Abstract
Enterococci are a common cause of bacteremia with E. faecalis being the predominant species followed by E. faecium. Because resistance to ampicillin and vancomycin in E. faecalis is still uncommon compared to resistance in E. faecium, the development of rapid tests allowing differentiation between enterococcal species is important for appropriate therapy and resistance surveillance. The E. faecalis OE PNA FISH assay (AdvanDx, Woburn, MA) uses species-specific peptide nucleic acid (PNA) probes in a fluorescence in situ hybridization format and offers a time to results of 1.5 hours and the potential of providing important information for species-specific treatment. Multicenter studies were performed to assess the performance of the 1.5 hour E. faecalis/OE PNA FISH procedure compared to the original 2.5 hour assay procedure and to standard bacteriology methods for the identification of enterococci directly from a positive blood culture bottle.
Protocol
1. Specimen Collection and Preparation
Collect venous blood from patients suspected of sepsis and put into two BACTEC (BD, Sparks, MD) blood culture bottles, one aerobic and one anaerobic, together comprising one blood culture set.
Place bottles into the BACTEC 9240 instrument at 35°C.
Incubate until the instrument is triggered into alarm due to growth of organisms in the blood culture bottle.
Remove bottle from the blood culture instrument.
2. Preparation of Reagents Prior to Staining
Prepare the wash solution by adding 4 mL of 60x Wash Solution followed by 240 mL of fresh deionized or distilled water directly to the Staining Dish.
Put the staining dish in the 55°C water bath to warm.
Store the remaining concentrate at 2-8°C.
Remove the Mounting Medium from the refrigerator and warm to room temperature.
3. Gram Staining
Gently swirl the blood culture bottle.
Using a needle and syringe or a subculturing apparatus, remove blood (approximately 5 mL) from the bottle and place in a sterile screw capped tube.
Using a sterile pipette, place one large drop of blood onto a glass microscope slide.
Air dry the slide and fix in methanol for 10 seconds.
Allow slide to air dry.
Perform a Gram stain on the blood film to determine the morphology of the organism growing in the blood culture bottle.
Using an oil immersion lens, view slide. Gram positive cocci in pairs and short chains observed on the Gram stain is most consistent with enterococcus species.
This Gram stain result then drives the selection of the proper PNA FISH probe kit to use for bacterial identification. This blood culture will be stained using the E. faecalis/OE PNA FISH stain.
4. PNA FISH Stain
Mix the tube containing the blood gently by swirling prior to smear preparation.
Place one drop of Fixation Solution on the well of a PNA FISH Microscope Slide.
Transfer 10 μL or one small drop from the tube with the positive blood culture to the Fixation Solution and mix gently to emulsify.
Fix the smears by heating them for 20 min. at 55°C on a heating plate.
5. Quality Control Material
One positive and one negative quality control slide must be tested with each batch of slides for staining.
Use E. faecalis/OE Control Slides purchased from AdvanDx for this purpose or prepare smears from liquid cultures of reference strains of E. faecalis and E. faecium as Positive Control either on separate slides or mixed on one slide and Staphylococcus spp or Streptococcus spp as Negative Control material.
The QC results should be able to monitor for appropriate testing conditions, particularly those affecting hybridization stringency and cell wall penetration, since PNA methodology is designed to optimize cell wall penetration
6. Hybridization
Add one drop of E. faecalis/OE PNA to the well on the microscope slide with the smear.
Add coverslip. Avoid air bubbles.
Incubate for 30 ± 5 min. at 55 ± 1°C on heating plate
Following incubation place the slides in slide carrier
7. Stringent Wash
Immerse slide carrier in the preheated Wash Solution at 55°C and carefully remove the coverslip. Often, the coverslip slides off by gently agitating the slide in the Wash Solution. Occasionally, the coverslip must be gently pushed off with forceps.
Incubate in the wash solution for 30 ± 5 min. at 55 ± 1°C.
Remove the slides from the wash solution
Allow the slide to air dry
8. Mounting
Add one drop of Mounting Medium to each slide
Add coverslip. Avoid air bubbles.
Examine the slide on a fluorescence microscope within 2 hours.
Do not expose the slides to direct sun light or other strong light sources as this may lead to fluorescence quenching.
9. Interpretation of Results
The fluorescence microscope used for slide examination must be equipped with the AdvanDx Dual Band Filter and a 60x or 100x oil objective. The QC slides should be examined first to confirm that hybridization did occur. E. faecalis should appear as bright green fluorescent cocci in multiple fields of view and the E. faecium will appear as bright red cocci. Non-enterococci control slide should appear nonfluorescent. After confirming the system in controls, the patient slides can be examined. At first the blood film will appear reddish but the bright red and green cocci will be quite apparent.
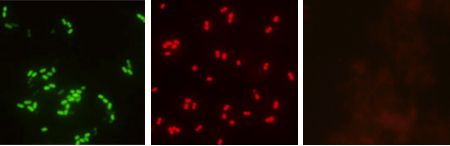 Figure 1.Representative examples of green-positive E. faecalis (left), red positive E. faecium (middle), and negative (right) test results.
Figure 1.Representative examples of green-positive E. faecalis (left), red positive E. faecium (middle), and negative (right) test results.
10. Representative Results
Three institutions were included in a multi-center clinical trial evaluating this PNA FISH stain and comparing a 2.5 hour protocol to the shortened 1.5 hour protocol that has just been reviewed. A total of 152 routine Gram positive cocci in pairs and chains (GPC) positive blood culture bottles were included in the studies. There was 100% (152/152) agreement between results of the modified and the original assay procedure for E. faecalis/OE PNA FISH: 41/41 E. faecalis; 33/33 other enterococci and 78/78 other GPC (Table 1). This staining method had exquisite sensitivity and specificity during this clinical trial (Table 2).
| Routine Methods | E. faecalis /OE PNA FISH Standard Procedure | E. faecalis/OE PNA FISH Short Procedure | |
| E. faecalis | 41 | 41 (Green Positive) | 41 (Green Positive) |
| E. faecium | 27 | 27 (Red Positive) | 27 (Red Positive) |
| E. casseliflavus | 2 | 2 (Red Positive) | 2 (Red Positive) |
| E. gallinarum | 2 | 2 (Red Positive) | 2 (Red Positive) |
| Other Enterococcus spp. | 2 | 2 (Red Positive) | 2 (Red Positive) |
| S. pneumoniae | 17 | 17 (Negative) | 17 (Negative) |
| S. viridans | 33 | 33 (Negative) | 33 (Negative) |
| S. mitis | 1 | 1 (Negative) | 1 (Negative) |
| S. pyogenes | 3 | 3 (Negative) | 3 (Negative) |
| S. bovis | 2 | 2 (Negative) | 2 (Negative) |
| S. salivarius | 1 | 1 (Negative) | 1 (Negative) |
| S. sanguinis | 2 | 2 (Negative) | 2 (Negative) |
| S. agalagtae | 7 | 7 (Negative) | 7 (Negative) |
| Other Streptococcus spp. | 7 | 7 (Negative) | 7 (Negative) |
| Abiotrophia spp. | 3 | 3 (Negative) | 3 (Negative) |
| Peptostrepococcus spp. | 2 | 2 (Negative) | 2 (Negative) |
| Total | 152 | 152/152 100% Agreement | 152/152 100% Agreement |
Table 1. Listing of bacteria tested using both the standard (2.5hr) and rapid (1.5 hr) protocol.
| Sensitivity E.faecalis | Sensitivity Other Enterococcus spp. | Specificity |
| 100% (41/41) 95% CI (93.0-100) | 100% (33/33) 33/33 95% CI (91.3-100) | 100% (78/78) 95% CI (96.2-100) |
Table 2. Sensitivity and Specificity of the 1.5 hour PNA FISH protocol.
Discussion
The E. faecalis/OE PNA FISH assay for enterococcus can provide identification 2-3 days earlier than standard culture methods. The shortened procedure for the assay (1.5 hourr) provides rapid identification that can be used for a better approach to antimicrobial therapy and patient outcome. One study to support this was performed by Forrest, et al. (6). Over two consecutive years beginning in 2005 the microbiology laboratory identified Gram positive cocci in pairs and chains growing in blood culture bottles by conventional microbiological methods and in 2006 adding the E. faecalis/OE PNA FISH. In addition a treatment algorithm developed by the institutions antimicrobial team (AMT) to effectively use the PNA FISH data generated by the laboratory in timely manner. Primary outcome assessed was time from blood culture draw to the implementation of effective antimicrobial therapy before and after PNA FISH was instituted into the laboratories workflow. Severity of illness, patient location and empiric antimicrobial therapy were measured. A total of 224 patients with hospital acquired enterococcal bacteremia were evaluated with 129 in the pre-intervention period and 95 in the PNA FISH period. PNA FISH identified E. faecalis 3 days earlier than conventional cultures (1.1 vs. 4.1 days, p<0.001). PNA FISH identified E. faecium a median 2.3 days earlier (1.1 vs. 3.4 days, p<0.001) and was associated with statistically significant reductions in time to initiating effective therapy (1.3 vs. 3.1 days, p<0.001) and decreased 30 day mortality (26% vs. 45%, p=0.04). This group concluded that the E. faecalis/OE PNA FISH assay in conjunction with an AMT treatment algorithm resulted in earlier initiation of appropriate empiric antimicrobial therapy for patients with hospital acquired E. faecium bacteremia.
Reagents must not be used after the expiration dates printed on the labels and reagents should not be modified in any way. They should remain in the refrigerator when not in use or they could lose potency.
Blood culture bottles need to be mixed prior to sampling.
It is important to not allow the dropper bottle tip to touch a blood smear because this may cause cross contamination of material between slides, or cause contamination of the reagent.
Make sure the water bath and the heating block are maintained at a constant 55°C.
Do not use filters other than the Dual Band Filter or colors will not be clearly discerned.
Do not use microscope slides other than the Microscope Slides or hybridization will not occur.
It is important that the microscope is functioning properly. Make sure the microscope bulb is within the approved number of hours for usage.
Some rare-occurring Enterococcus species are not detected by the PNA probe hybridizing to other Enterococcus species, such as E. asinii, E.dispar, E. haemoperoxidus, E. cecorum, E. columbae, E. saccharolyticus, E. solitarius and E. sulfurous.
E. moraviensis is identified as E. faecalis due to sequence similarities.
Some strains of Streptococcus anginosus produce a false green positive fluorescence due to sequence similarities.
VersaTREK blood culture bottles were not evaluated during the clinical studies. The performance to detect E. faecalis and other Enterococcus spp. with VersaTREK blood culture bottles is unknown.
False positive green autofluorescence may occur if a standard FITC filter is used instead of the Dual Band Filter.
False negative results may infrequently occur due to mixed growth or due to error in assay technique.
The type and condition of the instrumentation used will influence the visual appearance of the image obtained. The fluorescence may varydue to the type of microscope employed, the light source and the level of rRNA in the cells
Isolation on solid media is needed to differentiate mixed growth with other organisms and to identify positive blood cultures yielding a negative result.
The product has not been validated with specimens other than blood cultures.
Disclosures
The production of this video-article was sponsored by AdvanDx. Benjamin Crystal is an employee of AdvanDx, who makes the tools and reagents used in this article.
Acknowledgments
Studies were sponsored by AdvanDx.
References
- Oliveira K, Chapin KC, Musgnug MC, Haase G, Weber-Heynemann J, DeGirolami PC, Dakos J, Stender H, editors. Rapid identification and differentiation of Enterococcus faecalis and other Enterococcus species directly from positive blood cultures by dual color fluorescence in situ hybridization using PNA probes. 42rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002; San Diego. 2002. [Google Scholar]
- Chapin KC, Musgnug MC, Oliveira K, DeGirolami PC, Dakos J, Johansen JT, Procop GW, Wilson D, Padilla E, Gonzalez V, Stender H, editors. Multicenter evaluation of E. faecalis PNA FISH for rapid identification and differentiation of Enterococcus faecalis and other Enterococcus species directly from positive blood cultures. 103rd Annual Meeting of American Society; 2003; Washington DC. 2003. [Google Scholar]
- Rausei V, Youssef E, Morgan M, editors. Direct identification of Staphylococcus aureus, Enterococcus faecalis, and Candida albicans from blood cultures using fluorescence in situ hybridization with nucleic acid probes. 104th Annual Meeting of American Society for Microbiology; 2005; New Orleans, LA. 2005. [Google Scholar]
- Novak-Weekley SM, LaForga M, Carey L, Frazier L, editors. Validation of S. aureus PNA FISH, E. faecalis PNA FISH & C. albicans PNA FISH for Rapid Identification of Positive Blood Culture Bottles. 106th Annual Meeting of American Society for Microbiology; 2006; Orlando, FL. 2006. [Google Scholar]
- Johnson JK, Roberts AA, Forrest GN, Lincalis DP, Venezia RA, editors. Rapid Identification of Enterococcus Species in Positive Blood Cultures with Therapeutic Implications. 106th Annual Meeting of American Society for Microbiology; 2006; Orlando, FL. 2006. [Google Scholar]


