Abstract
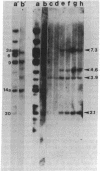
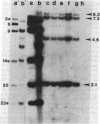
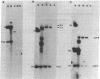
BT37 is a crown gall teratoma incited on tobacco by Agrobacterium tumefaciens containing pTi-T37, a nopaline-type Ti plasmid. Treatment of this cloned tumor tissue with kinetin at 1 mg/liter results in the formation of relatively normal-appearing shoots. These shoots can be induced to root and set viable seed. In contrast to BT37 tissue, the derived tissues are not phytohormone independent and do not produce nopaline. The reverted plants, like normal tobacco plants, are susceptible to infection by A. tumefaciens. This loss of tumorous traits is accompanied by the loss of most of the Ti plasmid sequences (T-DNA) found in BT37 DNA. Southern blot analysis indicates that the revertant tissues have lost the central portion of the T-DNA, which contains the “common DNA” sequences, a highly conserved region of the Ti plasmid that has been found to be incorporated into all tumors studied. Thus, these sequences appear necessary for oncogenicity and tumor maintenance and their loss is probably directly related to tumor reversal. The reverted plants as well as the plants obtained from seed, however, do retain sequences homologous to the ends of the T-DNA present in the parental teratoma. The persistence of foreign DNA sequences during the process of meiosis and seed formation has important implications for the possibility of the genetic engineering of plants.
Keywords: tumor reversal, Agrobacterium tumefaciens, genetic engineering of plants
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN A. C. The activation of two growth-substance systems accompanying the conversion of normal to tumor cells in crown gall. Cancer Res. 1956 Jan;16(1):53–56. [PubMed] [Google Scholar]
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Saiki R. K., Yadav N., Gordon M. P., Quetier F. T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4060–4064. doi: 10.1073/pnas.77.7.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Stabel S., Doerfler W. Revertants of adenovirus type 12-transformed hamster cell line T637 as tools in the analysis of integration patterns. J Virol. 1980 Oct;36(1):41–49. doi: 10.1128/jvi.36.1.41-49.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. S., Haapala D. K., Neubauer R. L., Fischinger P. J. Elimination of the sarcoma genome from murine sarcoma virus transformed cat cells. Science. 1976 Mar 26;191(4233):1264–1266. doi: 10.1126/science.1257745. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Genetello C., Engler G., van Vliet F., de Block M., Villarroel R., van Montagu M., Schell J. Spontaneous formation of cointegrates of the oncogenic Ti-plasmid and the wide-host-range P-plasmid RP4. Plasmid. 1978 Sep;1(4):456–467. doi: 10.1016/0147-619x(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Koekman B. P., Ooms G., Klapwijk P. M., Schilperoort R. A. Genetic map of an octopine TI-plasmid. Plasmid. 1979 Jul;2(3):347–357. doi: 10.1016/0147-619x(79)90018-0. [DOI] [PubMed] [Google Scholar]
- MENAGE A., MOREL G. SUR LA PR'ESENCE D'OCTOPINE DANS LES TISSUS DE CROWN-GALL. C R Hebd Seances Acad Sci. 1964 Dec 21;259:4795–4796. [PubMed] [Google Scholar]
- McPherson J. C., Nester E. W., Gordon M. P. Proteins encoded by Agrobacterium tumefaciens Ti plasmid DNA (T-DNA) in crown gall tumors. Proc Natl Acad Sci U S A. 1980 May;77(5):2666–2670. doi: 10.1073/pnas.77.5.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo D. J., Nutter R. C., Montoya A. L., Garfinkel D. J., Drummond M. H., Chilton M. D., Gordon M. P., Nester E. W. The boundaries and copy numbers of Ti plasmid T-DNA vary in crown gall tumors. Mol Gen Genet. 1980;177(4):637–643. doi: 10.1007/BF00272674. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Postle K., Chilton M. D., Blattner F. R., Powell A., Gordon M. P., Nester E. W. Recombination between higher plant DNA and the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6448–6452. doi: 10.1073/pnas.77.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Wood H. N., Braun A. C. Studies on the recovery of crown gall tumor cells. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3562–3564. doi: 10.1073/pnas.73.10.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. M., Montoya A. L., Nester E. W., Gordon M. P. Plant tumor reversal associated with the loss of foreign DNA. In Vitro. 1980 Jan;16(1):87–92. doi: 10.1007/BF02618202. [DOI] [PubMed] [Google Scholar]
- Yang F., McPherson J. C., Gordon M. P., Nester E. W. Extensive transcription of foreign DNA in a crown gall teratoma. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1273–1277. doi: 10.1016/0006-291x(80)90424-6. [DOI] [PubMed] [Google Scholar]
- Yang F., Merlo D. J., Gordon M. P., Nester E. W. Plasmid DNA of Agrobacterium tumefaciens detected in a presumed habituated tobacco cell line. Mol Gen Genet. 1980;179(1):223–226. doi: 10.1007/BF00268467. [DOI] [PubMed] [Google Scholar]
- Yang F., Montoya A. L., Merlo D. J., Drummond M. H., Chilton M. D., Nester E. W., Gordon M. P. Foreign DNA sequences in crown gall teratomas and their fate during the loss of the tumorous traits. Mol Gen Genet. 1980;177(4):707–714. doi: 10.1007/BF00272683. [DOI] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Holsters M., Kruger K., Depicker A., Schell J., Van Montagu M., Goodman H. M. Tumor DNA structure in plant cells transformed by A. tumefaciens. Science. 1980 Sep 19;209(4463):1385–1391. doi: 10.1126/science.6251546. [DOI] [PubMed] [Google Scholar]