Abstract
The membrane type 1 matrix metalloproteinase (MT1-MMP) is increased in left ventricular (LV) failure. However, the direct effects of altered MT1-MMP levels on survival, LV function, and geometry following myocardial infarction (MI) and the proteolytic substrates involved in this process remain unclear. MI was induced in mice with cardiac-restricted overexpression of MT1-MMP (MT1-MMPexp; full length human), reduced MT1-MMP expression (heterozygous; MT1-MMP+/−), and wild type. Post-MI survival was reduced with MT1-MMPexp and increased with MT1-MMP+/− compared with WT. LV ejection fraction was lower in the post-MI MT1-MMPexp mice compared with WT post-MI and was higher in the MT1-MMP+/− mice. In vivo localization of MT1-MMP using antibody-conjugated microbubbles revealed higher MT1-MMP levels post-MI, which were the highest in the MT1-MMPexp group and the lowest in the MT1-MMP+/− group. LV collagen content within the MI region was higher in the MT1-MMPexp vs. WT post-MI and reduced in the MT1-MMP+/− group. Furthermore, it was demonstrated that MT1-MMP proteolytically processed the profibrotic molecule, latency-associated transforming growth factor-1-binding protein (LTBP-1), and MT1-MMP-specific LTBP-1 proteolytic activity was increased by over fourfold in the post-MI MT1-MMPexp group and reduced in the MT1-MMP+/− group, which was directionally paralleled by phospho-Smad-3 levels, a critical signaling component of the profibrotic transforming growth factor pathway. We conclude that modulating myocardial MT1-MMP levels affected LV function and matrix structure, and a contributory mechanism for these effects is through processing of profibrotic signaling molecules. These findings underscore the diversity of biological effects of certain MMP types on the LV remodeling process.
Keywords: myocardial infarction, matrix, myocardial remodeling, ventricular function
one of the critical sequelae following a myocardial infarction (MI) are changes in left ventricular (LV) myocardial geometry and structure, which has been collectively termed LV remodeling. Increased induction of the family of proteases, the matrix metalloproteinases (MMPs), occurs in patients and animals in the context of post-MI remodeling (9, 10, 16, 27, 28). It is now becoming recognized that differential proteolytic profiles and a diversity of substrates and targets exist for the different MMP types (1, 2, 8, 13, 15, 17, 23, 26). Thus the determination of how certain MMP types specifically affect LV structure and function would provide critical insight into the pathogenesis of adverse LV remodeling such as that following an MI. One of the more unique MMP types, which is robustly increased in patients with LV remodeling and failure, is the membrane type 1 MMP (MT1-MMP) (19, 22). As the name implies, MT1-MMP is a transmembrane protein with a diversity of biological functions that include: 1) degradation of a spectrum of matrix structural proteins, 2) proteolytic processing of biologically active molecules such as growth factors and cytokines, and 3) activation of other MMPs. In animal models, MT1-MMP myocardial levels are increased early and appear sustained in the post-MI period coincident with adverse LV remodeling (6, 16, 20, 28). In addition, increased myocardial levels of MT1-MMP have been shown to adversely affect LV remodeling with aging and MI (20, 21). However, the comparative effects of augmenting and reducing MT1-MMP levels in the context of MI in terms of relevant functional outcomes such as survival, LV geometry and function, as well as biological outcomes such as determinants of myocardial matrix remodeling have not been examined. The central hypothesis of this study was that increased induction of MT1-MMP following MI would have adverse functional and biological consequences, whereas reduced induction of MT1-MMP would favorably affect these outcomes.
METHODS
Overview and rationale.
In these studies, MI was induced in mice through coronary ligation, and studies were performed at 14 days post-MI. This time point was chosen since it encompasses the initial phase of the MI wound-healing response and the initial trajectory of adverse LV remodeling (16, 27). These studies were performed in mice with cardiac-restricted overexpression (MT1-MMPexp), in mice heterozygous for MT1-MMP (MT1-MMP+/−), as well as in strain (FVB strain)-matched, wild-type (WT) mice. Survival, LV function and geometry, MT1-MMP levels and specific activity, and myocardial collagen content were then determined. In an additional cohort of mice from each group, in vivo localization of MT1-MMP was performed. Because MT1-MMP is a predominant pathway by which the proform of MMP-2 is proteolytically processed to active MMP-2 (24, 25) then the relative levels of these different MMP-2 forms were assessed. A fundamental structural event following MI is collagen accumulation within both the MI region and the remote viable myocardium. Signaling through the transforming growth factor (TGF)-β pathway constitutes a potent profibrotic stimulus, particularly in the context of MI (4, 7). One of the initial critical steps for matrix-bound TGF to become a competent profibrotic signaling molecule is through proteolytic release from the TGF latency-binding protein, LTBP-1 (4, 18, 29). Initial in vitro and in vivo studies have demonstrated an association between MT1-MMP and proteolytical processing of LTBP-1 (18, 20, 21). Accordingly, the present study examined the effects of modulating myocardial MT1-MMP levels upon specific LTBP-1 proteolysis and indexes of TGF signaling (Smad-3) following MI.
MT1-MMPexp.
The transgenic construct was developed expressing the human MT1-MMP coding sequence under control of the cardiac-restricted murine α-myosin heavy chain promoter, as described previously (20, 21). Routine genotyping was performed by PCR using primers for human MT1-MMP (5′-AAGCCTGGCTACAGCAAT-3′; 5′-GGCCTGCTTCTCATGG-3′) (21). Further confirmation studies for the full-length human MT1-MMP were performed by quantifying mRNA and protein levels as described in a subsequent section. The MT1-MMPexp negative mice were used as referent, WT sibling controls. The MT1-MMPexp mice displayed no obvious phenotypic abnormalities. MT1-MMPexp and WT mice were maintained until 3 mo of age and then randomized to undergo LV functional assessment and myocardial sampling or to undergo surgically induced MI.
MT1-MMP+/− expression.
The MT1-MMP-deficient transgenic mouse line was constructed by replacing a critical fragment (comprising the fourth exon) of the MT1-MMP gene by a neomycin cassette (29). Briefly, a 0.5-kb StuI fragment was excised from a genomic MT1-MMP clone and replaced by a neomycin cassette with the pgk1 promoter. The targeting construct was introduced into RW4 embryonic stem cells. Two clones were used for injections in blastocysts of C57BLy6 mice, one of these giving germ-line transmission. The mice are maintained in a heterozygous state, since homozygotes deficient of MT1-MMP develop malformations soon after birth (29). However, the heterozygote MT1-MMP+/− mice develop normally and express an ∼50% reduction in MT1-MMP levels. Accordingly, the MT1-MMP+/− mice were used for the present study.
Myocardial infarction.
Mice were anesthetized by isoflurane (2%), the LV was visualized, and the main left coronary artery was ligated (8.0 Neurilon; Ethicon, K801) (9, 10, 20, 21). The intraoperative mortality (first 24 h) was 15% and similar between groups. The mice were followed for 14 days post-MI at which time a second echocardiogram was performed. In an initial pilot study, WT mice (n = 8) underwent the surgical procedure whereby the suture was placed but the coronary was not ligated. In these sham control mice, LV echocardiography was performed before the surgical procedure and at 14 days following the sham procedure using the methodology outlined in a subsequent paragraph. This initial study demonstrated that LV end-diastolic volumes (44 ± 2 vs. 43 ± 2 μl) and LV ejection fraction (61 ± 3 vs. 63+2%) were equivalent at baseline and at 14 days postsham procedure (respectively, all P > 0.80). Thus, for the purposes of comparisons, the referent WT and transgenic control mice were unoperated. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, 1996) and the protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina (ARC no. 2389).
LV geometry and function.
Transthoracic echocardiography was performed to measure LV geometry and function (20, 21). Briefly, the mice were positioned on a feedback temperature-controlled operating table (Vestavia Scientific, Birmingham, AL) and maintained with 2% isoflurane anesthesia. Two-dimensional M-mode echocardiographic recordings were obtained using a 40-MHz scanning head with a spatial resolution of 30 μm (Vevo 2100 System; VisualSonics, Toronto, Ontario, Canada). With the use of long-axis views, LV end-diastolic volume and ejection fraction were computed. In addition, LV posterior and septal wall thickness were measured, and, with the use of these values and the volumetric measurements, LV mass was computed (20, 21). At 14 days post-MI, echocardiograms were repeated in identical fashion. Following the final set of measurements, and under a full surgical plane of anesthesia (4% isoflurane), the LV was removed, placed in iced saline, and processes as described below.
MMP targeted imaging studies.
An in vivo index of the regional distribution of myocardial MT1-MMP was determined in a subset of mice from each group using ultrasound and antibody-conjugated microbubbles (5, 11). Briefly, mice were anesthetized, and echocardiography was performed as described in the preceding paragraph. A biotinylated MT1-MMP antibody (custom rabbit polyclonal; Thermo Scientific Open Biosystems, Huntsville, AL) was conjugated to a streptavidin-coated microbubble contrast agent (Vevo MicroMarker Target-Ready Contrast Agent Kit; VisualSonics). Through an intravenous tail vein access, 70 μl of the antibody-conjugated microbubbles (3 μm diameter, 3.6 × 107 microbubbles) were injected over 2 s followed by an infusion of 50 μl of saline. Ultrasound cineloops were captured immediately before the injection, during the injection, and then 15 min following the injection. The preinjection cineloop from each animal was processed as a reference to which all other cineloops from the same animal were compared by digital subtraction to identify targeted microbubble contrast binding (5, 11).
Histomorphometry and immunohistochemistry.
For the histomorphometry studies, sections (5 μm) were stained with hematoxylin and eosin for measurement of MI size using computer-assisted planimetry (Sigma Scan; Media Cybernetics). LV sections were stained with picro-sirius red for fibrillar collagen, and the percent area of collagen within the remote and MI regions of the LV was computed (16, 21).
MT1-MMP activity LTBP-1 proteolytic processing.
LV myocardial extracts (50 μg) were incubated with a specific MT1-MMP fluorogenic substrate (MMP-14 Substrate I, catalog no. 444258; Calbiochem) described previously (6, 20, 21). The LV myocardial extracts (50 μg) were incubated (37°C) in the presence and absence of the MT1-MMP substrate, and excitation/emission were recorded (328/400, FluoStar Galaxy; BMG Labtech) continuously for up to 20 h (20, 21). Increasing concentrations of a recombinant active MT1-MMP construct (MT1-MMP Catalytic Domain, catalog no. 475935, 8–125 ng/m; Calbiochem) with a known catalytic activity resulted in a linear relationship with respect to fluorescence emission (y = 343x, r2 = 0.98, P = 0.001). The full-length sequence for LTBP-1 (AAI30290.1; NCBI) was first examined for MT1-MMP substrate binding/cleavage sites as described previously (21, 26). From these initial studies, a caged fluorogenic LTBP-1 peptide sequence that contained an MT1-MMP cleavage site was constructed (SCJJ-1; AnaSpec). Using approaches described previously (6, 20), and a specific excitation/emission wavelength (340/485 nm), 15 μM of LTBP-1 was exposed to increasing concentrations of the MT1-MMP catalytic domain. A clear fluorescent signal was detected with increasing concentrations of MT1-MMP, which was highly linear (y = 1041x, r2 = 0.99, P < 0.001) and was extinguished with a MT1-MMP neutralizing antibody, a MT1-MMP selective inhibitor, or a global MMP inhibitor (6, 20, 21). LV myocardial extracts (50 μg) were incubated (37°C), and fluorescence emission was recorded for up to 20 h. For both activity assays, utilizing known concentrations of the MT1-MMP catalytic domain and the fixed periods of incubation, an absolute catalytic activity for MT1-MMP- and LTBP-1-mediated proteolysis could be computed.
Myocardial zymography.
The LV myocardial homogenates (10 μg) were used to perform substrate zymography to assess the relative content of the gelatinases, MMP-2, and MMP-9 (6, 9, 10, 16, 19–22, 28). A positive control was used in all zymography measurements (2 μg, MMP-2/9 SE-244/237; BIOMOL).
MT1-MMP and LTBP-1 mRNA measurements.
LV myocardial homogenates were subjected to RNA extraction (RNeasy Fibrous Tissue Mini Kit; Qiagen, Valencia, CA), and the quantity and quality of the RNA were determined (Experion Automated Electrophoresis System; Bio-Rad Laboratories, Hercules, CA). RNA (1 μg) was reverse transcribed to generate cDNA (iScript cDNA Synthesis Kit; Bio-Rad). The cDNA was amplified with gene-specific primer/probe sets (TaqMan Universal PCR Master Mix: catalog no. 4364321; Applied Biosystems, Foster City, CA) using single-color real-time PCR (rtPCR, MyiQ; Bio-Rad). The specific TaqMan primer/probe sets (Applied Biosystems, Foster City, CA) were human MT1-MMP (catalog no. Hs0000237119_m1), mouse MT1-MMP (catalog no. Mm01318965_m1), mouse LTBP-1 (catalog no. Mm0049825_m1), and 18S rRNA (catalog no. 4333760F). Negative controls were run to verify the absence of genomic DNA contamination (reverse transcription control) and the absence of overall DNA contamination in the PCR system and working environment (template control). The rtPCR fluorescence signal was converted to cycle times and normalized to the 18S signal, and final expression levels were determined as a function of total RNA concentrations.
Smad-2 quantitation.
LV myocardial levels for a common intracellular convergence point of the TGF receptor transduction pathway, Smad-3, were examined (29). For these studies, LV myocardial extracts (20 μg) were subjected to immunoblotting for total Smad-3, and the membranes were then stripped and reprobed for phosphorylated Smad-3 (nos. 3102/3104, respectively, 1:1,000; Cell Signaling).
Data analysis.
LV function and geometry was compared between the referent control and MI groups using an ANOVA, and pairwise comparisons were performed by a Bonferroni-adjusted t-test. The zymographic/immunoreactive signals were analyzed using densitometric methods (Gel Pro Analyzer; Media Cybernetics) to obtain two-dimensional integrated optical density (IOD) values. The IOD values were then computed as a percent of control values where the control values were set to 100%, and comparisons were performed by a separate t-test. For the mRNA measurements, a Winsorized mean was used if extreme values existed in the data set. Between-group differences in these values were compared using ANOVA followed by Bonferroni-adjusted t-test. For the morphometric data, the results were first confirmed to conform to a Gaussian distribution and then subjected to ANOVA and finally to Tukey's test for mean separation. For the survival portion of the study, survival curves were constructed using Kaplan-Meier probability estimates, and 14-day post-MI survival was compared using a log rank survivor function. Values of P < 0.05 were considered statistically significant. All statistical procedures were performed using the STATA statistical software package (Statacorp, College Station, TX). Results are presented as means ± SE. Final sample sizes for each protocol/experiment are indicated in results or in the legends for Figs. 1–7.
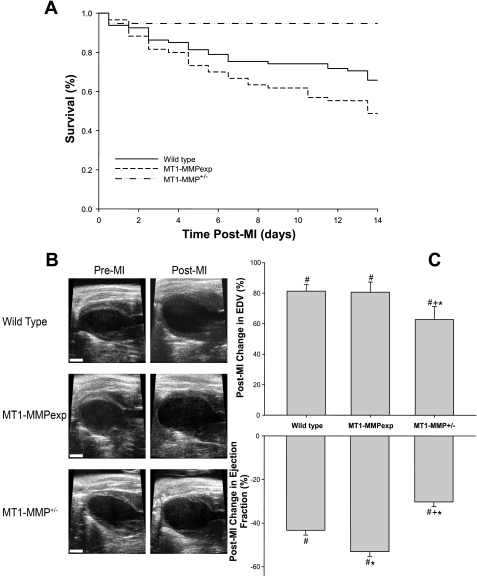
Fig. 1.
A: post-myocardial infarction (MI) 14-day survival curves for wild-type (WT) mice, cardiac-restricted overexpression of membrane type 1 matrix metalloproteinase [MT1-MMP (MT1-MMPexp)] mice, and mice heterozygous for MT1-MMP (MT1-MMP+/−). Post-MI survival was significantly lower in the MT1-MMPexp mice following MI compared with WT (P = 0.045). In marked contrast, post-MI survival was higher in the MT1-MMP+/− group compared with WT (P = 0.019) or MT1-MMPexp (P = 0.001). B: representative left ventricular (LV) long-axis echocardiographic views at end diastole before MI induction and at 14 days post-MI for the 3 groups. Significant LV dilation and posterior wall thinning at the site of the MI were readily evident in all groups (scale = 2 mm). C: changes in LV geometry as defined by end-diastolic volume (EDV) were computed on an individual basis from pre-MI values as was LV function as defined as ejection fraction. In the WT and MT1-MMPexp groups (n = 53 and 32, respectively), a significant and equivalent degree of LV dilation occurred, which was attenuated in the MT1-MMP+/− group (n = 18). LV ejection fraction fell in all groups post-MI but was lower in the MT1-MMPexp group and higher in the MT1-MMP+/− group. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
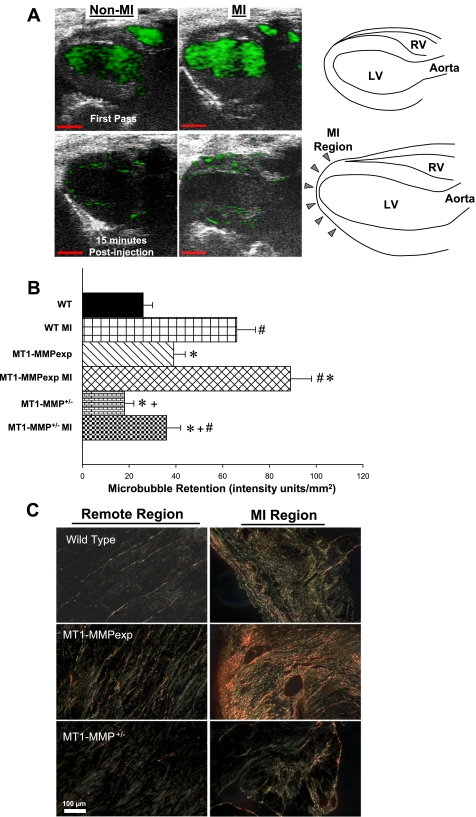
Fig. 2.
A: representative in vivo localization of MT1-MMP (40 MHz, 30 μm resolution) in a WT mouse without MI and in a WT mouse at 14 days post-MI. Images were obtained immediately following injection (First Pass) and at 15 min following tail vein injection of MT1-MMP antibody-conjugated echodense microbubbles (3 μm diameter, 3.6 × 107 microbubbles). Contrast retention at 15 min (green) was quantified. Schematics for orientation of these contrast echocardiograms are shown on the far right (scale = 2 mm). B: MT1-MMP-targeted contrast microbubble binding was quantified as contrast intensity within a region of interest (ROI) within the viable myocardium remote from the MI zone. Results were normalized by dividing the total intensity measured within each ROI by the area of the ROI. Values were computed for each non-MI group (n = 3/group) and post-MI groups (n = 5/group). In the viable myocardial region, microbubble retention was higher than respective non-MI values in all MI groups but was highest in the MT1-MMPexp group and lowest in the MT1-MMP+/− group. C: LV sections taken from the MI region and the remote region (septal wall) were stained with picro-sirius red and imaged under polarized light in WT mice (n = 5), MT1-MMPexp mice (n = 6), and MT1-MMP+/− mice (n = 4). As expected, increased collagen accumulation occurred within the MI region in all groups. However, increased collagen staining was observed in the MT1-MMPexp mice in both the remote and MI regions and appeared reduced in the MT1-MMP+/− mice. A minimum of 6 random fields from each region for each mouse was digitized and analyzed and compared with referent non-MI WT values (n = 5). This analysis is summarized in results (scale = 100 μm). P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
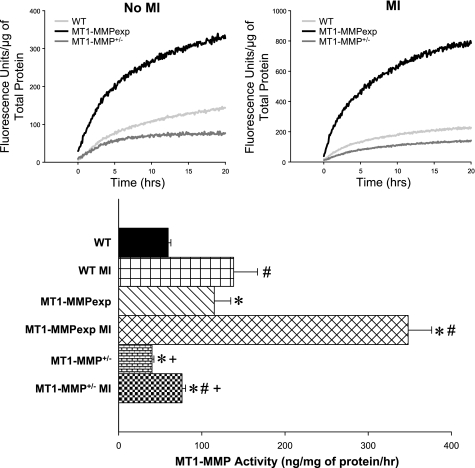
Fig. 3.
Top: LV myocardial extracts from WT, MT1-MMPexp, and MT1-MMP+/− mice with no MI and at 14 days post-MI (n = 8/group) were used to directly measure MT1-MMP catalytic activity using a calibrated and validated fluorogenic substrate. The reactions were monitored continuously for specific fluorescence emission, reflective of MT1-MMP proteolytic activity for up to 20 h. Significantly greater MT1-MMP activity occurred in the post-MI vs. non-MI samples; therefore, the fluorescence values are plotted using different scales. Bottom: time-averaged fluorescence (10–20 h) for each sample and actual MT1-MMP activity computed. At 14 days post-MI, LV myocardial MT1-MMP activity increased from respective non-MI values in all groups. However, MT-MMP activity was the highest in the MT1-MMPexp groups with and without an MI, whereas MT1-MMP activity was lowest in the MT1-MMP+/− groups. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
Fig. 4.
LV myocardial extracts from WT, MT1-MMPexp, and MT1-MMP+/− mice with no MI and at 14 days post-MI (n = 8/group) were subjected to gelatin zymography, which provides a measure of relative MMP-9 and MMP-2 levels. Relative MMP-9 levels were increased equivalently in the post-MI groups (data not shown). The molecular weight fractionation of the MMP-2 zymographic bands revealed a pronounced increase in the active form of MMP-2 (64 kDa) in the MT1-MMPexp groups, which was further increased post-MI. The active form of MMP-2 was reduced in the MT1-MMP+/− group post-MI. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
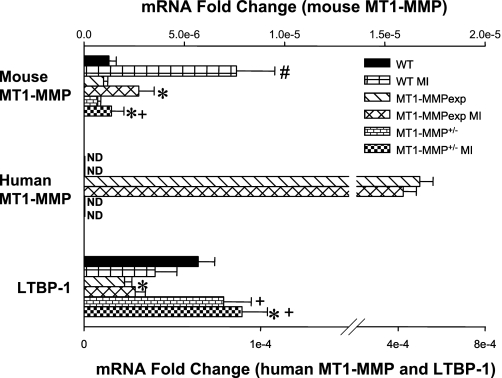
Fig. 5.
The relative mRNA levels for the endogenous mouse MT1-MMP, human MT1-MMP, and the mouse latency-associated transforming growth factor-binding protein-1 (LTBP-1) were computed using real-time PCR (rtPCR) from myocardial RNA extracted from WT, MT1-MMPexp, and MT1-MMP+/− mice with no MI and at 14 days post-MI (n = 6/group). The levels were normalized to 18S mRNA, and the degree of change was computed from the relative cycle time (Ct) values obtained for the mRNA of interest and 18S mRNA. In the post-MI WT group, a significant increase in the endogenous mouse MT1-MMP mRNA levels occurred, whereas these levels were reduced in both the MT1-MMPexp and MT1-MMP+/− post-MI groups. In the MT1-MMP+/− group, relative levels were reduced from WT values with no MI as well (P < 0.05). As expected, a robust signal for human MT1-MMP mRNA was obtained in both MT1-MMPexp groups and not detected (ND) in the WT or MT1-MMP+/− groups. LTBP-1 mRNA levels were reduced in both MT1-MMPexp groups compared with WT values. In contrast, LTBP-1 mRNA levels were increased in the MT1-MMP+/− group. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
Fig. 6.
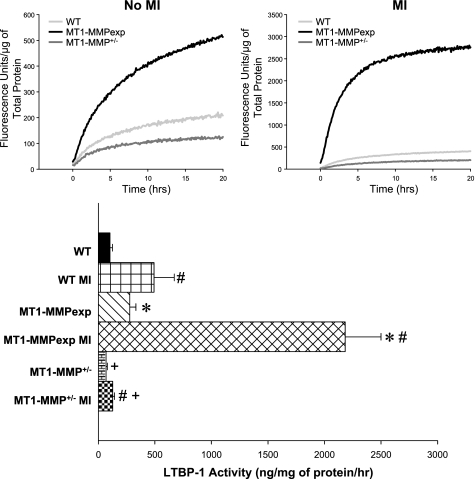
Top: LV myocardial extracts from WT, MT1-MMPexp, and MT1-MMP+/− mice with no MI and at 14 days post-MI (n = 8/group) were used to measure MT1-MMP-specific proteolysis using a LTBP-1 fluorogenic substrate. In the LV myocardial extracts, significantly higher MT1-MMP-mediated LTBP-1 proteolytic activity was obtained in the post-MI samples, necessitating the values being plotted using 2 different scales. Bottom: with the use of the MT1-MMP catalytic domain-LTBP-1 fluorescent standard curve, MT1-MMP-mediated LTBP-1 proteolytic activity was computed and was increased following MI in all groups. However, LTBP-1 proteolytic activity was highest in the MT1-MMPexp groups compared with WT and the lowest in the MT1-MMP+/− groups. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
Fig. 7.
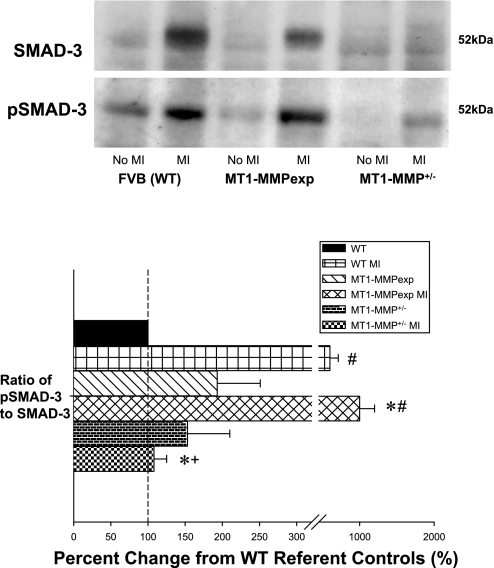
LV myocardial extracts from WT, MT1-MMPexp, and MT1-MMP+/− mice with no MI and at 14 days post-MI (n = 5/group) were used to measure a common Smad, Smad-3 in the classical TGF signaling pathway, by immunoblotting. Both total Smad-3 and the phosphorylated form of Smad-3 (pSmad-3) were quantified, and the ratio was computed. The ratio of phospho-to-total Smad-3 was increased to the greatest degree in the MT1-MMP post-MI group and was reduced substantially in the MT1-MMP+/− group. P < 0.05 vs. pre-MI values (#), vs. WT MI values (*), and vs. MT1-MMPexp MI values (+).
RESULTS
Post-MI survival.
A total of 90 WT mice, 77 MT1-MMPexp mice, and 26 MT1-MMP+/− mice underwent surgically induced MI, and the 14-day post-MI survival results are shown in Fig. 1. Overall post-MI survival in the WT mice was ∼60% and is consistent with this murine MI model (9, 10, 21). In the MT1-MMPexp group, 14-day post-MI survival was significantly lower than WT. In marked contrast, the post-MI survival was significantly higher in the MT1-MMP+/− mice. Postmortem analysis in the WT mice revealed that ∼60% of the deaths were due to myocardial rupture at the LV apical region, 30% were due to occult cardiac decompensation as evidenced by significant serous fluid accumulation within the thoracic space, and 10% revealed no significant transudate or serosanginuous fluid in the thoracic space; therefore, the deaths were presumed to be of an arrhythmic origin. Although the overall mortality was higher in the MT1-MMPexp mice, the distribution for the postmortem attribution cause of death was similar to WT (chi square analysis, P > 0.70). Similarly, although the overall post-MI mortality was significantly lower in the MT1-MMP+/− group, the postmortem cause of death attribution was similar to WT.
LV function and geometry.
LV function and geometry by echocardiography was examined at baseline (pre-MI) and at 14 days post-MI, and representative echocardiograms are shown in Fig. 1. Heart rates during these studies were equivalent across all groups (range 480–500 beats/min). In the WT, MT1-MMPexp, and MT1-MMP+/− groups, LV end-diastolic volume (42 ± 1 43 ± 2, and 42 ± 2 μl, respectively) and ejection fractions (64 ± 1, 61 ± 1, and 63 ± 1%, respectively) were equivalent before MI induction. The changes in LV end-diastolic volume and ejection fraction at 14 days post-MI in the surviving WT (n = 53), MT1-MMPexp (n = 32), and MT1-MMP+/− (n = 18) mice are summarized in Fig. 1. Significant post-MI LV dilation occurred in all groups post-MI, however, the degree of LV dilation was attenuated significantly in the MT1-MMP+/− group. LV ejection fraction fell in all groups post-MI, was significantly lower in the MT1-MMPexp group, and was significantly higher in the MT1-MMP+/− group. At baseline (pre-MI), LV mass computed by echocardiography was higher in the MT1-MMPexp mice compared with WT (92 ± 2 vs. 80 ± 2 mg, P < 0.05) but similar to WT in the MT1-MMP+/− group (79 ± 2 mg). At 14 days post-MI, LV mass increased from respective baseline values in all groups (WT 106 ± 3, MT1-MMPexp 118 ± 3, and MT1-MMP+/− 90 ± 3 mg, P < 0.05), which was higher in the MT1-MMPexp group compared with WT (P < 0.05) and lower in the MT1-MMP+/− group compared with respective WT or MT1-MMPexp values (P < 0.05).
MT1-MMP in vivo localization.
MT1-MMP antibody-conjugated microbubbles were injected, and ultrasound localization was performed in WT, MT1-MMexp, and MT1-MMP+/− mice without MI and at 14 days post-MI. Representative in vivo localization of MT1-MMP in a WT mouse without MI and in a WT mouse at 14 days post-MI is shown in Fig. 2. The contrast retention was quantified within the viable myocardium remote from the MI region, and summary results are shown in Fig. 2. In the viable myocardial region, microbubble retention was higher than respective non-MI values in all MI groups but was highest in the MT1-MMPexp group and lowest in the MT1-MMP+/− group.
Collagen morphometry.
Computed MI size was equivalent between the WT, MT1-MMPexp, and MT1-MMP+/− groups (35 ± 4, 38 ± 7, and 36 ± 4%, respectively, n = 5/group). Long-axis LV sections were stained with picro-sirius, and representative photomicrographs for the remote region (defined as the LV septum) and MI region are shown in Fig. 2. An observable increase in LV myocardial collagen occurred in the MT1-MMPexp mice under pre-MI conditions as well as following MI. In the non-MI groups, relative collagen content in the referent WT group was 0.63+0.06%, increased to 2.44 ± 0.15% in the MT1-MMPexp group (P < 0.05), and was reduced in the MT1-MMP+/− group (0.39 ± 0.02%, P < 0.05). At 14 days post-MI, fibrillar collagen was increased from respective non-MI values within the MI region in the WT, MT1-MMPexp, and MT1-MMP+/− groups (85.14 ± 0.88, 89.55 ± 1.07, and 77.03 ± 1.92%, respectively, P < 0.05) but was lower in the MT1-MMP+/− group compared with either WT or MT1-MMPexp values (P < 0.05). Within the remote region, fibrillar collagen was also increased from respective non-MI values in the WT, MT1-MMPexp, and MT1-MMP+/− groups (1.16 ± 0.10, 3.13 ± 0.25, 0.63 ± 0.19%, respectively, P < 0.05) and was significantly higher in the MT1-MMPexp group (P < 0.05) and significantly lower in the MT1-MMP+/− group (P < 0.05).
MT1-MMP activity.
MT1-MMP activity in LV myocardial extracts was assessed continuously for specific fluorescent emission over a 20-h period, and, through this approach, significant MT1-MMP activity was observed between the groups under referent control conditions (no MI) and at 14 days post-MI (Fig. 3). Under referent control conditions, myocardial MT1-MMP activity was increased substantially in the MT1-MMPexp group and was reduced in the MT1-MMP+/− group. Following MI, myocardial MT1-MMP activity increased in all groups but was substantially amplified in the MT1-MMPexp group and attenuated in the MT1-MMP+/− group.
Myocardial zymography.
LV myocardial extracts were subjected to gelatin zymography, and size-fractionated proteolytic bands were quantified to identify MMP-9 and MMP-2 levels in the three groups with no MI and at 14 days post-MI. Relative MMP-9 levels were increased equivalently in the post-MI groups (data not shown). However, significant changes in the total and the active form of MMP-2 (64 kDa) were observed and are summarized in Fig. 4. Total and active MMP-2 increased in all groups post-MI. However, the active form of MMP-2 was increased by approximately twofold in the MT1-MMPexp group and was reduced in the MT1-MMP+/− group post-MI.
MT1-MMP, LTBP-1 mRNA.
The relative mRNA levels for the endogenous mouse MT1-MMP, human MT1-MMP, and the mouse LTBP-1 were computed using rtPCR from myocardial RNA extracted from the WT, MT1-MMPexp, and MT1-MMP+/− groups with no MI and at 14 days post-MI (Fig. 5). In the post-MI WT group, a significant increase in the endogenous mouse MT1-MMP mRNA levels occurred, whereas these levels were reduced in both the MT1-MMPexp and MT1-MMP+/− post-MI groups. As expected, a robust signal for human MT1-MMP mRNA was obtained in both MT1-MMPexp groups and not detected in the WT or MT1-MMP+/− groups. LTBP-1 mRNA levels were reduced in both MT1-MMPexp groups compared with WT values. In contrast, LTBP-1 mRNA levels were increased in the MT1-MMP+/− group.
LTBP-1 proteolysis.
LV myocardial extracts from the three groups with no MI and at 14 days post-MI were used to measure MT1-MMP specific proteolysis using a specific LTBP-1 fluorogenic substrate (Fig. 6). Through continuous monitoring of fluorescence emission over a 20-h period, which would be reflective of MT1-MMP-mediated LTBP-1 proteolytic activity, significantly higher levels were observed in the MT1-MMPexp groups compared with WT and was further increased following MI. In marked contrast, MT1-MMP-mediated LTBP-1 proteolysis was reduced in the MT1-MMP+/− group.
Myocardial smad levels.
LV myocardial extracts from the three groups with no MI and at 14 days post-MI were used to measure Smad-3 levels. The ratio of phosphor-Smad-3 to total Smad-3 was computed as an index of TGF receptor signaling activity (Fig. 7). Although this ratio was increased in all groups post-MI, the phospho-Smad-3 ratio was increased to the greatest degree in the MT1-MMP post-MI group and was substantially reduced in the MT1-MMP+/− group.
DISCUSSION
Changes in the expression and activity of the large family of MMPs have been well documented in animal models and in clinical studies of LV remodeling (6, 9, 10, 16, 19–22, 28). Moreover, transgenic models and pharmacological MMP inhibition studies have demonstrated a cause-effect relation between MMP activity and adverse LV remodeling, particularly post-MI (9, 10, 16). One class of MMPs with a diverse substrate portfolio as well as unique functional aspects is the MT1-MMP (1, 2, 8, 13, 15, 17, 23, 26). In patients with LV remodeling and failure, as well as using post-MI transgenic reporter constructs, it has been demonstrated that significant MT1-MMP levels and MT1-MMP promoter activity occur (10, 19, 20). The present study examined the direct effects of cardiac-restricted overexpression of MT1-MMP (MT1-MMPexp) and heterozygous expression (MT1-MMP+/−) on the post-MI remodeling process. The unique and important findings of the present study were threefold. First, MT1-MMPexp reduced post-MI survival and worsened LV function, whereas MT1-MMP+/− improved post-MI survival, attenuated post-MI dilation, and improved LV function. Second, MT1-MMPexp increased myocardial fibrosis and MMP-2 activation, but MT1-MMP+/− reduced these indexes of myocardial matrix remodeling. Third, MT1-MMPexp increased proteolytic processing of LTBP-1 and increased the phosphorylation state of a common transduction convergence point of TGF signaling, Smad-3. LTBP-1 proteolytic processing and Smad-3 signaling were reduced with MT1-MMP+/− post-MI. These unique results demonstrated that altering MT1-MMP myocardial expression alters survival, LV remodeling, and profibrotic pathways following MI. Thus MT1-MMP likely contributes to the pathogenesis of adverse LV remodeling following MI.
Whereas past clinical and animals studies have provided an association between increased myocardial MT1-MMP and adverse LV remodeling (6, 16, 19, 22, 28), this study is the first to directly examine the effects of increased and reduced MT1-MMP myocardial levels following a common pathological stimulus, such as MI. The present study defined that changing MT1-MMP myocardial levels had direct effects on LV structure and function following MI. Moreover, the present study moved beyond measuring relative protein concentrations of MT1-MMP by identifying areas of MT1-MMP induction using an in vivo imaging modality as well as ex vivo through substrate-specific enzyme assays. Through these combined approaches, the results from the present study demonstrated that MT1-MMP overexpression directly resulted in increased competent enzyme within the myocardium and that heterozygous expression of MT1-MMP reduced relative levels and myocardial-specific MT1-MMP activity. In this manner, an absolute cause-effect relationship between changes in myocardial MT1-MMP levels to post-MI remodeling could be determined.
One of the first major observations from this study was that changes in MT1-MMP levels significantly affected early survival post-MI. There are likely several causes for this observation. First, MT1-MMP is a transmembrane protease, and therefore increased levels would disrupt critical cell-cell interactions, which in turn would alter myocardial conduction and increase arrhythmogenesis. Second, MT1-MMP can cause pericellular proteolysis and reduce cellular adhesion to the extracellular matrix, which in the context of MI could facilitate myocardial rupture. Third, increased myocardial MT1-MMP levels would likely alter the myocyte-matrix interface, diminish the efficiency of LV ejection, and accelerate the development of heart failure. Based upon postmortem findings, it is likely that all of these factors contributed to reduced post-MI survival in the MT1-MMPexp mice and improved survival in the MT1-MMP+/− mice. With the use of an MT1-MMP fluorogenic assay, the present study demonstrated concordant changes in MT1-MMP activity in the MT1-MMPexp and MT1-MMP+/− mice. These changes in myocardial MT1-MMP activity following MI were also directly associated with functional determinants of LV remodeling. Specifically, LV ejection fraction worsened with increased MT1-MMP activity, whereas LV ejection fraction was improved with reduced MT1-MMP activity in the post-MI period. Interestingly, although LV ejection fraction was lower in the MT1-MMPexp mice, the degree of LV dilation appeared similar to WT MI values. While remaining speculative, there are several possible reasons for this observation. First, in the MT1-MMPexp group, the mice with significant LV dilation were those that died early post-MI. Second, in the MT1-MMPexp group, the degree of myocardial collagen accumulation was higher, which may have prevented LV dilation. Another important inference that can be made from these observations is that, in the MT1-MMPexp mice, LV ejection fraction was lower at similar LV end-diastolic volumes. Thus increased myocardial MT1-MMP activity likely caused intrinsic defects in myocardial systolic performance.
MT1-MMP is an important pathway for proteolytically processing MMP-2 to the active form (24, 25). With the use of a zymographic approach that provides a sensitive means to identify and size fractionate MMP-2, higher levels of the active form of MMP-2 were observed in the MT1-MMPexp group and reduced in the MT1-MMP+/− group. These changes in the 68-kDa form would indicate the relative amounts of MMP-2 processed from the proform to the active form and thereby provided further in vivo evidence that MT1-MMP activity is directly associated with MMP-2 activation. Consistent with past studies, total myocardial MMP-2 levels were increased robustly following MI (9, 10, 16, 28). Interestingly, the highest levels of total MMP-2 occurred in the MT1-MMPexp group following MI, whereas total MMP-2 levels were unaffected by MT1-MMP heterozygous expression. These observations suggest that increased myocardial levels of MT1-MMP cause the activation of upstream MMP-2 transcriptional/translational events that are likely independent of the posttranslational processing of MMP-2. As discussed in a subsequent paragraph and reported by others previously (1, 2, 8, 13, 15, 23, 25, 26), a diverse portfolio of signaling molecules is likely processed by MT1-MMP, which may be responsible for altering upstream signaling pathways. Although this issue remains one of active investigation, there are several important conclusions that can be drawn regarding the findings from the present study. First, it is likely that altered expression of MT1-MMP in and of itself caused myocardial matrix instability and altered LV pump function. This conclusion is premised on the fact that past studies have demonstrated that significant cross talk between MT1-MMP and transmembrane matrix binding proteins (integrins) occurs (1, 2) as well as that MT1-MMP contributes to the phagocytosis of pericellular collagen fragments (1, 2). Second, because altered MT1-MMP myocardial levels in turn changed the activational state of MMP-2, then it is likely that this secondary proteolytic effect of MT1-MMP played a role in the changes in LV structure and function following MI.
One of the more unexpected outcomes from these MT1-MMP studies was the increased MT1-MMP myocardial levels caused increased myocardial collagen content, whereas reduced MT1-MMP myocardial levels directionally reduced collagen content post-MI. These findings in and of themselves challenge the canonical belief that myocardial induction of an MMP type, and directional changes in absolute MMP proteolytic activity, such as that of MT1-MMP, does not cause an absolute loss in extracellular matrix content. The present study examined a potential mechanistic basis by which MT1-MMP may actually provoke a profibrotic response through measuring critical components of the TGF pathway. TGF is synthesized as an inactive precursor bound to LTBP-1 through disulfide bonds and a cysteine-rich motif found within LTBP-1 (7, 18). Proteolytic processing of LTBP-1 and subsequent full activation and release of TGF in the interstitium is a critical step in this profibrotic signaling pathway. The present study was conducted with an ex vivo assay using an MT1-MMP cleavage site for LTBP-1 that falls within a Ca2+-binding epidermal growth factor-like protein-protein interaction domain of LTBP-1 (residues 1076–1117) (12). With the use of this approach, it was demonstrated that a significant induction of MT1-MMP-mediated LTBP-1 proteolysis occurs following MI and that myocardial overexpression of MT1-MMP increased and MT1-MMP heterozygous expression decreased this specific LTBP-1 peptide proteolysis. A previously performed cell-based study identified that LTBP-1 is a high-probability proteolytic substrate for MT1-MMP (26). The present study provides the first critical ex vivo studies to demonstrate that an MT1-MMP-LTBP-1 interaction occurs within the myocardium and was directly associated with changes in myocardial collagen content. In addition, increased MT1-MMP myocardial expression was associated with a significant reduction in LBTP-1 mRNA levels, whereas reduced MT1-MMP expression significantly amplified LTBP-1 mRNA content. These findings suggest that a feedback loop exists between MT1-MMP expression and the pathways that lead to LTBP-1 transcription, which in turn would hold biological relevance in terms of TGF-mediated signaling and post-MI remodeling. Because proteolysis of LTBP-1 would result in increased release of TGF and binding to cognate receptors, then it would follow that alterations in LTBP-1-mediated MT1-MMP proteolysis would directionally affect TGF-mediated signaling. Indeed, the present study demonstrated that the relative phosphorylation state of Smad-3, a common transduction element in the classical TGF signaling pathway (4, 7), was increased robustly post-MI, increased further with myocardial overexpression of MT1-MMP, and significantly fell with MT1-MMP heterozygous expression. These findings provide direct evidence that altered expression of a specific MMP type within the myocardium may actually yield directionally different effects in terms of profibrotic signaling and collagen accumulation, particularly following a pathological stimulus such as MI. However, although the present study demonstrated a likely interaction between MT1-MMP and LTBP-1, it is likely that there are other relevant proteolytic-substrate interactions for this transmembrane protease (1, 2, 8, 13, 15, 17, 23, 25). For example, TGF receptor signaling requires a complex formation of different TGF receptor subunits such as TGF-RI, TGF-RII, ALK1, and endoglin (4, 7, 12, 17, 18). Endoglin (CD150) is a high-molecular-weight integral glycoprotein that acts as a coreceptor with TGFR-II, and recent studies have documented a proteolytic interaction between MT1-MMP and this glycoprotein receptor (8). Specifically, in vitro studies have demonstrated that endoglin shedding is mediated by MT1-MMP through proteolytic processing of a specific extracellular domain sequence (8). MT1-MMP-mediated proteolytic release of soluble endoglin would have important consequences on TGF signaling. First, the shed extracellular endoglin domain will compete for TGF-binding sites on competent TGF receptor complexes. Second, MT1-MMP-mediated processing of the TGFR-II-endoglin receptor complex would result in an incompetent signaling moiety. Moreover, the present study focused on the MT1-MMP-TGF axis with respect to the profibrotic response, but it is likely that induction of the TGF signaling pathway would also cause a number of cellular responses, which would include fibroblast proliferation and hypertrophy of cardiocytes (3, 4, 14). In the present study, LV mass was higher with myocardial overexpression of MT1-MMP and lower with MT1-MMP heterozygous expression following MI, suggesting that MT1-MMP-mediated processing of LTBP-1 and subsequent activation of the TGF pathway also affected myocardial growth. Thus MT1-MMP likely modulates several important pathways in the myocardial remodeling response, which is induced following MI, and, based on the findings from the present study, a more comprehensive examination of other MT1-MMP-TGF proteolytic interactions would be warranted.
The findings of the present study clearly demonstrated that altering myocardial MT1-MMP expression has direct effects on LV structure and function following MI. While the MI process is fundamentally linked to a fibrotic response, the present study demonstrated that altering myocardial MT1-MMP levels directionally changed the fibrotic response following MI. These findings underscore the pleotropic nature of MT1-MMP in terms of simultaneously regulating both local proteolytic activity (MMP-2 activation) as well as profibrotic signaling (LTBP-1). The present study used a cardiac overexpression model of MT1-MMP, driven by a myosin heavy chain promoter. With the use of this construct, the preponderance of expression will be restricted to the cardiac myocyte. LV myocardial fibroblasts robustly express MT1-MMP, and increased fibroblast levels of MT1-MMP have been reported in patients with end-stage LV failure (10). The present study used a heterozygous transgenic construct of MT1-MMP, since this yielded a normal phenotype as opposed to the homozygous deletion, which is lethal (29). Thus reduced but not ablated expression of competent MT1-MMP was achieved. In the human MT1-MMP overexpression construct, endogenous mouse MT1-MMP levels were reduced, suggestive of compensatory feedback in MT1-MMP expression. Thus, as with any transgenic murine model, limitations and considerations exist with respect to interpretation. The present study suggests that MT1-MMP induction following MI causes a diverse number of effects that are likely to be regionally and substrate dependent. With the use of a microbubble localization method, in vivo validation of changes in MT1-MMP myocardial levels post-MI and in the transgenic constructs was demonstrated. However, the antibody-conjugated microbubbles can only traverse within the viable, perfused myocardium, and therefore the relative abundance of MT1-MMP levels within the MI region cannot be assessed by this approach. Nevertheless, the relative levels of MT1-MMP observed with this in vivo imaging method were concordant with the relative ex vivo myocardial mRNA and activity levels. Finally, it must be recognized that the present study examined only a limited number of potential MT1-MMP substrates at one point in time post-MI. In light of the fact that a number of MT1-MMP substrates have been identified that likely hold relevance to the post-MI remodeling process (1, 2, 8, 15, 17, 23, 25, 26) then proteolytic and temporal profiling of myocardial substrates would be an important future direction. These limitations not withstanding, the findings of this study challenge the original concept that MMPs, and in particular that of MT1-MMP, are restricted to degradation of a limited set of matrix structural proteins. In addition, these results suggest that developing targeted and selective genetic/pharmacological strategies to interrupt MT1-MMP myocardial expression and activity in the context of post-MI remodeling would be warranted.
GRANTS
This study was supported by NIH Grants HL-057952, HL-059165, and HL-095608 and by a Merit Award from the Veterans' Affairs Health Administration.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. S. S. Apte for providing the initial founders for the MT1-MMP-deficient mouse line.
REFERENCES
- 1. Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alphav integrin regulates cross-talk between alphavbeta3 and alpha2beta1 integrins in breast carcinoma cells. Exp Cell Res 291: 167–175, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol 19: 24–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brand T, Schneider MD. The TGF beta superfamily in myocardium: ligands, receptors, transduction and function. J Mol Cell Cardiol 27: 5–18, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decano JL, Moran AM, Ruiz-Opazo N, Herrera VL. Molecular imaging of vasa vasorum neovascularization via DEspR-targeted contrast-enhanced ultrasound micro-imaging in transgenic atherosclerosis rat model. Mol Imag Biol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deschamps AM, Yarbrough WM, Squires CE, Allen RA, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase (MT1-MMP) in ischemia and reperfusion: relation to interstitial MT1-MMP activity. Circulation 111: 1166–1174, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48: 504–511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res 70: 4141–4150, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nübe O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135–1142, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res 106: 796–808, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J 28: 2011–2017, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Koli K, Saharinen J, Hyytiäinen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech 52: 354–362, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci 122: 126–135, 2009 [DOI] [PubMed] [Google Scholar]
- 14. MacLellan WR, Brand T, Schneider MD. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ Res 73: 783–791, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Morrison CJ, Butler GS, Rodríguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol 21: 645–653, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta 1803: 39–54, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Saharinen J, Hyytiäinen M, Taipale J, Keski-Oja J. Latent transforming growth factor-beta binding proteins (LTBPs)–structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev 10: 99–117, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102: 1944–1949, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted over-expression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling following myocardial infarction. J Biol Chem 285: 30316–30327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Beck C, Bouges S, Stroud RE. Cardiac restricted over-expression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ Heart Fail 2: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spruill LS, Lowry AS, Stroud RE, Squires CE, Flack EC, Beck C, Ikonomidis JS, Crumbley AJ, McDermott PJ, Spinale FG. Membrane-type-1 matrix metalloproteinase in myocardial fibroblasts from patients with cardiomyopathy: persistent alterations in transcription and translation. Am J Physiol Cell Physiol 293: C1362–C1373, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114: 237–247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta 1803: 133–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tatti O, Vehviläinen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res 314: 2501–2514, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients following myocardial infarction: relation to left ventricular remodeling. Circulation 114: 1020–1027, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, Edmunds LH, Gorman RC, Spinale FG. Region and species specific induction of matrix metalloproteinases occurs with post-myocardial infarction remodeling. Circulation 107: 2857–2863, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA 97: 4052–4057, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.