Abstract
The interstitial cells of Cajal (ICC) are mesenchymal derived "pacemaker cells" of the gastrointestinal (GI) tract that generate spontaneous slow waves required for peristalsis and mediate neuronal input from the enteric nervous system1. Different subtypes of ICC form distinct networks in the muscularis of the GI tract 2,3. Loss or injury to these networks is associated with a number of motility disorders4. ICC cells express the KIT receptor tyrosine kinase on the plasma membrane and KIT immunostaining has been used for the past 15 years to label the ICC network5,6. Importantly, normal KIT activity is required for ICC development5,6. Neoplastic transformation of ICC cells results in gastrointestinal stromal tumor (GIST), that frequently harbor gain-of-function KIT mutations7,8. We recently showed that ETV1 is a lineage-specific survival factor expressed in the ICC/GIST lineage and is a master transcriptional regulator required for both normal ICC network formation and for of GIST tumorigenesis9. We further demonstrate that it cooperates with activating KIT mutations in tumorigenesis. Here, we describe methods for visualization of ICC networks in mice, largely based on previously published protocols10,11. More recently, the chloride channel anoctamin 1 (ANO1) has also been characterized as a specific membrane marker of ICC11,12. Because of their plasma membrane localization, immunofluorescence of both proteins can be used to visualize the ICC networks. Here, we describe visualization of the ICC networks by fixed-frozen cyrosections and whole mount preparations.
Keywords: Developmental Biology, Issue 53, mice, fluorescence microscopy, gastrointestinal track, motility, interstital cells of cajal, kit, ano1, pgp9.5
Protocol
1. Dissection of mouse GI tract
Euthanized mice by local IUCAC guidelines
Pin each limb on Styrofoam surface and rinse abdominal surface with 70% ethanol. Open abdominal cavity with long midline incision from the diaphragm to pubis. Make one cut at the distal esophagus and a second cut at the distal large intestine and remove the GI tract from stomach to anus en bloc, carefully cutting ligaments at the duodenum and cecum using small scissors. Place GI tract in a petri dish with PBS. Dissect away mesentery using tweezers (Figure 1). To separate stomach, small intestine, and large intestine, cut at pylorus and ileocecal junction. Rinse the lumen of each part of the GI tract with PBS using a 5ml syringe attached to a feeding needle (older mice) or blunt needle (younger mice).
Open stomach, small intestine, and large intestine along the mesenteric border. Cut two pieces for each part of the GI tract, one for whole mount visualization and one for cryosection.
2. Whole mount sample preparation and immunostaining
Place the entire piece of each part of GI tract into a 1.5 cm microfuge tube filled with 1.0 ml of ice cold acetone and fix samples for at least 1 hour at 4°C. Samples may be stored for up to 1 week at -20 °C. We typically store samples in acetone and proceed to prepare cyrosections.
Wash sample 2X with PBS. Place sample in petri dish with PBS. Under dissecting miscroscope, carefully scrape off mucosal layer with a scalpel by holding one end with a pair of tweezers, leaving the muscularis.
Muscle can be cut into 5mm pieces for staining with different antibodies. Do not store for more than 2 days in PBS before staining.
Block and incubation steps can be done in one well of a 24-well plate or in a 1.5ml microfuge tube. Block with 0.5 ml of blocking buffer (5% goat serum, 0.1% Triton X-100 in PBS for 1 hour at 4 °C).
Incubate with primary antibody diluted in blocking buffer and rotate overnight at 4 °C. We have successfully used ACK-2 rat anti-Kit, rabbit anti-Ano1, and rabbit anti-Pgp9.5 antibodies for whole mount immunostaining.
Wash for 5 min 2X with PBS on rotator at room temperature.
Incubate with secondary antibody diluted in blocking buffer for 2 hours at room temperature on rotator. We typically use Alexa Fluor 488 anti-rabbit and Alexa Fluor 594 anti-rat antibodies.
Wash for 15 min 3X with PBS on rotator at room temperature.
Mount on slide, serosal side on top to ensure the orientation of tissue during microscopy. This orientation will ensure the serosal side to face the coverslip and be encountered first as one focuses into the slide on a microscope. We do this on a dissecting microscope. Sometimes, we briefly examine the tissue under fluorescence microscopy to properly orient. Under fluorescence microscopy to visualize Kit or Ano1, one should encounter the ICC-MY network first from the side of the coverslip. Next, aspirate PBS as much as possible without completely drying. Add 50 μl of hard set mounting media (we use Prolong Gold) and gently place cover slip on top of tissue. Allow setting of mounting overnight at room temperature and store slides at -80 °C.
For microscopic examination, we used a wide-field microscope with a Z-drive. We used either a 20X air objective (NA 0.75, plan apochromat) or 60X oil objective (NA 1.4, plan apochromat) and took 2 μm or 1 μm Z-sections that span the entire muscularis. The images were subsequently deconvoluted using Autoquant deconvolution software. Alternatively, many others have used confocal microscopy instead with similar results.
3. Cryosection preparation and immunostaining
Place each part of the GI tract, serosal side down, flat on filter paper. Cut filter paper around the tissue. Place tissue on filter paper in 4% paraformaldehyde (PFA) in PBS (we dilute 32% paraformaldehyde in sealed vial into PBS right before use) for 2 hours at room temperature. Fixation can be done in a 6- or 12-well plate depending on size of tissue, or in a 1.5 ml microfuge tube. If done in plate, parafilm to minimize PFA exposure. Longer fixation times may result in antigen masking, particularly for the ACK-2 anti-Kit antibody.
Remove tissue from PFA and place in 30% sucrose in PBS. Allow tissue to sink overnight in 4 °C.
Equilibrate tissue with 1:1 ratio of 30% sucrose and O.C.T. for 1 hour. Then mount tissue vertically in cryomold filled with O.C.T. The longitudinal axis of the GI tract should be in parallel with the cyrosections. When examining mice with different genotypes or with different treatments, it may be useful to mount tissue from different mice onto same cryomold to ensure parallel processing. Freeze on dry ice and place in -80 °C freezer for storage.
Cut 10 μm cryosections onto slide and store at -80 °C freezer. We usually cut two sections per slide. We also usually perform H&E staining of one cryosection slide.
For immunofluorescence, first equilibrate slide at room temperature for 15 minutes. Then draw circle around tissue using PAP pen.
Block with 150 μl of blocking buffer (same as for whole mount immunostaining) for 1 hour.
Aspirate blocking buffer and add 150 μl primary antibody diluted in blocking buffer and incubate overnight at 4 °C in a humidified chamber.
Wash 5 min 2X with PBS.
Incubate with secondary antibody diluted in blocking buffer for 2 hours at room temperature. We typically use Alexa 488 anti-rabbit and Alexa 594 anti-rat antibodies.
Wash for 15 min 3X with PBS.
Aspirate slide without completely drying tissue. Add a drop of hard set mounting media with DAPI (we use Prolong Gold) and place #1.5 coverslip. Place slide at room temperature overnight to set and store in -80 °C freezer.
Acquire triple fluorescence images using DAPI, FITC, and Texas Red filter sets.
4. Representative Results:
Here, we use the large intestine as an example. H&E stain of a sucrose protected fixed frozen section used for immunofluorescence shows some artifacts compared to a reference paraffin section (Figure 2), where the yellow line demarks the myenteric plexus between the circular and longitudinal muscle layers and the green line demarks the border between the mucosa and muscularlis. Examining the cryosection, from the serosa to mucosa, you first encounter the thinner longitudinal muscle (LM) layer followed by the thicker circular muscle (CM) layer. The section should be in parallel with the longitudinal muscle so the nuclei of LM should be somewhat elongated while the nuclei of the CM should be small and round. Between the LM and CM are myenteric plexi made of neurons that stain with Pgp9.5. Pgp9.5 also stains neuronal processes throughout the CM. The myenteric ICC network (ICC-MY) surround the myenteric plexus and are made of multipolar cells. Within the longitudinal muscle layer, there are rare intramuscular ICCs (ICC-IMs) which are bipolar cells running in parallel with the muscle. Within the circular muscle layer, there are abundant ICC-IMs which are also bipolar cells that run parallel with the circular muscle and are cross-sectioned with this cut. At the junction of the muscularis and submucosa, the submucosal ICC network consisting of ICC-SMPs is a flat network of multipolar ICCs. Examining the whole mount Kit immunostaining of the large intestine, one should expect to encounter the ICC-MY network first, and then the ICC-IM of the CM, and ICC-SMP networks, as one focuses from the coverslip (Figure. 3). Volumetric measure of ICC networks using 3D reconstruction of whole-mount images have been described10. Using wide-field microscope, there is significant out-of-plane fluorescence in the raw images which can be removed after deconvolution (Figure 3). Incomplete stripping of mucosa will result in high background fluorescence. ANO1 is another marker of ICC’s and co-immunostaining of KIT and ANO1 shows complete overlap of ICC staining (Figure 4).
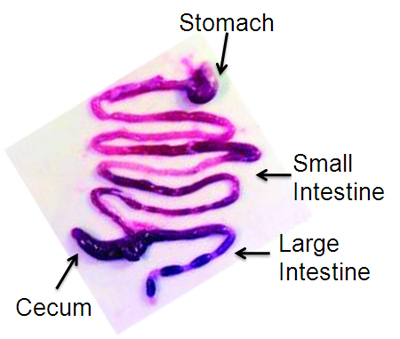 Figure 1. Representative dissected mouse gastrointestinal tract, reprinted with permission13.
Figure 1. Representative dissected mouse gastrointestinal tract, reprinted with permission13.
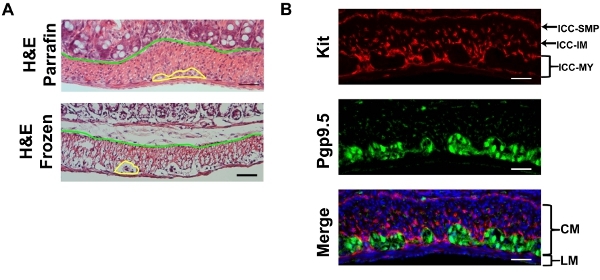 Figure 2. A) Representative H&E staining of the mouse large intestine of cryosections prepared as described and reference paraffin embedded section showing some shrinkage artifacts of cryosections. The yellow line demarks the myenteric plexus and the blue line separates the mucosa from the muscularis. B) Representative Kit (red, ACK-2 ) and Pgp9.5 (green) double immunofluorescence with DAPI (blue) counterstaining of the mouse large intestine. LM: longitudinal muscle CM: circular muscle. Scale bar 20 μm.
Figure 2. A) Representative H&E staining of the mouse large intestine of cryosections prepared as described and reference paraffin embedded section showing some shrinkage artifacts of cryosections. The yellow line demarks the myenteric plexus and the blue line separates the mucosa from the muscularis. B) Representative Kit (red, ACK-2 ) and Pgp9.5 (green) double immunofluorescence with DAPI (blue) counterstaining of the mouse large intestine. LM: longitudinal muscle CM: circular muscle. Scale bar 20 μm.
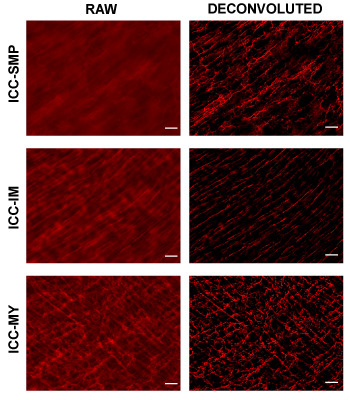 Figure 3. Representative Kit (red, ACK-2) whole-mount immunofluorescence of the same field at three focal planes, the ICC-MY plane at the LM/CM border, the ICC-IM plane in the CM, and the ICC-SMP plane at the CM/submucosa border. Raw images and deconvoluted images are shown. Scale bar 20 μm.
Figure 3. Representative Kit (red, ACK-2) whole-mount immunofluorescence of the same field at three focal planes, the ICC-MY plane at the LM/CM border, the ICC-IM plane in the CM, and the ICC-SMP plane at the CM/submucosa border. Raw images and deconvoluted images are shown. Scale bar 20 μm.
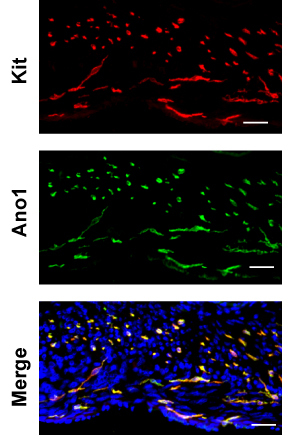 Figure 4. Representative double-immunofluorescence of Ano1 (green) and Kit (red, ACK-2) in large intestine. Scale bar 20 μm.
Figure 4. Representative double-immunofluorescence of Ano1 (green) and Kit (red, ACK-2) in large intestine. Scale bar 20 μm.
Discussion
Interstitial cells of Cajal were initially characterized by Santiago Ramóón y Cajal exactly one century ago using methylene blue and silver chromate stains of gastrointestinal muscularis. Cajal initially thought ICC’s were neurons based on their processes that are reminiscent of axons and dendrites. Over many ensuing years, study of ICC biology has been limited by lack of specific markers until the discovery of that KIT not only is expressed in ICC, but is also required for their development6. Since then, KIT immunofluorescence has been widely used in the study of ICC biology and has also led to the appreciation of ICC in other contractile organs such as the urinary bladder. Recently, ANO1 has been identified as a second reliable ICC marker.
There have been numerous publications over the past 15 years using immunofluorescence to identify ICC using various fixation and mounting techniques. In our hands "fixed-frozen" cryosections that have been prefixed with paraformaldehyde and sucrose protected works best compared to acetone or paraformaldehyde post-fixation after cryosectioning. For whole mounts, we have found that acetone fixation followed by mucosal dissection gives the most robust results.
Historically, the ACK-2 has been the Kit antibody of choice for mouse ICC identification. It is a rat monoclonal that recognizes the extracellular domain and it is also a blocking antibody that causes ileus when given to live mice. However, the ACK-2 epitope is slowly destroyed by paraformaldehyde fixation and is not rescued by standard antigen retrieval methods. We have found that the ACK-4 epitope is more resistant to paraformaldehyde fixation. Compared to fixed frozen blocks and sections, paraffin embedded blocks and sections preserves morphology better and is more amenable to long term archiving (Figure 4). Neither ACK-2 nor ACK-4 have been successfully used in paraffin sections. We have characterized that D13A2, a new rabbit monoclonal antibody, works exceptionally well in both fixed-frozen and paraffin sections of the GI tract.
Kit is expressed in several other cell types, including melanocytes, hematopoietic cells, germ cells, and in particular mast cells which are also found in the GI tract. Luckily, most mast cells are found is the mucosa and not in the muscularis. Double staining with rabbit anti-Ano1 and rat anti-Kit can eliminate non-specific staining of each antibody (Figure 4). It is also worth noting that the relative expression of Kit and Ano1 between subclasses of ICC seems different. For example, in the small intestine, Kit staining is much more intense in myenteric ICC compared to ICC of the deep muscle plexus whereas Ano1 staining is comparable.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the National Cancer Institute (K08CA140946, to Y.C.), (5F32CA130372, to P.C.), (R01CA102774 and R01HL055748 to P.B.), the Department of Defense (PC094302 to Y.C.), the Shuman Family Fund for GIST Research (to P.C.), and the Starr Cancer Consortium (to P.C., Y.C., C.L.S. and P.B.). We would like to thank Katia Manova, Ning Fan, and Mesurh Turkekul of the MSKCC Molecular Cytology core facility for help with cryosectioning and immunostaining.
References
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:602–611. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ordog T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- Hirota S. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Chi P. ETV1 is a lineage-specific survival factor in Gastrointestinal Stromal Tumour (GIST) Nature. 2010. Forthcoming. [DOI] [PMC free article] [PubMed]
- Kwon JG. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology. 2009;136:630–639. doi: 10.1053/j.gastro.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer G. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2003;100:6706–6711. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]


