Abstract
TNF-α is a proinflammatory cytokine that is involved in numerous pathological processes including chronic obstructive pulmonary disease (COPD). In the present study, we used a transgenic mouse model that overexpresses TNF-α in the lung (Tg+) to test the hypothesis that chronic exposure to TNF-α (as seen in COPD) reduces skeletal muscle force production and fatigue resistance, particularly under low Po2 conditions. At 7–12 mo, body and muscle weight of both extensor digitorum longus (EDL) and soleus were significantly smaller in Tg+ compared with littermate wild-type (WT) mice; however, the body-to-muscle weight ratio was not different between groups. EDL and soleus muscles were subjected to in vitro fatiguing contractile periods under high (∼550 Torr) and low Po2 (∼40 Torr). Although all muscles were less fatigue-resistant during low Po2 compared with high Po2, only the soleus fatigued more rapidly in Tg+ mice (∼12%) compared with WT at high Po2. The maximal tension of EDL was equally reduced in Tg+ mice (28–34% decrease from WT under both Po2 conditions); but for soleus this parameter was smaller only under low Po2 in Tg+ mice (∼31% decrease from WT). The peak rate of relaxation and the peak rate of contraction were both significantly reduced in Tg+ EDL muscles compared with WT EDL under low Po2 conditions, but not in soleus. These results demonstrate that TNF-α upregulation in the lung impairs peripheral skeletal muscle function but affects fast- and slow-twitch muscles differentially at high and low Po2.
Keywords: fatigue, contractility, fiber-type
skeletal muscle function can be severely affected by heart and lung disease (4, 31). These disorders develop distinct clinical symptoms; however, they all result in skeletal muscle dysfunction [e.g., decrease in submaximal and maximal force (1)]. Chronic obstructive pulmonary disease (COPD), which is associated with reduced exercise capacity and quality of life, can result in significant muscle wasting and atrophy (28). Peripheral muscle fatigue can often significantly limit exercise tolerance in COPD patients (10). It has been speculated that the sustained pulmonary inflammation may have remote effects on peripheral tissues via chronic inflammatory mediators released into the circulation in COPD patients (3, 16). Among them is TNF-α, a polypeptide cytokine that controls antitumor and immune responses, which has been implicated as a key cytokine correlated with the progression of muscular dysfunction in COPD patients (25). In fact, previous studies have shown that mice that overexpress TNF-α in the lung develop chronic pulmonary inflammation (21) and skeletal muscle wasting (15).
The main postulated mechanisms of TNF-α action on skeletal muscle have been related to decreased muscle regenerative capacity (15), increased protein catabolism (17), or decreased myofibrillar function mediated by free radical production (11, 16). It is known that reactive oxygen species (ROS) are constantly produced in skeletal muscle and that a transition to hypoxia can lead to an increased production of ROS (42). During repetitive contractions (e.g., exercise), the intracellular oxygen tension measured in humans can reach values close to anoxia [i.e., 1–3 Torr; (33)]. Furthermore, it has been shown that COPD patients can become rapidly hypoxic during exercise due to poor lung function (e.g., pulmonary emphysema) (19, 27). While the impaired contractile and performance characteristics of muscles from COPD patients or models of COPD have been documented, the causes of muscle dysfunction are unclear.
To further evaluate the effects of COPD on skeletal muscle function, we used a transgenic mouse model in which TNF-α was overexpressed in the lung (Tg+), thereby mimicking some of the pathophysiological conditions associated with COPD (9). We used this transgenic model to test the hypothesis that chronic exposure to TNF-α negatively affects skeletal muscle force production and fatigue resistance (i.e., muscle's ability to resist fatigue) in isolated fast- and slow-twitch muscles, particularly during low Po2 conditions. An in vitro isolated muscle model was used to avoid many of the confounding factors related to in vivo contracting muscle (i.e., cardiovascular or endocrine system), thereby allowing precise investigation of muscle contractile properties affected by TNF-α overexpression in the lung.
MATERIALS AND METHODS
Animal care and whole muscle isolation.
SP-C/TNF-α transgenic mice (Tg+) were obtained as a kind gift from Dr. Charles G. Irvin (Vermont Lung Center, University of Vermont), crossed with C57BL/6 mice, and screened by PCR analysis (15). Male mice of 7–12 mo old were used in this study. All procedures were approved by the University of California San Diego institutional animal care and use committee. Before each experiment, animals were anesthetized by intraperitoneal administration of ketamine (70 mg/kg) and xylazine (10 mg/kg). Both extensor digitorum longus (EDL; mostly composed of fast-twitch fibers) and soleus (mostly composed of slow-twitch fibers) were carefully removed from both hindlimbs and mounted in experimental chambers (model 800MS; Danish Myo Technology, Aarhus, Denmark) in the presence of Tyrode's solution (in mM: 121 NaCl, 5 KCl, 0.4 NaH2PO4, 1.8 CaCl2, 0.5 MgCl2, 24 NaHCO3, 5.5 glucose, 0.1 EGTA) bubbled continuously with 95% O2-5% CO2 (pH 7.4, room temperature). For each muscle, the tendon located on one end of the muscle was attached to a mobile lever arm that was designed to change the muscle length. The other end was attached to a stationary force transducer (force range between 0 and 1,600 mN) previously weight calibrated (1 to 40 g range) to convert voltage into grams of tension development.
Experimental procedure.
The muscles were electrically stimulated (S48 stimulator; Grass Technologies, West Warwick, RI) using square-wave pulses (EDL: 40 V, 250-ms train duration, 1-ms pulse duration, 150 Hz; soleus: 40 V, 500-ms train duration, 1-ms pulse duration, 80 Hz). Under these stimulation parameters, both EDL and soleus muscles at 34–35°C develop maximal tetanic tension, respectively. An A-D converter (model MP100WSW; Biopac Systems, Santa Barbara, CA) was used to convert the analog to digital data, and Acknowledge III 3.2.6 software (Biopac Systems) was used to analyze the results. After being mounted, muscle optimal length (L0) was set, and the muscle was allowed to rest for 20 min at room temperature (22°C). The temperature was then increased to 34–35°C, and two contractile bouts (separated by 60-min rest at room temperature) were conducted. One contractile period was under high Po2 (when the solution was bubbled with 95% O2-5% CO2), and the other under low Po2 (when the solution was bubbled with 5% O2-5% CO2, balanced with N2) conditions in a blocked order design (i.e., 50% of muscles used were tested under high Po2 in the first contractile bout followed by a second bout under low Po2, and the other 50% were tested in the reverse sequence). Maintaining muscles at room temperature during the 60-min rest period minimized the tissue degradation over the time period to ensure the full recovery of the muscle function as shown previously (35). Oxygen tensions were measured using a fiber optic oxygen sensor (OXYMICRO; World Precision Instruments, Sarasota, FL) immersed in the experimental chamber solution. The Po2 values under high and low Po2 conditions were ∼550 Torr and ∼40 Torr, respectively. After achieving the experimental Po2, muscles were equilibrated for 3 min prior to each contractile period. The contractile period consisted of a series of repeated tetanic contractions with increasing train frequency (0.125, 0.166, 0.25, 0.33, 0.5 contractions/s) every min until the initial contraction had fallen to 60% (fatigue point). The time period between the first contraction and the last contraction at the fatigue point was defined as time to fatigue.
Data analysis.
After the experimental procedure, the muscles were blotted dry and weighed, and tension development (in mN) was normalized with respect to the muscle cross-sectional area (mN/mm2) (30). The cross-sectional area (in mm2) was calculated by dividing muscle mass (in mg) by the product of L0 (in mm) times the density of the muscle (1.06 mg/mm3) (30). The experimental results are presented as means ± SE. Differences among individual variables were analyzed using paired Student's t-test for comparison between two muscle groups in the same animal. For multiple comparisons, one-way ANOVA followed by the Tukey test or two-way ANOVA followed by a Bonferroni test was used. The animal was treated as a random variable, with control and treatment being factors of interest. Analyses were carried out using PRISM 4 software. P < 0.05 was considered to be statistically significant.
RESULTS
Effects of chronic production of lung TNF-α on body and skeletal muscle weight.
The transgenic animals used in this study are known to contain high amounts of TNF-α in the lung (∼100 pg/ml in bronchoalveolar lavage fluid) and in blood (∼9 pg/ml in serum) (15). These animals showed chronic pulmonary inflammation with reduced body and muscle weights (15). As shown in Table 1, ∼17% reduction of the body weight and ∼12% reduction of isolated muscle weight (Table 1) were observed in Tg+ mice compared with their littermate wild-type (WT) mice. However, the muscle weight-to-body weight ratio was not changed (Table 1) in Tg+ mice compared with WT mice.
Table 1.
Whole body and muscle weights
| Muscle Weight, mg |
Muscle Weight/Body Weight, mg/g |
L0, mm |
Cross-Sectional Area, mm2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Body Weight, g | EDL | Soleus | EDL | Soleus | EDL | Soleus | EDL | Soleus |
| WT | 35.9 ± 1.3 (8) | 10.7 ± 0.3 (9) | 10.5 ± 0.2 (9) | 0.30 ± 0.01 | 0.29 ± 0.01 | 11.6 ± 0.4 | 12.0 ± 0.3 | 0.88 ± 0.04 | 0.83 ± 0.03 |
| Tg+ | 28.5 ± 0.6* (7) | 9.3 ± 0.3† (7) | 9.3 ± 0.5† (7) | 0.31 ± 0.01 | 0.32 ± 0.02 | 10.4 ± 0.3 | 10.1 ± 0.2 | 0.84 ± 0.05 | 0.87 ± 0.05 |
Values are means ± SE; number in parenthesis are the number of animals analyzed (for body wt) or number of muscles analyzed (for muscle wt). L0, muscle optimal length; EDL, extensor digitorum longus; WT, wild type; Tg+, transgenic mouse model that overexpresses TNF-α in the lung.
P < 0.05 vs. WT, Student's t-test;
P < 0.05 vs. WT, one-way ANOVA.
Contractile performance of the isolated muscles.
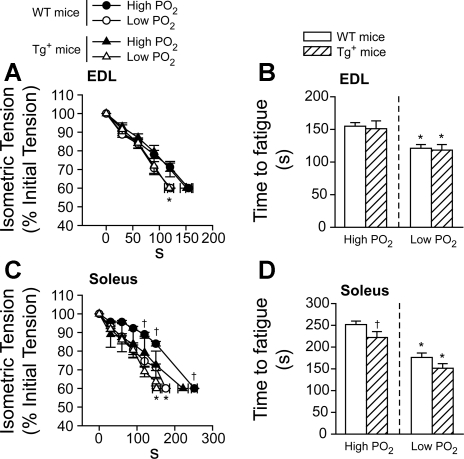
Muscle fatigue is defined as the muscle's inability to maintain the initial maximal force development over time, which corresponds to an exercise-induced decrease in the muscle power. In our studies, muscles (EDL and soleus) were electrically stimulated in two consecutive repetitive contractile periods until they reached the fatigue point (for details, see materials and methods). A longer time to reach the fatigue point represented an increased fatigue resistance. For EDL, the low Po2 condition significantly reduced the time to fatigue within the mouse groups (Tg+ vs. WT, P < 0.05, Fig. 1, A and B). However, there were no changes observed in the isometric tension developed at different time points in the contractile period (Fig. 1A). The time to fatigue (Fig. 1B) was also not changed between the groups of mice (WT and Tg+) at either high or low Po2 conditions in EDL muscles. Regarding the soleus (Fig. 1, C and D), similar to EDL, the decrease in the oxygen tension (low Po2) in the chamber significantly reduced the time to fatigue in both WT and Tg+ (P < 0.05, Fig. 1, C and D), compared with high Po2. Compared with the repetitive contraction protocols at high Po2 between the groups, relative tension development was significantly reduced in soleus muscle from Tg+ mice during the time period between 120 s and the time at fatigue point, compared with WT mice (P < 0.05, Fig. 1C). Time to fatigue was also significantly reduced in soleus muscle from Tg+ mice compared with WT mice (252 ± 8 s vs. 222 ± 14 s for WT and Tg+, respectively; P < 0.05, Fig. 1D). However, there was no difference in time to fatigue under low Po2 conditions (Fig. 1, C and D) in soleus between WT and Tg+ mice.
Fig. 1.
Tension development and time to fatigue during a repetitive contractile period on extensor digitorum longus (EDL; A and B) and soleus (C and D) muscles. Relative tension (%) was developed at different time points of the contractile period in wild-type (WT; circles, A and C) and transgenic model of pulmonary TNF-α overexpression (Tg+) mice (triangles, A and C) (solid symbols: high Po2; open symbols: low Po2). Time to fatigue under different Po2 in WT (open bars) and Tg+ (crossed bars) mice (B and D). Data were averaged from 9 muscles of 5 mice for WT and 7 muscles of 5 mice for Tg+. *P < 0.05, low vs. high Po2 condition, two-way repeated-measures ANOVA; †P < 0.05, Tg+ vs. WT under the same Po2 condition, two-way repeated-measures ANOVA.
Contractile parameters during each contractile bout.
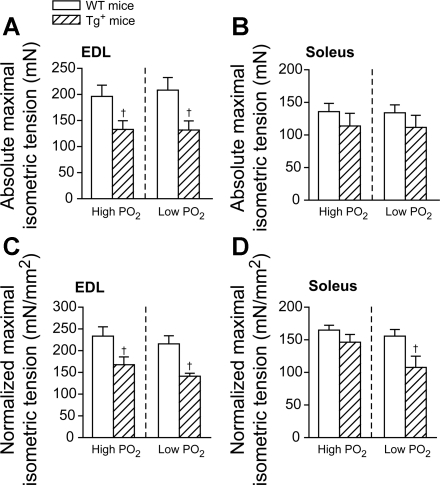
To investigate whether the contractile properties of the muscles from Tg+ mice were changed from control, both absolute tension development and the tension development normalized by the muscle cross-sectional area were calculated. In the normalized tension, any individual changes of each muscle force were minimized and not directly related to the muscle weight (20). Fig. 2 summarized the isometric tension development for the first tetanic contraction of each contractile bout, which represented an unfatigued state of the muscle.
Fig. 2.
Maximal initial isometric tension from EDL (A and C) and soleus (B and D) in both WT (open bars) and Tg+ (crossed bars) mice under different oxygen tensions. Isometric tension is represented by either the absolute tension development (in mN; A and B) or the tension development normalized by the cross-sectional area (in mN/mm2; C and D). †P < 0.05, Tg+ vs. WT under the same Po2 condition, one-way ANOVA. Data were averaged from 9 muscles of 5 mice for WT and 7 muscles of 5 mice for Tg+.
For EDL muscle, as shown in Fig. 2, A and C, both absolute tension (in mN) and tension normalized by the cross-sectional area (in mN/mm2), were significantly reduced in the Tg+ mice compared with WT mice under both high (∼28–32% decrease; P < 0.05) and low Po2 (∼34–37% decrease; P < 0.05). There was no significant change in both absolute and normalized tension development between Po2 conditions within the same group (Fig. 2, A and C).
For soleus muscle, unlike EDL, the absolute tension development was not different between groups (WT vs. Tg+) at both high and low Po2 conditions (P > 0.05, Fig. 2B). The initial normalized tension in Tg+ mice at high Po2 was also not different from WT (P > 0.05, Fig. 2D). However, at low Po2, normalized tension in Tg+ mice was significantly smaller than WT (∼31% decrease, P < 0.05; Fig. 2D). Similar to the EDL muscle, there was no significant change in the absolute or normalized tension development between the two Po2 conditions in either Tg+ or WT mice (Fig. 2, B and D).
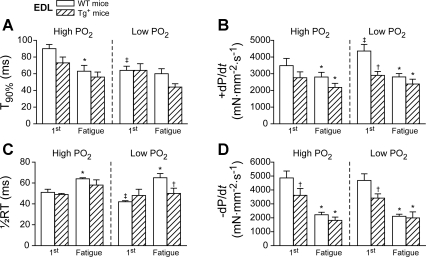
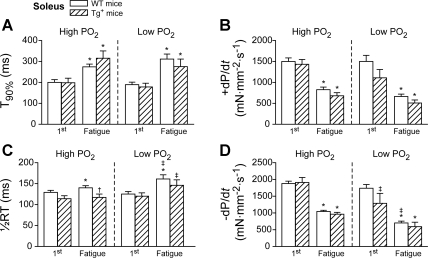
To identify the contractile mechanisms regarding the decreased isometric tension in Tg+ mice (e.g., excitation-contraction coupling and force generated by cross bridges) some contractile parameters were measured during the period of contraction (i.e., during electrical stimulation) and during relaxation (after electrical stimulation) of the first (unfatigued state) and the last contraction (defined as fatigued state) in each work period. The results were summarized in Fig. 3 (EDL) and Fig. 4 (soleus). In both figures, the contraction period was analyzed using the time to reach 90% of the peak tension from the start (T90%; Figs. 3A and 4A) and the peak rate of contraction (+dP/dt, the maximal first derivative of the force development during the contraction, Figs. 3B and 4B), the relaxation period represented by one-half relaxation time (1/2RT, the 50% of the time between the peak of the developed force and the resting state, Fig. 3C and 4C), and the peak rate of relaxation (−dP/dt, the maximal first derivative of the force development during the relaxation, Figs. 3D and 4D).
Fig. 3.
Contractile parameters of the first and the last (fatigue point) contractions during the repetitive contractile period in EDL muscle from WT (open bars) and Tg+ (crossed bars) mice. Time to reach 90% of the peak tension (T90%) (A); peak contraction rate (+dP/dt) (B); half relaxation time (1/2RT) (C); peak relaxation rate (−dP/dt) (D). *P < 0.05, fatigue vs. the first contraction within the same group, paired t-test; †P < 0.05, Tg+ vs. WT within the same group, two-way ANOVA; ‡P < 0.05 low vs. high Po2 within the same panel, two-way repeated-measures ANOVA. Data were averaged from 9 muscles of 5 mice for WT, and 7 muscles of 5 mice for Tg+.
Fig. 4.
Contractile parameters of the first and the last (fatigue point) contractions during the repetitive contractile period in soleus muscle from WT (open bars) and Tg+ (crossed bars) mice. Time to reach 90% of the peak tension (T90%) (A); peak contraction rate (+dP/dt) (B); half relaxation time (1/2RT) (C); peak relaxation rate (−dP/dt) (D). *P < 0.05, fatigue vs. the first contraction within the same group, paired t-test; ‡P < 0.05 low vs. high Po2 within the same panel, two-way repeated-measures ANOVA. Data were averaged from 9 muscles of 5 mice for WT, and 7 muscles of 5 mice for Tg+.
When the contractile parameters of unfatigued contractions were compared between the WT and Tg+ groups, only −dP/dt was significantly smaller in Tg+ than in WT under both Po2 conditions in EDL (−3,615 ± 489 vs. −4,862 ± 496 mN·mm−2·s−1 for high Po2 and −3,415 ± 304 vs. −4,684 ± 474 mN·mm−2·s−1 for low Po2, P < 0.05; Fig. 3D). Interestingly, Tg+ soleus did not show any difference in contractile parameters compared with WT during the contraction and relaxation period of any unfatigued contractions (Fig. 4, A–D). This was quite consistent with the isometric tension development at high Po2 in soleus (Fig. 2B).
However, when the contractile parameters were compared between high and low Po2 during unfatigued contractions, the EDL demonstrated significant differences under low Po2 compared with high Po2 conditions. In the EDL muscle from WT mice, T90% was significantly reduced (90 ± 5 vs. 64 ± 5 ms for high vs. low Po2, Fig. 3A, P < 0.05) and +dP/dt was significantly increased at low Po2 (3,481 ± 433 vs. 4,355 ± 401 mN·mm−2·s−1 for high vs. low Po2, P < 0.05; Fig. 3B). No change in these parameters was detected in Tg+ mice.
In contrast to the EDL, neither T90% nor +dP/dt was changed in the soleus from WT and Tg+ mice at low Po2. However, during the relaxation period, only in Tg+ mice −dP/dt from soleus muscle was significantly reduced under low Po2 (−1,909 ± 149 vs. −1,288 ± 296 mN·mm−2·s−1 for high vs. low Po2, P < 0.05; Fig. 4D).
The contractile properties of the last contraction (fatigue point) showed the expected differences in the contraction and relaxation periods in both groups of mice. Specifically at the fatigue point, +dP/dt was significantly decreased in both EDL and soleus from WT and Tg+ mice (i.e., slowing of contraction, P < 0.05; Figs. 3B and 4B). The 1/2RT was increased, while −dP/dt was decreased, slowing down the relaxation (P < 0.05; Fig. 3, C and D for EDL and Fig. 4, C and D for soleus). Regarding contractile properties, no significant differences between WT and Tg+ mice (Figs. 3 and 4) were found at the fatigue point in both EDL and soleus muscles.
DISCUSSION
Our study showed that chronic production of TNF-α in the lung resulted in specific fiber-type-related muscle dysfunctions. Particularly, the maximal isometric tension development was decreased in muscle primarily composed of fast-twitch fibers (EDL), and fatigue resistance was reduced in muscle primarily composed of slow-twitch fibers (soleus).
Morphological effects.
TNF-α upregulation in the lung reduced body and muscle weight (Table 1), which agreed with a previous report using the same mouse model (15). Although atrophy is characterized by a reduced muscle weight compared with overall body weight (40), there was no difference in the ratio of muscle to body weight between groups (Table 1), which suggests that the TNF-α effects were likely not related to the muscle atrophy per se. In other studies, the level of other cytokines including interleukin IL-6, interferon-γ, and TGF-β remained the same in some skeletal muscles (i.e., quadriceps) from patients with COPD compared with control (3), while in other skeletal muscles (i.e., intercostal muscles) from COPD patients, the expression of cytokines, including IL-6 and -1β, were significantly increased (5). However, the level of TNF-α seemed to be more responsive to COPD conditions and was significantly changed in both quadriceps and intercostal muscles from those patients (3, 5). Thus, although other inflammatory cytokines may be produced in Tg+ mice, the major factor that was changed in our transgenic mouse model was the chronic elevation of TNF-α, so that the pathophysiological effects noted in the present study on skeletal muscle function were likely most dependent on the TNF-α elevation. Additionally, these animals had a life-long elevated TNF-α production, which could cause adaptations of other tissues as well. In fact, this lifelong TNF-α overexpression is different from normal COPD progression. However, the effect of this chronic transgenic overexpression of TNF-α on skeletal muscle function should be similar to the effect of elevated levels of TNF-α seen in many COPD patients. In our model, both body and muscle mass were reduced in lung-specific Tg+ mice (Table 1). In other similar models, research showed that there were no morphological differences between cardiac-specific Tg+ mice and WT littermates (16). However, these cardiac-specific Tg+ mice were 5 to 10 mo younger than the lung-specific Tg+ animals used in the present investigation. Therefore, the reduced mice size and function in the present model could be a result of a longer period of TNF-α exposure.
Skeletal muscle performance.
Skeletal muscle contains fast-twitch (glycolytic) and slow-twitch (oxidative) fibers. The mouse EDL and soleus were representatives of fast- and slow-twitch muscles, respectively (6, 22, 29). Our data suggested that EDL from Tg+ had a similar fatigue resistance indicated by time to fatigue, compared with WT under both Po2 conditions (Fig. 1, A and B). However, the soleus from Tg+ was significantly less fatigue resistant (∼12%) compared with WT, but only under high Po2 conditions (Fig. 1, C and D). This was likely because in slow-twitch fibers, the mitochondrial volume and density are fundamentally more important during repetitive contractions than in fast-twitch fibers (18). Furthermore, it has been shown that either mice or cultured skeletal muscle cells (i.e., C2C12) exposed chronically to TNF-α, have decreased mitochondrial protein content and biogenesis (32, 37). It is highly likely that the decreased fatigue resistance of Tg+ soleus under high Po2 in the present study was a result of a reduced aerobic capacity caused by chronic exposure to TNF-α present in the blood, which was not an important component of fatigue in a fast-twitch muscle (i.e., EDL) (6, 22, 29).
To mimic a hypoxic environment that would occur during exercise in COPD patients (34), the external Po2 in our setup was decreased to ∼40 Torr prior to the contractile periods; however, the core of the muscle underwent much more hypoxic conditions during contractions (2). Thus, our data demonstrate that both EDL and soleus were less fatigue resistant under low Po2 compared with high Po2 conditions (Fig. 1). There was no difference in the time to fatigue between WT and Tg+ at low Po2 in both EDL and soleus (Fig. 1, B and D), since the mitochondria activity was likely highly restricted in such conditions (12, 36). These results strengthened the point that chronic TNF-α exposure affected fatigue in the slow-twitch muscle by decreasing the aerobic capacity.
Contractile differences.
The effects of acute and chronic treatments of TNF-α on skeletal muscle function are contradictory in the literature (16, 31). While isolated EDL and soleus were not affected in mice that overexpressed TNF-α in cardiac muscle (16), acute exposure of exogenous TNF-α reduced the tension in single fast-twitch fibers (31), similar to the present investigation [i.e., 30% (Fig. 2, A and C) compared with 20% as reported (31)]. This acute effect resulted in a substantial decrease in either myosin ATPase activity or thin filament Ca2+ sensitivity (31). This could involve the generation of nitric oxide (NO) and ROS. Under our protocol, the 60-min rest period did not cause any substantial decline in tension over time, i.e., 60- min under 95% O2 bubbling at room temperature was sufficient for a full functional recovery in both EDL and soleus. In our model under both Po2 conditions, we observed a decreased tension in Tg+ EDL (Fig. 2, A and C) and a decreased −dP/dt (Fig. 3D), but neither T90% nor +dP/dt was changed (Fig. 3, A and B). This indicated that the rate of maximal tension development was not changed in Tg+ EDL under both Po2 conditions. The decreased −dP/dt under both Po2 conditions could relate to the reduced cross bridge dissociation rate or reduced Ca2+ uptake rate (26, 39). While both absolute and normalized maximal tensions were decreased in Tg+ EDL under both Po2 conditions (Fig. 2, A and C), it is likely that Ca2+ uptake rate could not be reduced (39). Thus, the reduced tension of Tg+ EDL was associated with a decreased cross bridge dissociation rate, which could be affected by either NO or ROS as shown in the literature (14, 24, 38, 39). These results suggested that chronic production of TNF-α likely affected the cross bridge dissociation rate of Tg+ EDL under both Po2 conditions, consistent with the hypothesis by Reid et al. (31) that TNF-α negatively affected either myosin ATPase activity or the thin-filament Ca2+ kinetics.
Furthermore, since the −dP/dt in Tg+ EDL was decreased by a similar level under both Po2 conditions compared with WT EDL, the present results suggested that fast-twitch muscles from Tg+ mice were not more susceptible to low Po2. Fast-twitch muscle preferentially utilizes glycolytic metabolism during a short period of contractions. The EDL maximal tension from Tg+ and WT was not changed at low Po2 compared with high Po2 (Fig. 2, A and C). These results agreed with a previous study on isolated dog muscles at different Po2 conditions (13). Interestingly, the rate of tension development was increased (i.e., decreased in T90% and increased +dP/dt; P < 0.05 in Fig. 3, A and B) at low Po2 compared with high Po2 in WT EDL. Previous research has shown that low Po2 increases Ca2+ release from the SR through ROS production (7, 8). It is possible that this increased rate of tension development could be a response of increased ROS level during hypoxia (42).
For soleus, unlike EDL, the maximal tension and the contractile parameters were not significantly different between WT and Tg+ mice at both high and low Po2 conditions (Figs. 2B and Fig. 4). These results suggested that TNF-α did not affect either excitation-contraction coupling or myofilament activation in a slow-twitch muscle. However, the normalized tension was reduced in Tg+ soleus compared with WT at low Po2 (Fig. 2D). The only contractile difference between high and low Po2 was the reduced −dP/dt. These data suggested that slow-twitch muscles from Tg+ mice had the similar contractile dysfunction to EDL, but this only occurred under hypoxia.
Perspectives and Significance
We used a transgenic model of pulmonary TNF-α overexpression (Tg+) mice, which mimics the conditions in some COPD patients (9), to study skeletal muscle contractile function and fatigue. These mice chronically produced TNF-α in their lungs, leading to a marked pulmonary inflammation (15). The results of the present study demonstrated distinct effects of TNF-α overexpression on muscles of different fiber-type composition at both high and low Po2. Muscle composed of primarily slow-twitch fibers were principally less fatigue resistant, while muscle composed primarily of fast-twitch fibers demonstrated more contractile dysfunction. These results may have important implications for understanding complications in peripheral skeletal muscles from patients with COPD.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant PPG-HL-091830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the assistance of Harrieth Wagner for conducting animal breeding and PCR analysis of the transgenic mice. We thank Drs. Peter Wagner and Ellen Breen for research support.
REFERENCES
- 1. Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S. Induction of MuRF1 is essential for TNF-α-induced loss of muscle function in mice. J Mol Biol 384: 48–59, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil 26: 225–235, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Barreiro E, Schols AM, Polkey MI, Galdiz JB, Gosker HR, Swallow EB, Coronell C, Gea J. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 63: 100–107, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Braith RW, Limacher MC, Leggett SH, Pollock ML. Skeletal muscle strength in heart transplant recipients. J Heart Lung Transplant 12: 1018–1023, 1993 [PubMed] [Google Scholar]
- 5. Casadevall C, Coronell C, Ramirez-Sarmiento AL, Martinez-Llorens J, Barreiro E, Orozco-Levi M, Gea J. Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur Respir J 30: 701–707, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du W, Frazier M, McMahon TJ, Eu JP. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 128: 556S–558S, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Espinosa A, Leiva A, Pena M, Muller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol 209: 379–388, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-α produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 280: L39–L49, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol 107: 832–840, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-α acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Hogan MC, Stary CM, Balaban RS, Combs CA. NAD(P)H fluorescence imaging of mitochondrial metabolism in contracting Xenopus skeletal muscle fibers: effect of oxygen availability. J Appl Physiol 98: 1420–1426, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hogan MC, Welch HG. Effect of altered arterial O2 tensions on muscle metabolism in dog skeletal muscle during fatiguing work. Am J Physiol Cell Physiol 251: C216–C222, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589: 2119–2127 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 35: 689–696, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Li X, Moody MR, Engel D, Walker S, Clubb FJ, Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-α causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor-α. FASEB J 12: 871–880, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibers. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Make B, Krachman S, Panos RJ, Doherty DE, Stoller JK. Oxygen therapy in advanced COPD: in whom does it work? Semin Respir Crit Care Med 31: 334–342, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101: 898–905, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest 96: 250–259, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murakami S, Fujino H, Takeda I, Momota R, Kumagishi K, Ohtsuka A. Comparison of capillary architecture between slow and fast muscles in rats using a confocal laser scanning microscope. Acta Med Okayama 64: 11–18, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Nogueira L, Figueiredo-Freitas C, Casimiro-Lopes G, Magdesian MH, Assreuy J, Sorenson MM. Myosin is reversibly inhibited by S-nitrosylation. Biochem J 424: 221–231, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Pinto JR, Veltri T, Sorenson MM. Modulation of troponin C affinity for the thin filament by different cross-bridge states in skinned skeletal muscle fibers. Pflügers Arch 456: 1177–1187, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 42: 461–471, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflügers Arch 449: 505–517, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Rabinovich RA, Figueras M, Ardite E, Carbo N, Troosters T, Filella X, Barbera JA, Fernandez-Checa JC, Argiles JM, Roca J. Increased tumour necrosis factor-α plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J 21: 789–794, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Rabinovich RA, Vilaro J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 16: 123–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsey KA, Bakker AJ, Pinniger GJ. Fiber-type dependence of stretch-induced force enhancement in rat skeletal muscle. Muscle Nerve 42: 769–777, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Reid MB, Feldman HA, Miller MJ. Isometric contractile properties of diaphragm strips from alcoholic rats. J Appl Physiol 63: 1156–1164, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-α: involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, Galdiz J, Wouters EF, Langen RC, Schols AM. TNF-α impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J 24: 5052–5062, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96: 1916–1926, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, Barbera JA, Nadal J, de Jover L, Rodriguez-Roisin R, Wagner PD. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 1726–1734, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol 248: C265–C270, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Stary CM, Hogan MC. Effect of varied extracellular Po2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Tang K, Wagner PD, Breen EC. TNF-α-mediated reduction in PGC-1α may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222: 320–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vandenboom R. The myofibrillar complex and fatigue: a review. Can J Appl Physiol 29: 330–356, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Westerblad H, Allen DG. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol 474: 291–301, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. J Appl Physiol 53: 335–345, 1982 [DOI] [PubMed] [Google Scholar]
- 41. Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M. Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol 100: 1520–1526, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol 289: C207–C216, 2005 [DOI] [PubMed] [Google Scholar]