Abstract
The isolated, ventilated and instrumented mouse lung preparation allows steady and pulsatile pulmonary vascular pressure-flow relationships to be measured with independent control over pulmonary arterial flow rate, flow rate waveform, airway pressure and left atrial pressure. Pulmonary vascular resistance is calculated based on multi-point, steady pressure-flow curves; pulmonary vascular impedance is calculated from pulsatile pressure-flow curves obtained at a range of frequencies. As now recognized clinically, impedance is a superior measure of right ventricular afterload than resistance because it includes the effects of vascular compliance, which are not negligible, especially in the pulmonary circulation. Three important metrics of impedance - the zero hertz impedance Z0, the characteristic impedance ZC, and the index of wave reflection RW - provide insight into distal arterial cross-sectional area available for flow, proximal arterial stiffness and the upstream-downstream impedance mismatch, respectively. All results obtained in isolated, ventilated and perfused lungs are independent of sympathetic nervous system tone, volume status and the effects of anesthesia. We have used this technique to quantify the impact of pulmonary emboli and chronic hypoxia on resistance and impedance, and to differentiate between sites of action (i.e., proximal vs. distal) of vasoactive agents and disease using the pressure dependency of ZC. Furthermore, when these techniques are used with the lungs of genetically engineered strains of mice, the effects of molecular-level defects on pulmonary vascular structure and function can be determined.
Protocol
In this protocol we demonstrate an isolated, ventilated, perfused mouse lung preparation that has previously been used to quantify the impact of pulmonary emboli and chronic hypoxia on pulsatile pulmonary vascular pressure-flow relationships (Tuchscherer, Webster, & Chesler, 2006; Tuchscherer et al., 2007). In brief, the mouse lungs are surgically isolated from surrounding tissues, placed in a heated chamber (IL-1; Harvard Apparatus, Holliston, MA) and ventilated (Ventilatory Control Module (VCM)-R with timer counter module (TCM); Harvard Apparatus). The lung vasculature is perfused with heated RPMI 1640 cell culture medium with 3.5% Ficoll using a syringe pump (Cole-Parmer, Vernon Hills, IL) to generate the steady flow waveforms or a high-frequency oscillatory pump (Bose -Electro Force, Eden Prairie, MN) in parallel with the syringe pump to create pulsatile pulmonary vascular flow waveforms. Pressure transducers (P75, Harvard Apparatus) measure the instantaneous pulmonary artery pressure (PAP) and left atrial pressure (LAP). Instantaneous flow rate (Q) is measured with an in-line flow meter (Transonic Systems, Inc., Ithaca, NY). Pulsatile pressure-flow relationships are derived from these measurements, which provide insight into pulmonary vascular physiology and pathology and right ventricular afterload.
1. Equipment:
Isolated lung set-up including mouse ventilator
Data acquisition system and computer with LabView program
Two pressure transducers and flowmeter for perfusate flow
Pressure transducer and flowmeter (pnemotachometer) for airway flow
High-frequency oscillatory pump and computer with Win Test program
Boom/zoom Microscope, lamp
Heating bath with high output pump for IL-1 system
2. Preparing the IL-1 system
Distilled water heated to 37°C by the heating bath is circulated into the interior of the IL-1 system.
All pumps, transducers and the IL-1 cannula are connected via clean tubing and all tubing is flushed with distilled water heated to 37°C. Air bubbles, which could travel into the lung and cause edema, must be removed. Tubing from the oscillatory flow pump to the flow sensor and from the flow sensor to the pulmonary artery cannula are flushed with 1% PBS.
The P75 pressure transducers are zeroed by closing the valve to the cannula, opening the valves to the atmosphere and then pushing the automatic zero button on the PLUGSYS amplifier. Then, the valve to the atmosphere is closed and the valve to the cannula opened.
The ceramic porous piece in the ventilation pathway of the IL-1 system is wetted to provide humidity.
3. Solutions
Prepare 3.5% by volume Ficoll-RPMI solution and sterile filter the media. Filtering the media ensures there are no large particles that could unintentionally embolize the lung. Using sterile media also reduces the likelihood of sudden edema developing in the lung. Fill one 10 ml syringe with RPMI for surgery and one 60 ml syringe with RPMI for each experimental trial. Heat the perfusate in a 37 °C water bath.
Prepare 1ml of heparin 500IU/100g body weight of the mouse (approximate). The heparin salt is 158IU/mg. For a ~25 g mouse, mix 1.25 mg heparin salt with 1ml PBS solution in a small microcentrifuge tube.
4. Ventilating a Mouse
After an intraperitoneal injection of 150 mg pentobarbital in solution per kilogram into the mouse, ensure deep anaesthesia by performing a hard pinch on a paw. If there is no reaction, prepare the mouse for surgery by pinning its front paws to corkboard for stability. Note: Throughout the experiment anesthetic depth is monitored by carefully observing the animal. The pins in the paws and frequent incisions act as noxious stimuli, the lack of response to which confirms that the animal remains in a surgical plane of anesthesia.
Spray the chest with 95% alcohol to wet down the fur and use straight forceps to grab the skin at the neck. Cut a 1 cm opening in the skin using the straight scissors.
Once the inside of the neck is exposed, remove all the white glandular tissue and superficial muscle, looking for the esophagus and trachea. Isolate the esophagus and trachea from tissue on both sides and posteriorly.
Insert the small bent forceps under the trachea and grab a piece of suture on the other side. Pull the suture under the trachea and tie a loose surgeons knot. Do not tighten the suture or tie the knot.
Cut a small angled "v" in the trachea with small scissors; do not cut all the way through the trachea. Move the cork board and mouse to the heated IL-1 system. Using the two blunt forceps, grab the tracheal cannula and the trachea below the "v". Then, slide the tracheal cannula into the trachea through the "v" opening. Tighten the suture around trachea and tracheal cannula to secure the cannula inside the trachea. Tie the knot.
Begin ventilation (50% inspiration, 90 breaths/min, with deep inspiration) with room air.
5. Perfusing a Ventilated Mouse
5.1. Access the right ventricle to inject heparin
Spray mouse chest with alcohol again to re-wet the fur. Remove all of the skin on the chest above the ribs using straight forceps and straight scissors. Cut upward along the sternum. Lift the skin on each side and then cut the skin following the line of the lower ribs.
Grasp the xiphoid process at the bottom of the sternum with tweezers and cut a hole into the diaphragm using the straight scissors. Grab the diaphragm with the tweezers and cut it away from the ribs.
Grasp the xiphoid process again with the tweezers (left hand) and use the ball-tip, angled scissors to cut up the sternum and through ribs, being careful not to cut the lungs, the heart or blood vessels (use the ball-tip on the scissors to guide you). There will be blood but as long as the cutting edge of the scissors is against the sternum, the heart and lungs will not be cut.
Grasp the ribs on the left side and cut away as much of the ribs as necessary to expose the heart. Slowly inject the right ventricle with 0.1 ml heparin solution. This step is important to preventing blood clots in the lung, which damage endothelial cells and will impair perfusion. The heparin must be injected while the heart is still beating.
5.2. Cannulate the main pulmonary artery
Cut away the rest of the ribs (left and right side) using the back (rounded) end of the forceps to gently push the lungs away from the rib wall. Grabbing the lungs themselves will damage the delicate tissue. Incidental contact between the scissor tips and the lung tissue will also cause damage.
Move the microscope into place over lung. Cut away the glandular and fatty tissue on top of the heart. Use the tweezers to pull it away from the arteries and veins and then cut with the spring scissors while the tissue is in tension.
Using another blunt set of tweezers, scoop from right to left from the top of heart under left atrium/ventricle to get the tip of the tweezers under the aorta and pulmonary artery (PA). Do this carefully- there should not be resistance to the tweezers. Using blunt tweezers reduces the likelihood of accidentally puncturing the pulmonary artery or aorta.
Once the tweezer tip is under the aorta and PA, grab a piece of suture and pull through. Tie a loose surgeons knot. Do not tighten the suture or tie the knot.
Optional: use the angled scissors to remove the lower half of the body. Cut down through the ribs and spine; cutting the aorta and vena cava will cause a significant amount of blood to flow -- use a Q-tip to staunch the flow. Place in a bag for disposal.
Prime PA cannula with 4 ml from a 10 ml syringe of RPMI. Double check that all tubing is free of air bubbles. Perfusion of the lung with distilled water or bubbles will result in edema.
Cut a small notch in the right ventricular free wall and insert the PA cannula, aiming down and to the right. The tip of the cannula should be visible through the transparent wall of the PA. Infuse a small amount of RPMI to confirm your location. Tighten the suture around the cannula, aorta and PA and tie the knot. Note that at this point, the aorta is also tied off so actual perfusion should not begin until the left atrium is cannulated.
5.3. Cannulate the left atrium
Cut a notch in lower part of left ventricle and inert the left atrial (LA) cannula, aiming upwards. Slight pressure may be needed to open the mitral valve in this direction. In the correct location, the cannula tip will slip through and be secure without suture.
5.4. Begin perfusion
Manually infuse RPMI from the 10 ml syringe at 0.3 ml per minute until RMPI is evident in the outflow tubing (pink-ish color in contrast to the clear PBS). If there is no flow in the outflow tubing, re-position the LA cannula. If no outflow can be obtained with re-positioning, check for a leak in the pulmonary artery. A leak or tear in the pulmonary artery cannot be repaired; this is cause for aborting the experiment.
Connect a 60 ml syringe to the PA cannula through the IL-1 system and start 1ml/min infusion of perfusate, checking for leakage and making sure the lungs turn white, which shows that RPMI is replacing blood in the lungs. Perfuse with slow flow for two minutes.
6. Measuring Pulsatile and Steady Pulmonary Pressure-Flow Relationships
For the pulsatile flow studies, first set the oscillatory pump piston displacements at the desired levels for each frequency in the WinTest program based on previous experiments. Due variability in lung structure and mechanics, the displacements have to be adjusted for each mouse. Set steady flow to the desired level. Open the valve to the oscillatory pump and start recording data right before running the oscillatory flow profile (Wintest program). Open the data file and plot the experimental flow (Q) in Excel. Adjust the oscillatory pump piston displacements at each frequency (Wintest program) so that Q max and Q min are as desired.
For steady flow trials, close the valve to the oscillatory pump. If this valve is left open, the oscillatory pump acts as a capacitor, dampening the changes from one flow rate to another. Collect data for at least 10 seconds at each flow rate or until the PA pressure does not change by more than 5%.
For either pulsatile or steady flow measurements, old ventilation at a constant pressure before data collection. Resume ventilation immediately after data collection.
Watch for RPMI in the airway tubing; this is evidence of edema and is cause for aborting the experiment. Also, do not let RPMI reach the airway pressure or flow sensors because it will damage the transducers.
7. Representative Results:
Representative Steady Results:
In the isolated lung set-up the experimenter has the ability to independently control not only the pulmonary flow Q but also the airway pressure Pair and left atrial pressure LAP. This is advantageous because Q, Pair and LAP influence the pulmonary vasculature and the resulting pulmonary artery pressure PAP. Another advantage is that results obtained are independent of sympathetic nervous system tone 1, volume status, and anesthesia 2.
PAP changes induced by stair-step changes in Q for fixed Pair and either fixed or varying LAP are shown in Figure 1. Note that in the isolated lung preparation, the LAP cannula is typically connected to tubing that directs the perfusate into a waste container. With this tubing in place, LAP is linearly dependent on Q due to Poiseuille flow. However, the height of the outlet and waste container can be manually adjusted to provide a constant, non-zero LAP or the tubing can be removed to allow zero LAP that is independent of Q.
Representative Pulsatile Results:
While arbitrary pulsatile flow waveforms can be generated with this system, we typically generate flow of the form Q = 3 + 2 sin (2fπt) ml/min at frequencies of f = 1, 2, 5, 10, 15 and 20 Hz to assess the linearized impedance of the pulmonary vasculature (Figure 2: top panel). From the resulting PAP, LAP and Q measurements, pulmonary vascular impedance magnitude (Z) and phase (θ) are calculated by first decomposing one full sinusoidal cycle of ΔP = PAP-LAP and Q at each imposed sinusoidal flow rate frequency into a series of sinusoidal harmonics using a Fourier transform. The ratio of the pressure transform to flow transform yields the pulmonary vascualar impedance, PVZ = FFT (ΔP)/FFT(Q), which has magnitude Z and phase θ. Input impedance Z0, characteristic impedance ZC, and index of wave reflection RW, are calculated from the impedance magnitude. In particular, Z0 is calculated from Z at the 0th harmonic (f = 0 Hz) averaged over all frequencies, ZC is calculated as the average of Z between the first minimum (5 Hz) and 20 Hz, and RW is calculated as (Z0-ZC)/(Z0+ZC) 3.
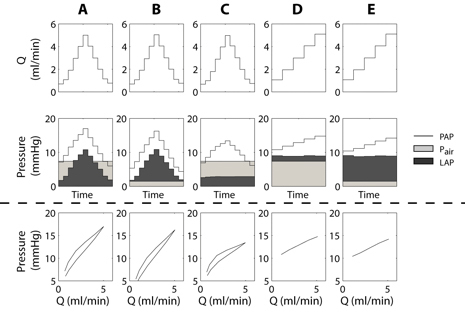 Figure 1. Steady flow waveforms (top row) and resulting pressures (second row: PAP, Pair, LAP) as a function of time with different combinations of LAP and Pair. The bottom row shows PAP vs. Q. In (A) and (B), LAP increases and decreases with Q because the outflow tubing was in place. This tubing was removed for (C) so that LAP is constant and independent of Q. In (D) and (E) the height of the outlet tubing was adjusted manually so that LAP is higher but independent of Q. Pair was either at end-inspiratory (A, C, D) or end-expiratory (B, E) pressure.
Figure 1. Steady flow waveforms (top row) and resulting pressures (second row: PAP, Pair, LAP) as a function of time with different combinations of LAP and Pair. The bottom row shows PAP vs. Q. In (A) and (B), LAP increases and decreases with Q because the outflow tubing was in place. This tubing was removed for (C) so that LAP is constant and independent of Q. In (D) and (E) the height of the outlet tubing was adjusted manually so that LAP is higher but independent of Q. Pair was either at end-inspiratory (A, C, D) or end-expiratory (B, E) pressure.
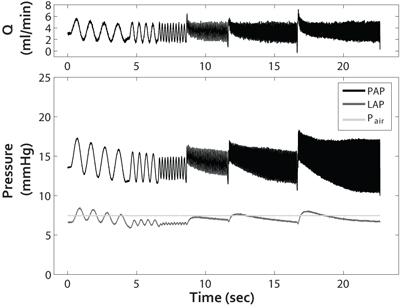 Figure 2. Pulsatile flow waveform Q (top panel) and resulting pressures (bottom panel: PAP, LAP and Pair) as a function of time. From these pulsatile pulmonary pressure-flow relationships, PVZ can be calculated, which reflects the total right ventricular afterload.
Figure 2. Pulsatile flow waveform Q (top panel) and resulting pressures (bottom panel: PAP, LAP and Pair) as a function of time. From these pulsatile pulmonary pressure-flow relationships, PVZ can be calculated, which reflects the total right ventricular afterload.
Discussion
Critical steps in the surgery
It is critical that care is taken when cutting the rib cage away from the lungs. The lungs must be fully exposed and uninhibited by surrounding tissues during inflation but not damaged during the process of isolation. The use of a flat object like the back end of the forceps can be used to hold the lungs away from the chest wall so that there is a clear path for scissors to cut. Another critical step is the placement of the suture around the pulmonary artery and the aorta. Using a blunted straight tweezers will reduce the risk of puncturing the pulmonary artery. The final critical step during the surgery is the placement of the cannulas. If the cannulas are too high above the plane of the great vessels as they exit the heart, the cannulas can pull on either the pulmonary artery or pulmonary veins. If the cannula in the left atrium is too low, it can block flow into the left lung. As a consequence, more flow goes to the right lung, increasing PAP and hastening the development of edema in the right lung.
Critical steps during data collection
Steady flow data collection should be performed quickly, especially for high flow rates, so that the exposure of the lung vasculature to high fluid shear stresses is minimized. In our experience, high shear stresses lead to lung edema. Also, rapid increases in shear stress can cause lung edema. For steady flow conditions, an increase in flow of 6 ml/min/min does not cause edema. Steady flow rates over 5 ml/min can be obtained without edema in certain conditions. We have perfused lungs of control and chronically hypoxic mice with steady flow rates as high as 10 ml/min successfully.
Frequency limitations
The highest frequency tested by us is typically 20 Hz because we use a flow waveform Q = 3 + 2 sin (2fπt) ml/min. The pump we describe here can generate oscillations at higher frequencies (at least 50 Hz), however the trade-off is decreased stroke length, i.e., change in Q. A more physiological flow waveform in which the magnitude of flow oscillation decreases with increasing frequency could likely be simulated with this system. Alternatively, a custom perfusion pump could be used with the same surgical isolation and ventilation procedures described here. The frequency range of the pressure transducers (P75, Harvard Apparatus, Holliston, MA) is reported as 0-100 Hz. The actual frequency response of the transducers is dependent on the stiffness and size of the tubing used to connect the transducers to the PA and LA cannulas. Using metal tubing instead of polyethylene tubing would increase the response of the system. However it is not possible to use completely rigid tubing because flexibility in cannula location and placement are needed during surgery. Nevertheless, higher frequency response transducers and/or more rigid tubing would increase the signal-to-noise in the pressure measurements and enable PVZ to be obtained at higher frequencies.
Applications
This isolated lung preparation has been used to investigate the effect of pulmonary embolism 4 as well as chronic hypoxia 5 on pulsatile pulmonary pressure-flow relationships. It also was used to investigate the effects of vasoactive agents in the pulmonary circulation 6 and to quantify the proximal and distal pulmonary vascular effects of acute rho kinase inhibition 7. This technique can be used to quantify pulmonary vascular physiology in inbred or outbred strains of mice or genetically engineered mice 8. The interpretation of the pressure-flow data obtained with this isolated lung preparation is not complicated by differences in heart rate or cardiac output between strains of mice. It is important to note that the impedance spectra obtained in an isolated, ventilated perfused lung in response to a non-physiological waveform should not be directly compared to those obtained in an in vivo preparation in response to a normal heart beat. Also, in vivo, ventilation is by negative, not positive, pressure and the viscosity of blood is approximately 4-fold the viscosity of RPMI with Ficoll.
Significance
Using the isolated, ventilated, perfused mouse lung preparation, we have been able to show that smooth muscle cell de-activation by acute rho-kinase inhibition has no direct effect on the compliance of the large, conduit arteries that significantly impact RV afterload 7. The clinical importance of proximal artery compliance has been increasingly recognized 9-11. Additionally, decreased main pulmonary artery compliance has been shown to be an excellent predictor of mortality in pulmonary arterial hypertension 10,11. The main cause of death from pulmonary hypertension is right ventricular failure; however increased mean pulmonary artery pressure alone is not sufficient to cause failure 12. A more effective measure of total right ventricular afterload is PVZ, which depends on both proximal compliance and distal resistance and is calculated from pulsatile pulmonary pressure-flow relationships such as can be obtained in mouse lungs with this protocol.
Disclosures
No conflicts of interest declared.
Acknowledgments
This research was supported by the National Institutes of Health grant R01HL086939 (NCC).
References
- Pace J. Sympathetic control of pulmonary vascular impedance in anesthetized dogs. Circ Res. 1971;29:555–568. doi: 10.1161/01.res.29.5.555. [DOI] [PubMed] [Google Scholar]
- Ewalenko P, Stefanidis C, Holoye A, Brimioulle S, Naeije R. Pulmonary vascular impedance vs. resistance in hypoxic and hyperoxic dogs: effects of propofol and isoflurane. J Appl Physiol. 1993;74:2188–2193. doi: 10.1152/jappl.1993.74.5.2188. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF, Hartley C, McDonald DA. McDonald's blood flow in arteries : theoretical, experimental, and clinical principles. 5th edn. Arnold; 2005. [Google Scholar]
- Tuchscherer HA, Webster EB, Chesler NC. Pulmonary Vascular Resistance and Impedance in Isolated Mouse Lungs: Effects of Pulmonary Emboli. Annals of Biomedical Engineering. 2006;34:660–668. doi: 10.1007/s10439-005-9050-z. [DOI] [PubMed] [Google Scholar]
- Tuchscherer HA, Vanderpool RR, Chesler NC. Pulmonary vascular remodeling in isolated mouse lungs: Effects on pulsatile pressure-flow relationships. Journal of Biomechanics. 2007;40:993–1001. doi: 10.1016/j.jbiomech.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Vanderpool R, Naeije R, Chesler N. Impedance in Isolated Mouse Lungs for the Determination of Site of Action of Vasoactive Agents and Disease. Ann Biomed Eng. 2010 doi: 10.1007/s10439-010-9960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool R, Kim A, Molthen R, Chesler N. Effects of acute rho kinase inhibition on chronic hypoxia-induced changes in proximal and distal pulmonary arterial structure and function. Journal of Applied Physiology. 2010 doi: 10.1152/japplphysiol.00533.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bizri N. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res. 2008;102:380–388. doi: 10.1161/CIRCRESAHA.107.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion H, Michelakis E, Hassoun P. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- Gan C. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, Nishimura R, Sorajja P, Cha S, McGoon M. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Bogaard H. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]


