterial and jugular catheters. As animal stress (handling) and anesthesia alter glucose flux and insulin sensitivity, a model that minimizes these factors is favored and utilized in this procedure.
The insulin clamp is commonly used in diabetes, cardiovascular and pharmaceutical investigations. Modifications to the procedure include the addition of isotopic tracers (radioactive or stable). These are powerful in that they allow the investigator to determine how specific tissues and biochemical pathways are altered in response to the clamp. Common isotopes include the use of 2-[14C]-deoxyglucose to examine whole body and tissue specific glucose disposal as well as [3H]-glucose for the measurement of glucose flux. Excellent reviews of methodological and reporting issues when performing the insulin clamp procedure by Wasserman et al.10,11 are highly recommended.
Disclosures
Procedures were approved by the University of Calgary Animal Care and Use Committee and abide by the Canadian Association for Laboratory Animal Science guidelines for experimentation. The authors have no conflicting interests or disclosures.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research and Genome Canada.JS holds salary support awards from the Alberta Heritage Foundationfor Medical Research, Heart and Stroke Foundation of Canadaand the Canadian Diabetes Association. Special thanks to Dr. David Wasserman and Bingle Bracy for teaching this procedure to the Shearer laboratory.
References
- Halseth AE, Bracy DP, Wasserman DH. Limitations to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am J Physiol Endocrinol Metab. 2000;279:E1064–E1071. doi: 10.1152/ajpendo.2000.279.5.E1064. [DOI] [PubMed] [Google Scholar]
- Halseth AE, Bracy DP, Wasserman DH. Limitations to muscle glucose uptake due to high fat feeding. Am. J. Physiol. 2000;279:E1064–E1071. doi: 10.1152/ajpendo.2000.279.5.E1064. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Cho H. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Dubois MJ. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med. 2006;12:549–556. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]
- Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001;281:693–703. doi: 10.1152/ajpendo.2001.281.4.E693. [DOI] [PubMed] [Google Scholar]
- Herbach N. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes. 2007;56:1268–1276. doi: 10.2337/db06-0658. [DOI] [PubMed] [Google Scholar]
- Maeda N. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes. 2006;55:3594–3603. doi: 10.2337/db06-0667. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Ayala JE, McGuinness OP. Lost in translation. Diabetes. 2009;58:1947–1950. doi: 10.2337/db09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]


