Abstract
Allogeneic hematopoietic stem cell transplantation (AHSCT) offers the best chance of cure for many patients with congenital and acquired hematologic diseases. Unfortunately, transplantation of alloreactive donor T cells which recognize and damage healthy patient tissues can result in Graft-versus-Host Disease (GvHD)1. One challenge to successful AHSCT is the prevention of GvHD without associated impairment of the beneficial effects of donor T cells, particularly immune reconstitution and prevention of relapse. GvHD can be prevented by non-specific depletion of donor T cells from stem cell grafts or by administration of pharmacological immunosuppression. Unfortunately these approaches increase infection and disease relapse2-4. An alternative strategy is to selectively deplete alloreactive donor T cells after allostimulation by recipient antigen presenting cells (APC) before transplant. Early clinical trials of these allodepletion strategies improved immune reconstitution after HLA-mismatched HSCT without excess GvHD5, 6. However, some allodepletion techniques require specialized recipient APC production6, 7and some approaches may have off-target effects including depletion of donor pathogen-specific T cells8and CD4 T regulatory cells9.One alternative approach is the inactivation of alloreactive donor T cells via induction of alloantigen-specific hyporesponsiveness. This is achieved by stimulating donor cells with recipient APC while providing blockade of CD28-mediated co-stimulation signals10.This "alloanergization" approach reduces alloreactivity by 1-2 logs while preserving pathogen- and tumor-associated antigen T cell responses in vitro11. The strategy has been successfully employed in 2 completed and 1 ongoing clinical pilot studies in which alloanergized donor T cells were infused during or after HLA-mismatched HSCT resulting in rapid immune reconstitution, few infections and less severe acute and chronic GvHD than historical control recipients of unmanipulated HLA-mismatched transplantation12. Here we describe our current protocol for the generation of peripheral blood mononuclear cells (PBMC) which have been alloanergized to HLA-mismatched unrelated stimulator PBMC. Alloanergization is achieved by allostimulation in the presence of monoclonal antibodies to the ligands B7.1 and B7.1 to block CD28-mediated costimulation. This technique does not require the production of specialized stimulator APC and is simple to perform, requiring only a single and relatively brief ex vivo incubation step. As such, the approach can be easily standardized for clinical use to generate donor T cells with reduced alloreactivity but retaining pathogen-specific immunity for adoptive transfer in the setting of AHSCT to improve immune reconstitution without excessive GvHD.
Keywords: Immunology, Issue 49, Allogeneic stem cell transplantation, alloreactivity, Graft-versus-Host Disease, T cell costimulation, anergy, mixed lymphocyte reaction.
Protocol
1. Preparation of PBMC
Procedures for good aseptic technique and universal precautions must be adhered to during the conduct of this protocol.
Isolate PBMC from healthy volunteer donors by density gradient centrifugation using Ficoll-Hypaque. Alternatively cryopreserved PBMC can be resuscitated.
Count viable PBMC after staining with Trypan Blue using a hemocytometer. Resuspend PBMC in complete culture media (CM) at a concentration of 1 million viable cells/mL in a 15mL Falcon tube.
2. Setting Up the Bulk Alloanergizing Co-culture
PBMCs from two separate donors are needed to set up the alloanergization cultures. Cells from one donor will serve as the responder and cells from another donor will serve as the stimulator (termed the First Party stimulator).
Place 10 million stimulator PBMC at 1 million/ml in a 15 mL Falcon tube and add 100mg of anti-B7.1 and 100mg of anti B7.2 antibodies to block costimulation. Gently agitate the tube and incubate for 30 minutes. Incubation conditions for this and all subsequent steps are 37°C/5% CO2/80% humidity
Irradiate stimulator PBMC (33 Gy) and add to a T-25 50cm3 cell culture flask with a gas-permeable cap. Label the flask "Bulk alloanergizing co-culture".
Add 10mL of responder PBMC (at 1 million/ml in CM) to the flask.
Add a further 100mg each of anti-B7.1 and anti B7.2 antibodies and mix gently prior to placing the flask upright in an incubator for 72 hours
3. Setting Up the Bulk Control Co-culture
Place 10 million stimulator PBMC (at 1 million/ml) in a 15mL Falcon tube.
Irradiate 10 million stimulator PBMC (33 Gy).and add to a 50cm3 cell culture flask. Label "Bulk control co-culture".
Add 10mL of responder PBMC at 1 million/ml in CM to the flask and mix gently prior to placing the flask upright in an incubator for 72 hours.
4. Setting Up the Primary Mixed Lymphocyte Reaction (MLR) to Measure the Efficacy of Co-stimulatory Blockade
The efficacy of costimulatory blockade is measured in a primary MLR which is set up using PBMC from the bulk alloanergizing and control co-cultures
The primary MLR is set up on the same day that the bulk cultures have been set up
First label one U bottomed 96 well plate "Primary MLR" for each of the three time points to be measured (Day 4, 5 and 6 after plates are set up).
Decant 5mL from each of the bulk control and alloanergizing co-cultures into 15mL Falcon tubes.
Pipette 200mL of cell suspension from each Falcon tube into triplicate wells on each plate. Label these wells "no costimulatory blockade (CSB)" for the cells from the bulk control co-cultures and "CSB" for the cells from the bulk alloanergizing co-cultures
Add 200mL of CM to 6 wells on each plate as negative controls. 200mL PBS is added to all empty wells to reduce evaporation. Place plates in the incubator.
5. Setting Up the Secondary MLR to Measure the Efficacy of Alloanergization
72 hours after setting up the bulk co-cultures, residual alloresponses in alloanergized PBMCs are measured in a secondary MLR. In the secondary MLR, PBMC from both the bulk alloanergizing co-culture and the bulk control co-culture are washed and restimulated with irradiated allostimulator PBMC
To set up the secondary MLR label one U bottomed 96 well plate "Secondary MLR" for each of the three time points to be measured (Day 3, 5 and 7 after set up).
Remove 5mL from each of the bulk control and alloanergizing co-cultures, transfer to 15mL Falcon tubes and centrifuge for 5-10 minutes at 2000 rpm.Aspirate the supernatant carefully, disrupt the pellet, add 10mL of CM and centrifuge the cells again. Repeat this washing step.
Resuspend the cells in 1mL of CM. Count the viable cells using Trypan Blue staining, and adjust the cell concentration to 1 million viable responder cells/mL.
Pipette 100mL of cell suspension from each Falcon tube into triplicate wells of each plate. Label these wells "First Party (FP) allorestimulation"
Pipette 100mL of cell suspension from each Falcon tube into a further 3 wells on each plate and label these wells "CD3/28 stimulation"
To prepare stimulator cells for the secondary MLR, isolate PBMC from fresh blood (or resuscitate cryopreserved PBMC) from the same healthy donor used to supply first party stimulator PBMCs in the alloanergizing and control cultures.
Suspend PBMC from the FP donor at a concentration of 1 million/mL and irradiate (33Gy)
Add 100mL of irradiated stimulator cells to wells labeled "FP allorestimulation"
For positive controls, add 1 mL each of CD3 and CD28 monoclonal antibodies and 98mL of CM to wells labeled "CD3/28 stimulation". Add 200 mL of CM to 6 wells on each plate as a negative control and 200mL of PBS to empty wells. Place the plates in the incubator.
6. Measuring the Specificity of Alloanergization
The specificity of alloanergization is determined by comparing proliferation of responder cells taken from the completed alloanergizing and control co-cultures after stimulation with irradiated PBMC obtained from a "third party" healthy donor (i.e. a different donor to the First Party) in a secondary MLR.
Label three 96 well plates "Secondary MLR Specificity" Day 3, 5 and 7.
Remove 5mL from each of the bulk control and alloanergizing co-cultures transfer to 15mL Falcon tubes and centrifuge for 5-10 minutes at 2000 rpm. Aspirate the supernatant carefully, disrupt the pellet, add 10mL of CM and centrifuge the cells again. Repeat this washing step.
Resuspend the cells in 1mL of CM. count using Trypan Blue staining, and adjust the cell concentration to 1 million viable responder cells/mL.
To prepare third party stimulator cells for the secondary MLR, isolate PBMC from fresh blood (or resuscitate cryopreserved PBMC) from a new healthy donor. Pipette 100mL of cell suspension from each Falcon tube into triplicate wells of each plate. Label these wells "TP allostimulation"
Suspend PBMC from a TP donor at 1 million/mL and irradiate (33 Gy)
Add 200mL of irradiated TP stimulator cells to wells labeled "TP allostimulation"
The specificity of alloanergization may also be determined by comparing proliferation of responder cells taken from the completed alloanergizing and control co-cultures after stimulation with an infectious pathogen such as CMV. For this step it is essential to use responder PBMC from donors with previously demonstrated proliferative responses to CMV lysate.
Label three 96 well plates "Secondary MLR CMV" Day 3, 5 and 7.
To prepare the responder cells for the secondary MLR to measure CMV responses, transfer 5 ml from each of the bulk control and alloanergizing co-cultures to 15 ml tubes and wash, count and resuspend at 1 million cells/ml in CM as previously described.Pipette 100mL of cell suspension from each Falcon tube into a 3 wells on each plate.
Add 0.1 mL of CMV lysate in 100ul CM to wells
Add 200 mL of CM to 6 wells on all plate as a negative control and add 200mL of PBS to empty wells. Place the plates in the incubator.
7. Measuring Proliferation in the Primary and Secondary MLRs
Responder cell proliferation is measured by thymidine incorporation assay in the primary and secondary MLRs. Proliferation is measured at Day 4, 5 and 6 after the Primary MLR plates are set up and at Day 3, 5 and 7 after the Secondary MLR plates are set up.
1mCi of tritiated thymidine is added to wells 16 hours prior to harvesting
After the addition of tritiated thymidine, the plate is incubated for a further16 hours then harvested onto a filter mat using a Tomtec harvester (or similar). Air-dry the filtermat for one hour then place in a sample bag, add 5 mL scintillation fluid and seal. Read the plate in a Wallac Micro beta scintillation counter or similar.
8. Calculating the Efficacy of Co-stimulatory Blockade in the Primary MLR
The percentage inhibition (PI) of primary alloresponses is calculated as 100 x [mean cpm in allostimulation wells (bulk alloanergy co-culture PBMC) - mean cpm in allostimulation wells (bulk control co-culture PBMC)}
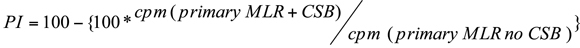
9. Calculating the Efficacy of Alloanergization in the Secondary MLR
The PI of secondary alloresponses is calculated as ![]()
10. Calculating the Specificity of Alloanergization in the Secondary MLR
After restimulation of cells with CD3 and CD28, TP allostimulators or CMV antigen, the PI of responses to these stimuli may also be calculated using this formula. This gives a measure of the specificity of the alloanergization process
11. Generating Alloanergized Donor PBMC for Clinical Use After Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
Alloanergized donor PBMC may be generated for clinical use after HLA-mismatched allogeneic HSCT using the protocol outlined above
When generating cells for clinical use, responder PBMC are obtained from the HSCT donor and stimulator PBMC from the HSCT recipient prior to transplant.
When generating PBMC for clinical use, all steps are performed under Good Manufacturing Process conditions in accredited stem cell processing facilities.
Additional quality control assays are performed including endotoxin assays and gram stain and culture to meet safety criteria prior to the release of cells for clinical use
12. Representative Results
Using HLA-mismatched stimulator and responder PBMC, the presence of costimulatory blockade in the primary MLR reduces mean alloproliferation of responder PBMC at Day 5 to around 30% (+/- 10%, mean +/- standard deviation) of that seen in control primary MLR in the absence of costimulatory blockade. This is equivalent to a mean inhibition of primary alloproliferation of around 70% (+/- 10%) at D5, Figure 1 A and B.
In the secondary MLR at Day 5, FP alloproliferation of alloanergized PBMC is typically 10-15% (+/- 10%) of that seen with control PBMC equivalent to a mean inhibition of proliferation of 85-90% (+/- 10%), Figure 2A and B. This demonstrates that alloanergized PBMC are hyporesponsive to FP allostimulators.
In contrast alloanergized PBMC typically retain 70-100% of their proliferation to mitogens (CD3/CD28 antibodies), TP allostimulators and CMV lysate (in CMV-reactive donors). This demonstrates that hyporesponsiveness of alloanergized PBMC is specific to FP alloantigens.
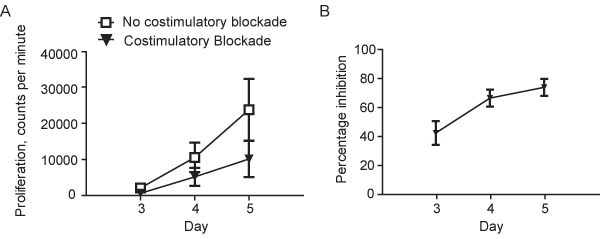 Figure 1. A.Proliferation (determined by thymidine incorporation) in a Primary Mixed Lymphocyte Reaction using HLA-mismatched stimulator and responder peripheral blood mononuclear cells (PBMC) in the absence or the presence of costimulatory blockade using humanized monoclonal anti-B7.1 and -B7.2 antibodies. Results are shown as mean (+/-sd) for 8 representative experiments using unique stimulator-responder pairs. B. Percentage inhibition of alloproliferation in primary MLRs performed in the presence of costimulatory blockade using humanized monoclonal anti-B7.1 and -B7.2 antibodies. Results are shown as mean (+/-sd) for the same 8 unique stimulator-responder pairs depicted in Figure 1A.
Figure 1. A.Proliferation (determined by thymidine incorporation) in a Primary Mixed Lymphocyte Reaction using HLA-mismatched stimulator and responder peripheral blood mononuclear cells (PBMC) in the absence or the presence of costimulatory blockade using humanized monoclonal anti-B7.1 and -B7.2 antibodies. Results are shown as mean (+/-sd) for 8 representative experiments using unique stimulator-responder pairs. B. Percentage inhibition of alloproliferation in primary MLRs performed in the presence of costimulatory blockade using humanized monoclonal anti-B7.1 and -B7.2 antibodies. Results are shown as mean (+/-sd) for the same 8 unique stimulator-responder pairs depicted in Figure 1A.
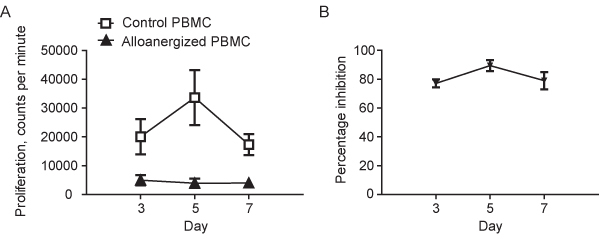 Figure 2. A. Proliferation (determined by thymidine incorporation) in secondary MLRs where responder PBMC from primary MLRs performed in the absence (control PBMC) or the presence of costimulatory blockade (alloanergized PBMC) are restimulated with irradiated first party stimulators. Results are shown as mean (+/-sd) for the 8 unique stimulator-responder pairs depicted in Figure 1. B. Percentage inhibition of First Party alloproliferation in secondary MLRs where responder PBMC from primary MLRs performed in the absence (control PBMC) or the presence of costimulatory blockade (alloanergized PBMC) are restimulated with irradiated first party stimulators. Results are shown as mean (+/-sd) for the 8 unique stimulator-responder pairs depicted in Figure 1 and Figure 2A.
Figure 2. A. Proliferation (determined by thymidine incorporation) in secondary MLRs where responder PBMC from primary MLRs performed in the absence (control PBMC) or the presence of costimulatory blockade (alloanergized PBMC) are restimulated with irradiated first party stimulators. Results are shown as mean (+/-sd) for the 8 unique stimulator-responder pairs depicted in Figure 1. B. Percentage inhibition of First Party alloproliferation in secondary MLRs where responder PBMC from primary MLRs performed in the absence (control PBMC) or the presence of costimulatory blockade (alloanergized PBMC) are restimulated with irradiated first party stimulators. Results are shown as mean (+/-sd) for the 8 unique stimulator-responder pairs depicted in Figure 1 and Figure 2A.
Discussion
The induction of alloantigen-specific hyporesponsiveness, or anergy, in donor PBMC via allostimulation in the presence of co-stimulatory blockade is a simple technique for the generation of donor PBMC with reduced alloreactivity. We have developed the process in the laboratory and are currently applying the strategy in the clinic to generate donor PBMC with reduced alloreactivity for infusion after HLA-mismatched allogeneic HSCT. The aim of using such therapy is to improve immune reconstitution without excess toxicity. Although our current clinical application of the strategy is limited to the setting of allogeneic HSCT, the approach could be applied to other settings where tissue damage is caused by unwanted T cell responses, such as rejection of solid organ transplantation or autoimmune conditions.
Several reagents can be used for blockade of CD28-mediated co-stimulatory signals during the alloanergization co-culture process. Here we describe our current protocol using clinical-grade humanized murine monoclonal antibodies directed against the ligands of CD28 (B7.1 and B7.2). Non-clinical grade murine anti-human B7.1 and B7.2 antibodies are commercially available from several manufacturers. Alternatively, the fusion protein Cytotoxic T Lymphocyte Antigen (CTLA) 4-Immunoglobulin (Ig) can be used to block CD28-costimulation during the alloanergization process. CTLA4-Ig, which consists of the extracellular portion of the CTLA4 molecule linked to the IgG Fc gamma receptor, binds with high affinity to B7.1 and B7.2. The use of CTLA4-Ig results in similar efficacy and specificity of alloanergization of human PBMCs.
The technique of alloanergization is simple to perform. Fresh or previously cryopreserved stimulator PBMCs can be used with no significant variation in the outcome of the process. The requirement for only a single relatively brief ex vivo incubation step with no cell sorting procedures minimizes potential for cell death and bacterial contamination of cells prior to infusion which makes the strategy relatively simple to apply at a clinical scale.
One limitation of the approach we describe (and other approaches to selectively reduce alloreactivity of human cells) is the absence of a real-time assay to determine residual alloreactivity. Proliferation assays take 3-5 days to perform to confirm the reduction of alloreactivity and cells generated for clinical use must be infused prior to the results of these assays being available. Furthermore, measurement of proliferation of PBMCs by thymidine incorporation, although easy to perform, measures proliferation of B cells and other cell subsets in addition to T cell proliferation. CFSE dye dilution of responder PBMCs can be used as an alternate assay and permits determination of T cell subset-specific alloproliferation. One way to improve the clinical application of our strategy approach would be to develop and validate an assay of alloreactivity that takes only a few hours to perform which could be performed prior to release of alloanergized cells for clinical use.
In addition to demonstrating the efficiacy of the strategy it is also important to confirm the specificity alloanergization process. This can easily be done by determination of the relative preservation of alloresponses specific to third party allostimulators and when CMV-reactive donor cells are being alloanergized, by the preservation of proliferative responses to CMV
Disclosures
No conflicts of interest declared.
Acknowledgments
Supported by the National Institutes of Health (U19 CA100625 and R21 CA137645). JKD was supported by the Leukemia & Lymphoma Society and the American Society of Blood and Marrow Transplantation/OtsukaNew Investigator Award.
References
- Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- Keever CA. Immune reconstitution following bone marrow transplantation: Comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989;73:1340–1350. [PubMed] [Google Scholar]
- Bacigalupo A. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423–1428. [PubMed] [Google Scholar]
- Horowitz MM. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- Andre-Schmutz I. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- Amrolia PJ. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrolia PJ. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102:2292–2299. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- Perruccio K. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2008;40:76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Mielke S. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110:1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribben JG. Complete blockade of B7 family-mediated costimulation is necessary to induce human alloantigen-specific anergy: a method to ameliorate graft-versus-host disease and extend the donor pool. Blood. 1996;87:4887–4893. [PubMed] [Google Scholar]
- Davies JK, Yuk D, Nadler LM, Guinan EC. Induction of alloanergy in human donor T cells without loss of pathogen or tumor immunity. Transplantation. 2008;86:854–864. doi: 10.1097/TP.0b013e3181861b6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JK. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 phase 1 studies. Blood. 2008;112:2232–2241. doi: 10.1182/blood-2008-03-143636. [DOI] [PMC free article] [PubMed] [Google Scholar]


