Abstract
Background
New roles for flavonoids, as developmental regulators and/or signalling molecules, have recently been proposed in eukaryotic cells exposed to a wide range of environmental stimuli. In plants, these functions are actually restricted to flavonols, the ancient and widespread class of flavonoids. In mosses and liverworts, the whole set of genes for flavonol biosynthesis – CHS, CHI, F3H, FLS and F3′H – has been detected. The flavonol branch pathway has remained intact for millions of years, and is almost exclusively involved in the responses of plants to a wide array of stressful agents, despite the fact that evolution of flavonoid metabolism has produced >10 000 structures.
Scope
Here the emerging functional roles of flavonoids in the responses of present-day plants to different stresses are discussed based on early, authoritative views of their primary functions during the colonization of land by plants. Flavonols are not as efficient as other secondary metabolites in absorbing wavelengths in the 290–320 nm spectral region, but display the greatest potential to keep stress-induced changes in cellular reactive oxygen species homeostasis under control, and to regulate the development of individual organs and the whole plant. Very low flavonol concentrations, as probably occurred in early terrestrial plants, may fully accomplish these regulatory functions.
Conclusions
During the last two decades the routine use of genomic, chromatography/mass spectrometry and fluorescence microimaging techniques has provided new insights into the regulation of flavonol metabolism as well as on the inter- and intracellular distribution of stress-responsive flavonols. These findings offer new evidence on how flavonols may have performed a wide array of functional roles during the colonization of land by plants. In our opinion this ancient flavonoid class is still playing the same old and robust roles in present-day plants.
Keywords: Auxin transport, early flavonoid genes, evolution of early terrestrial plants, flavonol metabolism, Myb genes, ROS scavengers, stress-responsive flavonoids, sub-cellular flavonoid distribution, UV-B screening
INTRODUCTION
Flavonoids have long been reported as displaying a variety of functional roles in higher plants in response to a wide range of environmental stimuli (for reviews, see Dixon and Paiva, 1995; Winkel-Shirley, 2002; Taylor and Grotewold, 2005; Roberts and Paul, 2006), but less is known about how this vast class of phenylpropanoids may perform such a multiplicity of functions (Close and McArthur, 2002; Hernández et al., 2009; Agati and Tattini, 2010). The significance of their UV screening functions in photoprotective mechanisms has to be considered with some caution (Harborne and Williams, 2000; Agati and Tattini, 2010). The key role of flavonoids in UV-B protection has been conclusively assessed by examining Arabidopsis mutants lacking or possessing the ability to synthesize flavonoids (Li et al., 1993; Bieza and Lois, 2001), but these experiments failed to address the controversial issue of how flavonoids actually perform their photoprotective functions. It is worth noting that all higher plants are capable of synthesizing flavonoids, and that the UV-induced upregulation of flavonoid biosynthesis does not correlate with tolerance to high light in some species (Semerdjieva et al., 2003; Tattini et al., 2005, 2006).
The high level accumulation of flavonoids in the vacuole of epidermal cells exposed for short periods to unnatural levels of sunlight irradiance does not necessarily support a primary function for flavonoids as UV-screening pigments in photoprotection (Ryan et al., 2001, 2002). Landry et al. (1995) provided compelling evidence that Arabidopsis mutants defective in sinapate biosynthesis were more sensitive to UV-B radiation than flavonoid-deficient mutants, and suggested for flavonoids a major role in countering UV-B-induced oxidative damage. This suggestion is consistent with sinapic acid derivatives having higher molar extinction coefficients (ɛ) than flavonoids (namely kaempferol and quercetin derivatives in Arabidopsis; Li et al., 1993; Lillo et al., 2008) in the 290–320 nm waveband. It is conceivable that flavonoids are not primarily aimed at avoiding the generation of reactive oxygen species (ROS), by merely decreasing the flux of highly energetic solar wavelengths in the leaf, but, rather, reduce ROS formed as a consequence of UV-B penetration in ROS-generating cells.
More recently, UV-B irradiance has been conclusively reported to enhance the biosynthesis of most quercetin derivatives in Arabidopsis, and quercetin displays ɛmax at the longest wavelengths (with the exception of myricetin derivatives; Harborne and Williams, 2000) among the thousands of flavonoid structures encountered in plants cells. The question of UV-B-induced accumulation of flavonoids, mostly flavonols, which have an absorbance minimum in the UV-B region of the solar spectrum was posed earlier by Caldwell et al. (1983), and still needs to be conclusively answered. Cockell and Knowland (1999) noted that the induction spectrum for a compound's biosynthesis should overlap with its absorbance spectrum to rule out conclusively a specific screening function for it.
The actual significance of the screening functions for flavonols in UV-B protection has recently been questioned by Gerhardt et al. (2008), who detected a steep increase in the ratio of quercetin to acylated kaempferol derivatives in response to UV radiation. Coumaroyl derivatives of kaempferol display a far greater ability to absorb UV-B wavelengths (Strack et al., 1988; Tattini et al., 2007), but a dramatically lower antioxidant potential than quercetin glycosides (Rice-Evans et al., 1996). More recently, a steep enhancement in the biosynthesis of quercetin has been observed in leaves exposed to full sunlight in the presence or absence of UV radiation (Agati et al., 2009, 2011), and PAR (photosynthetic active radiation, over the 400–700 nm waveband) strongly modulated the UV-dependent accumulation of quercetin glycosides (Götz et al., 2010). Remarkably, root-zone salinity stress and UV-B radiation enhanced the biosynthesis of quercetin glycosides similarly in both shaded and fully sun-exposed leaves of Ligustrum vulgare, and these flavonols accumulate greatly in the mesophyll, not only in the epidermal cells (Agati et al., 2011).
Overall, these findings led to the hypothesis that UV screening is just one, possibly not the most important, function served by flavonols in photoprotection (Cockell and Knowland, 1999; Agati and Tattini, 2010). An increasing body of evidence suggests for flavonoids, particularly flavonols, an antioxidant function in photoprotection (Close and McArthur, 2002; Ryan et al., 2002; Schmitz-Hoerner and Weissenbock, 2003; Agati et al., 2009, 2011) as well as in response to a wide array of stress agents of different origin (Lillo et al., 2008; Akhtar et al., 2010; Løvdal et al., 2010), but the actual significance of their ROS-scavenging activity in an in planta situation is still a matter of controversy (Winkel-Shirley, 2002; Hernández et al., 2009).
Recently, flavonols have been additionally reported to be capable of regulating key developmental processes in eukaryotic cells faced with environmental-induced changes in cellular redox homeostasis (for review articles, see Williams et al., 2004; Peer and Murphy, 2006). It is worth noting that flavonol metabolism is regulated by redox-controlled MYB transcriptor factors (Dubos et al., 2010), but the regulatory functions ascribed to flavonols go beyond their capacity to reduce different forms of reactive oxygen. Flavonols behave as developmental regulators because of their great affinity for a wide array of proteins that control signalling cascades vital to cell growth and development (DeLong et al., 2002; Taylor and Grotewold, 2005; Peer and Murphy, 2006). Hatier and Gould (2008) have also hypothesized for anthocyanins (polyhydroxylated B-ring flavonoids sensu strictu) a role as modulators of stress signals, a function that depends only in part on their capacities to scavenge H2O2, thought to diffuse at considerable rates out of the chloroplast during severe stress conditions (Mullineaux and Karpinski, 2002; Mubarakshina et al., 2010).
The issue of the functional roles of flavonoids in plant–environment interactions has attracted scientists world-wide during the last three decades from both an evolutionary and physiological point of view, but we are far from being able to give conclusive answers. There are still major concerns regarding the localization/functional relationship of flavonoids, and the strikingly different capacity of different flavonoid structures (i.e. glycosides vs. aglycones) to reduce ROS as well as to inhibit the phosphorylation of different proteins (Jacobs and Rubery, 1988; Mathesius et al., 1998; Besseau et al., 2007; Ringli et al., 2008). For example, most flavonoid aglycones have the capacity to regulate the activity of different protein kinases in animals (as well as of the auxin efflux facilitator, PIN, proteins, located at the plasma membrane in plant cells; Brown et al., 2001), but very few flavonoid glycosides, the forms usually detected in plant tissues, display an effective regulatory potential for kinase activity (Mathesius et al., 1998; Ringli et al., 2008).
In this brief review article we explore the significance of new functional roles recently proposed for the old class of flavonols in plant–environment interaction (see, among others, Taylor and Grotewold, 2005; Peer and Murphy, 2007; Buer et al., 2010) in the light of early hypotheses for their primary roles during the colonization of land by plants (Swain, 1986; Stafford, 1991). Our discussion is based upon the following observations: flavonol biosynthetic genes were already present in mosses and liverworts; the flavonol branch pathway has remained intact for millions of years, and is almost exclusively involved in the responses of present-day plants to stress agents of different origin; flavonols are not as efficient as most other secondary metabolites in absorbing wavelengths in the UV-B spectral region; and stress-responsive flavonols display the greatest potential for both countering increases in ROS concentration and regulating the development of individual organs and the whole plant.
FLAVONOLS IN EARLY AND CURRENT-DAY TERRESTRIAL PLANTS: OLD HYPOTHESES AND NEW EVIDENCE FOR THEIR FUNCTIONAL ROLES
Stafford (1991) raised serious concerns about the primary UV-B screening function served by flavonoids during the evolution of early terrestrial plants. She speculated that the concentration of flavonoids would have been very low in liverworts and mosses, because ‘early’ (in the sense proposed by Rausher, 2006) flavonoid enzymes were not as efficient as current enzymes at constituting an effective filter against UV-B irradiance. Agati and Tattini (2010) have recently noted that a leaf flavonoid concentration as low as a few micromoles, on a dry mass basis, may result in a much greater concentration, on a molar basis, in the epidermal cells, as actually required for constituting an effective shield against the UV-B wavelengths (Edwards et al., 2008). Nevertheless, a primary UV-B screening function for flavonols in the photoprotection of early land plants is actually questionable for several reasons (Winkel-Shirley, 2002).
Early terrestrial plants lost the mycosporin-like amino acid (MAA) in favour of flavonol metabolism, although MAAs are more effective than flavonols in absorbing the short solar wavelengths reaching the leaf surface. Cockell and Knowland (1999) argued that UV-screening flavonoids evolved from other physiological roles to later fulfil a UV screening function, probably following the evolution of different branches of both the general phenylpropanoid (which may lead, for example, to the synthesis of effective UV-absorbers, such as acylated flavonoids; Strack et al., 1988; Harborne and Williams, 2000; Tattini et al., 2007) and the flavonoid biosynthetic branch pathways. Their suggestion is consistent with the ancient class of flavonols, particularly the dihydroxy B-ring quercetin derivatives (the almost ubiquitous flavonoid in higher plants) having molar extinction coefficients in the 290–390 nm spectral region, 35 % smaller than that of monohydroxy B-ring flavones, such as derivatives of apigenin (Tattini et al., 2004).
It may not be a mere coincidence that UV-B-responsive flavonols display the greatest antioxidant potential, but not the greatest UV-B-attenuating capacity (Harborne and Williams, 2000; Ryan et al., 2002; Tattini et al., 2004; Gerhardt et al., 2008). Stafford (1991) argued that the epidermal cells, the vacuole of which has long been reported (erroneously) to be the exclusive site of flavonoid accumulation, themselves have to be protected, not only aimed at preserving the underlying (sensitive) tissues from photo-oxidative damage. Her suggestion is strongly corroborated by the steep increase in the ratio of dihydroxy B-ring-substituted flavonoids (which display ɛmin in the 290–320 nm spectral region) to hydroxycinnamates (ɛmax between 290 and 320 nm) in tissues and organs exposed to the greatest flux of UV-B radiation (Olsson et al., 1999; Tattini et al., 2000; Agati et al., 2002). Tattini et al. (2000) and Agati et al. (2002) suggested that in highly specialized glandular trichomes of Phillyrea latifolia, which are autonomous in phenylpropanoid biosynthesis, the exclusive UV-induced accumulation of flavonoids (namely, dihydroxy B-ring-substituted quercetin 3-O-glycosides and luteolin 7-O-glycosides), apparently at the expense of caffeic acid derivatives, was primarily for protecting glandular trichomes from oxidative damage, while losing the greatest effectiveness in screening out the highly energetic solar short wavelengths from reaching the underlying tissues.
The capacity of flavonoids to inhibit the generation of ROS (through the complexation of Cu and Fe ions, which may lead to the catalytic production of both the hydroxyl radical and the hydroxyl anion, in the well-known Fenton/Haber–Weiss reactions; see Hernández et al., 2009) and to reduce ROS, once formed, was considered of key value during the colonization of land by plants (Swain, 1986). Swain's idea conforms to (1) radiation and desiccation, common themes in early land plant evolution, imposing a very severe oxidative stress (Rothschild and Mancinelli, 2001); and (2) the ancient class of flavonols displaying an effective antioxidant capacity (Winkel-Shirley, 2002). The presence of the OH group in the 3-position of the flavonoid skeleton (Fig. 1) is the key structural feature responsible for the peculiar ability of flavonols to chelate transition metal ions, and, hence, to inhibit the generation of free radicals, as well as to reduce ROS once formed (Rice-Evans et al., 1996; Brown et al., 1998; Melidou et al., 2005; Agati et al., 2007). Nevertheless, the flavonols usually found in leaf tissues are the glycosylated forms, so that the most reactive/antioxidant group (the OH group in the 3-position in the A-ring of the flavonoid skeleton) is actually ‘silenced’ (Fig. 1). Noticeably, in response to various environmental stimuli (Gerhardt et al., 2008; Lillo et al., 2008; Jaakola and Hohtola, 2010), plants almost exclusively synthesize quercetin 3-O-glycosides, in which the presence of a catechol group in the B-ring of the flavonoid skeleton is responsible for the superior capacity to chelate transition metal ions and to reduce various forms of ROS, as compared with monohydroxy B-ring-substituted flavone or flavonol glycosides (Fig. 1; Tattini et al., 2004; Melidou et al., 2005; Agati et al., 2009).
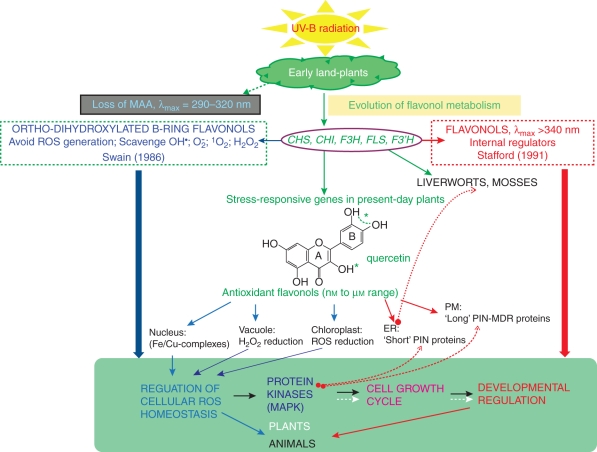
Fig. 1.
A schematic diagram showing the functional roles served by flavonols in early and present-day terrestrial plants, based on a top-down approach. Quercetin derivatives (asterisks indicate the actual functional groups) in the nanomolar to micromolar range may regulate both the cellular redox homeostasis and developmental processes. In plants, quercetin derivatives may inhibit the phosphorylation of auxin efflux facilitator proteins located at both the endoplasmic reticulum (ER) and the plasma membrane (PM). The presence of the whole set of genes for quercetin biosynthesis, coupled with the occurrence of ‘short’ PIN proteins at the ER (the site of flavonoid biosynthesis) detected in liverworts and mosses, suggests ancestral functions for flavonols as developmental regulators. Quercetin derivatives have also been shown to tightly control the oxidative stress-induced MAPK activities in animals, but conclusive evidence for this functional role in plants is still lacking (dotted arrows at the bottom).
It is worth noting that the whole set of genes responsible for the biosynthesis of quercetin derivatives – CHS, CHI, F3H, FLS and F3'H (encoding chalcone synthase, chalcone isomerase, flavanone 3-hydroxylase, flavonol synthase and flavonoid 3'-hydroxylase, respectively) – was already present in liverworts and mosses (Fig. 1; Markham, 1988; Rausher, 2006). Interestingly, these early/old genes (sensu Rausher, 2006) are induced early by high light, at least in Arabidopsis (van Tunen et al., 1988; Vanderauwera et al., 2005), and are the most responsive genes in current-day plants suffering from a wide range of environmentally induced oxidative damage (Fig. 1; Walia et al., 2005; Hannah et al., 2006; Lillo et al., 2008; Olsen et al., 2009; Akhtar et al., 2010; Agati et al., 2011). R2R3 MYB transcription factors, which control the biosynthesis of flavonols, were already present in mosses, are strongly induced by UV-B radiation and are themselves controlled by changes in cellular redox homeostasis (Rabinowicz et al., 1999; Heine et al., 2006; Falcone Ferreyra et al., 2010). R2R3 Myb genes have been proposed as having been involved in the protection of early land plants from pathogens (Rabinowicz, 1999), but new evidence leads to hypothesizing for them other regulatory functions, through the flavonol-mediated control of plant form and, possibly, of ROS homeostasis (Close and McArthur, 2002; Taylor and Grotewold, 2005; Fujita et al., 2006; Dubos et al., 2010). The observation that the flavonol metabolic pathway has remained intact for millions of years is consistent with natural selection having favoured secondary metabolites with multiple functional roles to protect plants from unpredictable injuries of different origin (Izhaki, 2002). We therefore conclude that the flavonol biosynthetic branch pathway represents a robust character in land plants, as having conferred adaptability to species in an ever-changing environment, over an extraordinarily extended time scale (Lesne, 2008).
Stafford (1991) also hypothesized flavonoids as having served an ‘internal’ function during the evolution of early land plants, based upon their ability to inhibit polar auxin transport (PAT; Jacobs and Rubery, 1988), a role fully accomplished by flavonols in the manomolar range. This issue has been explored in depth during the last decade (for reviews, see Peer and Murphy, 2007; Buer et al., 2010), and flavonols have been conclusively shown to behave as endogenous regulators of auxin movement, at the inter- and intracellular level. Arabidopsis mutants defective in the first enzyme of flavonoid biosynthesis, CHS, display phenotypes with altered growth (Brown et al., 2001; Buer and Muday, 2004; Besseau et al., 2007). It is noted that quercetin is a much more potent inhibitor of PAT as compared with kaempferol (Jacobs and Rubery, 1988), as a consequence of a greater ability to inhibit the activity of protein kinases (DeLong et al., 2002) – which is conferred by the catechol group in the B-ring of the flavonoid skeleton – and, hence, of both PIN and MDR-glycoproteins (multidrug-resistant proteins), the auxin efflux facilitator proteins (Peer et al., 2004; Geisler et al., 2005; Bandyopadhyay et al., 2007). Jansen et al. (2001) have suggested that the widely reported UV-B-induced increase in the quercetin to kaempferol ratio may offer protection against UV-B stress, as a consequence of the contrasting effects of the two flavonols on the peroxidase-mediated oxidation of indole acetic acid (IAA). Quercetin is an inhibitor and kaempferol is a cofactor of IAA oxidase (Furuya et al., 1962), and flavonols might have served these ancestral functions to regulate the levels of free IAA in early land plants, such as in the liverworts (Cooke et al., 2002).
Recently, Friml and Jones (2010) have reported that PIN5, an atypical member of the PIN protein family, is associated with the endoplasmic reticulum (ER), the putative site of flavonoid biosynthesis (Fig. 1). The finding that these ‘short’ PIN proteins (which also include PIN6 and PIN8), which have been suggested to mediate intracellular auxin homeostasis (Mravec et al., 2009), were the only PINs present in mosses is consistent with Stafford's idea of flavonoids as physiological regulators during the evolution of early terrestrial plants (Fig. 1), although a direct effect of flavonols on the activity of short PINs has not been proven yet. The new evidence of ER-located PINs also addresses the important question of ‘how much free flavonoids remains in the cytoplasm to modulate the trafficking or the activity of auxin transporters’ posed by Taylor and Grotewold (2005). The ‘long’ PIN and MDR-P glycoproteins that act in concert at the plasma membrane (PM) to regulate the cell–cell movement of auxin (Fig. 1; Geisler et al., 2005, Titapiwatanakum et al., 2009) and, hence, basipetal auxin transport, occurred at a later stage during the evolution of land plants (Friml and Jones, 2010).
Actually, flavonols are good candidates to affect greatly the stress-induced redistribution of growth, the so-called ‘flight’ strategy of sessile organisms (Potters et al., 2009). Stress-induced morphogenic responses (Potters et al., 2007) have been reported to reflect molecular processes, such as increased ROS production and altered phytohormone transport and metabolism, which can be tightly controlled by the stress-responsive antioxidant flavonols (Peer and Murphy, 2006; Pritzsche and Hirt, 2006; Beveridge et al., 2007). Thibaud-Nissen et al. (2003) have suggested that flavonoids play a role in the regulation of the redox activity associated with the induction of cell division and somatic embryogenesis. We note that antioxidant flavonols in the high nanomolar to low micromolar concentration range may perform these regulatory functions – which depend upon their ‘antioxidant structure’, but go beyond their mere ability to scavenge ROS (Fig. 1) – as earlier speculated by Stafford (1991).
Nevertheless, how the control exerted by flavonols on auxin movement directly translates to developmental events at the level of the whole plant is still to be explored in depth and little, merely correlative, evidence has been shown for Arabidopsis only (Besseau et al., 2007; Buer and Djordjevic, 2009). This complex issue will be unlikely to be addressed simply by analysing the growth responses of Arabidopsis mutants lacking or not the ability to synthesize flavonols, particularly when grown under unnatural sunlight irradiance (Jansen, 2002). Indeed, high sunlight induces the synthesis of both auxin and quercetin derivatives, and increases the activity of phenol-oxidizing peroxidases (Jansen et al., 2001; Friml, 2003; Buer and Muday, 2004; Besseau et al., 2007). Quercetin displays a great capacity for fine regulating auxin gradients as well as the local auxin concentrations – by inhibiting PAT and peroxidase-mediated IAA oxidation – that represent the actual determinants for different morphological responses (Jansen, 2002), such as the outgrowth of axillary buds (Bennet et al., 2006; Dun et al., 2006; Lazar and Goodman, 2006). Actually, low doses of UV-B irradiance have been reported to alter the whole-plant architecture profoundly, with more axillary branching being associated with an increase in UV-B-absorbing compounds (Hectors et al., 2007). Above-ground biomass production and leaf size have been shown to correlate negatively with both the quercetin glycoside concentration and the ratio of quercetin to kaempferol in Trifolium repens, and ecotypes with a constitutively superior quercetin concentration were more resistant to both UV-B and drought stresses than the fast-growing ecotypes (Hofmann et al., 2001; Hofmann and Jahufer, 2011).
INTEGRATING OLD HYPOTHESES AND NEW EVIDENCE FOR THE FUNCTIONAL ROLES OF FLAVONOLS
At the time of Stafford's and Swain's hypotheses on the functional roles served by flavonols in the response of early terrestrial plants faced with an abrupt increase in UV-B irradiance, the central issue of their inter- and intracellular distribution (a pre-requisite to explain how this class of flavonoids is capable of multiple functions) was still unresolved. At the present time, the availability of both confocal laser scanning and wide-field deconvolution fluorescence microimaging has allowed exploriation of the occurrence of flavonols in different leaf tissue layers and cellular compartments (Fig. 1). Feucht et al. (2004) and Polster et al. (2006) have detected flavonols in the nucleus of mesophyll cells, and hypothesized that they protect DNA from oxidative damage. A nuclear localization of flavonoid enzymes in Arabidopsis is consistent with control exerted by flavonoids in the transcription of genes required for growth and development (Saslowsky et al., 2005). Flavonoids have long been detected in chloroplasts (and chloroplasts have been additionally reported as capable of flavonoid biosynthesis; Zaprometov and Nikolaeva, 2003), and chloroplast flavonols underwent H2O2-induced oxidation (Takahama, 1984). More recently, Agati et al. (2007), using three-dimensional deconvolution fluorescence microscopy, were able to visualize, in vivo, the reduction of singlet oxygen by dihydroxylated B-ring flavonoids (quercetin and luteolin glycosides) associated with the chloroplast envelope in P. latifolia leaves. Antioxidant flavonols have recently been found in the vacuoles of both epidermal and mesophyll cells in leaves exposed to visible sunlight (Agati et al., 2009, 2011). These findings led to the hypothesis that the unanticipated key role of the vacuole in the control of cellular ROS homeostasis (Mittler et al., 2004) might be mediated by flavonols (in addition to anthocyanins) in the peroxidase-mediated reduction of H2O2 (Yamasaki et al., 1997; Takahama, 2004; Hatier and Gould, 2008). Flavonoids have also been detected at the PM (Peer et al., 2001) and, hence, well sited to regulate polar auxin transport by interacting with PM-located PIN and MDR-glycoproteins (Titapiwatanakum et al. 2009), but an additional role as ROS scavengers for PM flavonols has recently been proposed (Erlejman et al., 2004; Korn et al., 2008). We note, however, that the intracellular detection of flavonoids by fluorescence microscopy still generates conflicts, as all the flavonol aglycones, but only the ortho-dihydroxylated flavonoid glycosides, can form adducts with the Naturstoff reagent, the probe commonly used to induce flavonoid ‘pseudo-fluorescence’ (Agati et al., 2007, 2009).
Reductionism supersedes present-day approaches to study plant systems biology (Lucas et al., 2011), and great efforts have been made to determine both the actors in play (e.g. metabolites in the top-down approach proposed in Fig. 1) and where they play (the distribution of inter- and intracellular metabolites), to support conclusively the early views for the functional roles of flavonoids during the evolution of early land plants (Fig. 1; Swain, 1986; Stafford, 1991). Hernández et al. (2009) have recently explored the issue of to what extent the flavonoids play an antioxidant role in the in planta condition, and concluded that their ROS-reducing ability was of minor significance. They have suggested that the products of flavonoid oxidation have to be detected within the main sources of ROS to prove conclusively they have performed a reducing activity. They also suggested the minor significance of the H2O2-reducing activity of vacuolar flavonoids, as the amount of H2O2 entering the vacuole is probably low and possible only when the tonoplast membrane is disrupted. Agati and Tattini (2010) have recently noted that the products of flavonol oxidation are unlikely to be observed in healthy leaf cells, as flavonoid radicals may be recycled back to their reduced forms by ascorbate in different sub-cellular compartments. Ascorbic acid is a very poor substrate for vacuolar guaiacol-peroxidases as compared with dihydroxy B-ring flavonols, and ascorbate has long been suggested to behave as a secondary antioxidant, involved in the recycling of flavonoid radicals to their reduced forms (Sakihama et al., 2000).
The actual significance of flavonols as detoxifying agents against ROS is further complicated by taking into account the wide array of antioxidant defences operating in plants, the activity and/or the concentration of which may change profoundly in response to environmental injuries of different origin. Nevertheless, Hatier and Gould (2008) have suggested that under severe excess light stress, inactivation of antioxidant enzymes may occur (Casano et al., 1997; Streb et al., 1997; Karpinski et al., 1999) concomitantly with the greatest upregulation of flavonoid biosynthesis. Recently, Fini et al. (2011) reported that UV-B radiation and root-zone salinity induced a decline in ascorbate peroxidase (APX) activity on a relatively long-term basis (3 weeks), and such depletion was paralleled by the accumulation of quercetin-3-O-glycosides. Conversely, early experiments by Landry et al. (1995) showed that the UV-B-induced enhancement of the activity of APX was much greater in the Arabidopsis tt5 mutant than in wild-type plants. It may be speculated that flavonols may constitute a secondary antioxidant defence system, even on a temporal basis, with their biosynthesis being activated upon drastic alterations in cellular ROS/REDOX homeostasis (Taylor and Grotewold, 2005; Akhtar et al., 2010; Dubos et al., 2010), following the depletion of primary antioxidant defences. An inherently lower capacity to avoid the penetration of highly energetic UV wavelengths into the leaf coupled with a constitutively reduced activity of antioxidant enzymes has been reported to be responsible for the increased biosynthesis of quercetin glycosides and damage to membrane lipids in some woody species (Tattini et al., 2005, 2006). Interestingly, the greatest compartment-specific increase of ascorbate have recently been detected in the vacuole in Arabidopsis and Nicotiana tabacum leaves suffering from severe excess light stress (Zechmann et al., 2011), and ascorbic acid displays a very low affinity for vacuolar peroxidases.
Excess light is the very condition that leads, on one hand, to the greatest H2O2 production and H2O2-induced inactivation of chloroplast antioxidants (Karpinski et al., 1999; Mullineaux and Karpinski, 2002; Mubarakshina et al., 2010) and, on the other hand, to the massive accumulation of ‘antioxidant’ flavonols (Tattini et al., 2005; Agati et al., 2011). Taken together, these findings may in part answer the question posed by Hernández et al. (2009) regarding a link between the biological properties of stress-responsive flavonoids and their spatio-temporal correlation with oxidative stress events. H2O2 has been definitively reported to cross cellular membranes through aquaporins/peroxyporins (Bienert et al., 2007; Maurel et al., 2009), and H2O2 may be a threat for a cell in a very low (a few micromolar) concentration range (Mittler et al., 2004; Cheeseman, 2007). Gould et al. (2002) have provided compelling in vivo evidence for vacuolar anthocyanins as scavengers of H2O2 generated upon mechanical injury.
Finally, we note that the capacity of antioxidant flavonols to inhibit the generation of ROS through the complexation of Cu/Fe ions – an antioxidant function in the sense proposed by Halliwell (2009) – which has been reported to be of great value in preserving animal cells from oxidative damage (Mladĕnka et al., 2010) – should also be taken into account in order to assess conclusively their overall antioxidant role in an in planta condition.
CONCLUSIONS
Assessing the relative significance of the various potential functions attributable to flavonols in the responses of higher plants to a wide range of environmental stimuli will represent a tremendous task for both plant biologist and plant ecophysiologists in the near future. The matter is complicated not only because of the occurrence of flavonoids in different plant organs and cellular compartments, but also considering that key components of the antioxidant machinery may be affected to very different extents, depending on the severity of the stress. The relationship between primary antioxidant defences and flavonol metabolism is an additional issue to be addressed not only at the molecular level, by examining the transcript or mRNA abundance, but also at the level of protein abundance and, hence, of enzyme activity.
In the meantime, relevant ‘free-of-scale’ issues have to be taken into account: genes devoted to the biosynthesis of flavonoids with the potential of displaying multiple functional roles (at both the cell and whole-plant level) were present at the very beginning of the appearance of plants on land, and are still the most responsive genes to abiotic and biotic stresses in current-day plants; the amplification of Myb genes occurred between 250 and 550 million years ago (after the divergence of vascular plants from bryophytes; Rabinowicz et al., 1999), and the functions of several R2R3 Myb genes – that are strongly induced by stress agents of different origin and regulate the biosynthesis of flavonols – make them ideal candidates to be key players in the evolution of plant form and metabolic plasticity (Dubos et al., 2010); quercetin derivatives, which are almost ubiquitously distributed in higher plants, display similar functions in animals and plants (DeLong et al., 2002; Williams et al. 2004; Taylor and Grotewold, 2005; Lamoral-Theys et al., 2010). Surprisingly, the relatively new issue of flavonoid modulation of mitogen-activated protein kinase (MAPK) signalling cascades, which have long been reported to be of vital significance in animal cell functioning (Fig. 1; for reviews, see Williams et al., 2004; Lamoral-Theys, 2010), still needs to be explored in an in planta situation (Peer and Murphy, 2006).
The routine use of genomic, chromatography/mass spectrometry and fluorescence microimaging techniques during the last two decades has provided strong, new evidence about how flavonols may have performed a wide range of functional roles during the colonization of land by plants. In our opinion, this ancient flavonoid class is still playing the same ‘old’ and ‘robust’ roles in present-day plants.
ACKNOWLEDGEMENTS
Work in the authors' laboratory has been partially supported by grants from Ente Cassa di Risparmio di Firenze and Uniser Consortium Pistoia. We thank the reviewers and the Handling Editor, Professor Smirnoff, for their valuable suggestions for improving this paper.
LITERATURE CITED
- Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytologist. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- Agati G, Galardi C, Gravano E, Romani A, Tattini M. Flavonoid distribution in tissues of Phillyrea latifolia as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochemistry and Photobiology. 2002;76:350–360. doi: 10.1562/0031-8655(2002)076<0350:fditop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Agati G, Matteini P, Goti A, Tattini M. Chloroplast-located flavonoids may scavenge singlet oxygen. New Phytologist. 2007;174:77–89. doi: 10.1111/j.1469-8137.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Agati G, Stefano G, Biricolti S, Tattini M. Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Annals of Botany. 2009;104:853–861. doi: 10.1093/aob/mcp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. Journal of Plant Physiology. 2011;168:204–212. doi: 10.1016/j.jplph.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Akhtar TA, Lees HA, Lampi MA, Enstone D, Brain RA, Greenberg BM. Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemma gibba. Plant, Cell and Environment. 2010;33:1205–1219. doi: 10.1111/j.1365-3040.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Blakeslee JJ, Lee OR, et al. Interactions of PIN and PGP auxin transport mechanisms. Biochemical Society Transactions. 2007;35:137–141. doi: 10.1042/BST0350137. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Besseau S, Hoffmann L, Geoffry P, Lapierre C, Pollet B, Legrand M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. The Plant Cell. 2007;19:148–162. doi: 10.1105/tpc.106.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM. Common regulatory themes in meristem development and whole-plant homeostasis. Current Opinion in Plant Biology. 2007;10:44–51. doi: 10.1016/j.pbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller ALB, Kristiansen KA, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Bieza K, Lois R. An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiology. 2001;126:1105–1115. doi: 10.1104/pp.126.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu(II) ions: implication for their antioxidant properties. Biochemical Journal. 1998;359:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Rashotte AM, Murphy AS, et al. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiology. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Djordjevic MA. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. Journal of Experimental Botany. 2009;60:751–763. doi: 10.1093/jxb/ern323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the responses of Arabidopsis roots to gravity and light. The Plant Cell. 2004;16:1191–1205. doi: 10.1105/tpc.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. Journal of Integrative Plant Biology. 2010;52:96–111. doi: 10.1111/j.1744-7909.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiologia Plantarum. 1983;58:445–450. [Google Scholar]
- Casano LM, Gómez LD, Lascano HR, Gonzáles CA, Trippi VS. Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplast exposed to photooxidative stress. Plant and Cell Physiology. 1997;38:433–440. doi: 10.1093/oxfordjournals.pcp.a029186. [DOI] [PubMed] [Google Scholar]
- Cheeseman JM. Hydrogen peroxide and plant stress: a challenging relationship. Plant Stress. 2007;1:4–15. [Google Scholar]
- Close DC, McArthur C. Rethinking the role of many plant phenolics – protection from photodamage not herbivores? Oikos. 2002;99:166–172. [Google Scholar]
- Cockell MM, Knowland J. Ultraviolet radiation screening compounds. Biological Reviews. 1999;74:311–345. doi: 10.1017/s0006323199005356. [DOI] [PubMed] [Google Scholar]
- Cooke TJ, Poli D, Sztein AE, Cohen JD. Evolutionary patterns in auxin action. Plant Molecular Biology. 2002;49:319–338. [PubMed] [Google Scholar]
- DeLong A, Mockaitis K, Christensen S. Protein phosphorylation in the delivery of and response to auxin. Plant Molecular Biology. 2002;49:285–303. [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. The Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends in Plant Science. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Dun EA, Ferguson BJ, Beveridge CA. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms. Plant Physiology. 2006;142:812–819. doi: 10.1104/pp.106.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards WR, Hall JA, Rowlan AR, et al. Light filtering by epidermal flavonoids during the resistant response of cotton to Xanthomonas protects leaf tissues from light-dependent phytoalexin toxicity. Phytochemistry. 2008;69:2320–2328. doi: 10.1016/j.phytochem.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Erlejman AG, Verstraiten SV, Fraga CG, Oteiza PI. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radical Research. 2004;38:1311–1320. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Rius S, Emiliani J, et al. Cloning and characterization of a UV-B-inducible maize flavonol synthase. The Plant Journal. 2010;62:77–91. doi: 10.1111/j.1365-313X.2010.04133.x. [DOI] [PubMed] [Google Scholar]
- Feucht W, Treutter D, Polster J. Flavonol binding of nuclei from tree species. Plant Cell Reports. 2004;22:430–436. doi: 10.1007/s00299-003-0705-7. [DOI] [PubMed] [Google Scholar]
- Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behavior. 2011;6:709–711. doi: 10.4161/psb.6.5.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J. Auxin transport: shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J, Jones AR. Endoplasmic reticulum: the rising compartment in auxin biology. Plant Physiology. 2010;150:458–462. doi: 10.1104/pp.110.161380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Furuya M, Galston AW, Stowe BB. Isolation from peas of co-factors and inhibitors of indolyl-3-acetic acid oxidase. Nature. 1962;193:456–457. doi: 10.1038/193456a0. [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, et al. Cellular efflux of auxin catalysed by the Arabidopsis MDR/RGP transporter AtPGP1. The Plant Journal. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt KK, Lampi MA, Greenberg BM. The effect of far-red light on plant growth and flavonoid accumulation in Brassica napus in the presence of ultraviolet B radiation. Photochemistry and Photobiology. 2008;84:1445–1454. doi: 10.1111/j.1751-1097.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- Götz M, Albert A, Stich S, et al. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heinh. leaf rosettes; cumulative effects after a whole vegetative growth period. Protoplasma. 2010;243:95–103. doi: 10.1007/s00709-009-0064-5. [DOI] [PubMed] [Google Scholar]
- Gould KS, McKelvie J, Markham KR. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant, Cell and Environment. 2002;25:1261–1269. [Google Scholar]
- Halliwell B. The wanderings of a free radical. Free Radical Biology and Medicine. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Hannah MA, Weise D, Freund S, Fiehn O, Heyer AG, Hincha DK. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiology. 2006;142:98–112. doi: 10.1104/pp.106.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Hatier J-HB, Gould KS. Foliar anthocyanins as modulators of stress signals. Journal of Theoretical Biology. 2008;253:625–627. doi: 10.1016/j.jtbi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Hectors K, Prinsen E, De Coen W, Jansen MAK, Guisez Y. Arabidopsis thaliana plants acclimated to low doses of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytologist. 2007;175:255–270. doi: 10.1111/j.1469-8137.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- Heine GF, Hernandez JM, Grotewold E. Two cysteines in plant R2R3 MYB domains participate in REDOX-dependent DNA binding. Journal of Biological Chemistry. 2006;279:37878–37885. doi: 10.1074/jbc.M405166200. [DOI] [PubMed] [Google Scholar]
- Hernández I, Alegre L, van Breusegem F, Munné-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends in Plant Science. 2009;14:125–132. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Hofmann RW, Campbell BD, Fountain DW, et al. Multivariate analysis of intraspecific responses to UV-B radiation in white clover (Trifolium repens L.) Plant, Cell and Environment. 2001;24:917–927. [Google Scholar]
- Hofmann RW, Jahufer MZZ. Tradeoff between biomass and flavonoid accumulation in white clovers reflects contrasting plant strategies. PloS ONE. 2011;6:e18949. doi: 10.1371/journal.pone.0018949. doi:10.1371/journal.pone.0018949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhaki I. Emodin – a secondary metabolite with multiple ecological functions in higher plants. New Phytologist. 2002;155:205–217. [Google Scholar]
- Jaakola L, Hohtola A. Effect of latitude on flavonoid biosynthesis in plants. Plant, Cell and Environment. 2010;33:1239–1247. doi: 10.1111/j.1365-3040.2010.02154.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Natural occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jansen MAK. Ultraviolet-B-radiation on plants: induction of morphogenic responses. Physiologia Plantarum. 2002;116:423–439. [Google Scholar]
- Jansen MAK, van der Noort RA, Tan A, et al. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiology. 2001;126:1012–1023. doi: 10.1104/pp.126.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingle G, Greissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Korn M, Peterek S, Mock H-P, Heyer AG, Hincha DK. Heterosis in freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant, Cell and Environment. 2008;31:813–827. doi: 10.1111/j.1365-3040.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoral-Theys D, Pottier L, Dufrasne F, et al. Natural polyphenols that display anticancer properties through inhibition of kinase activity. Current Medicinal Chemistry. 2010;17:812–825. doi: 10.2174/092986710790712183. [DOI] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiology. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar G, Goodman HM. MAX1, a regulator of flavonoid pathway, controls vegetative bud outgrowth in Arabidopsis. Proceedings of the National Academy of Science, USA. 2006;103:472–476. doi: 10.1073/pnas.0509463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne A. Robustness: confronting lessons from physics and biology. Biological Reviews. 2008;83:509–532. doi: 10.1111/j.1469-185X.2008.00052.x. [DOI] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B radiation. The Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant, Cell and Environment. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- Løvdal T, Olsen KM, Slimestad R, Verheul M, Lilli C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry. 2010;71:605–613. doi: 10.1016/j.phytochem.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Lucas M, Laplaze L, Bennett MJ. Plant systems biology: network matters. Plant, Cell and Environment. 2011;34:535–555. doi: 10.1111/j.1365-3040.2010.02273.x. [DOI] [PubMed] [Google Scholar]
- Markham KR. Distribution of flavonoids in the lower planta and its evolutionary significance. In: Harborne JB, editor. The flavonoids: advances in research since 1980. London, UK: Chapman & Hall; 1988. pp. 427–468. [Google Scholar]
- Mathesius U, Schlman HRM, Spain HP, Saufter CO, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. The Plant Journal. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- Maurel C, Santoni V, Luu D-T, Wudick MM, Verdoucq L. The cellular dynamics of plant aquaporin expression and functions. Current Opinion in Plant Biology. 2009;12:690–698. doi: 10.1016/j.pbi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Melidou M, Riganakos K, Galaris D. Protection against DNA damage offered by flavonoids in cells to hydrogen peroxide: the role of iron chelation. Free Radical Biology and Medicine. 2005;39:1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vandarauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plants Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mladĕnka P, Zatloukalová , Filipskỳ T, Hrdina R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radical Biology and Medicine. 2010;49:963–975. doi: 10.1016/j.freeradbiomed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Mravec J, Skůpa P, Bailly A, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- Mubarakshina MM, Ivanov BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A. Production and diffusion of chloroplastic H2O2 and its implication to signalling. Journal of Experimental Botany. 2010;61:3577–3587. doi: 10.1093/jxb/erq171. [DOI] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Current Opinion in Plant Biology. 2002;5:43–48. doi: 10.1016/s1369-5266(01)00226-6. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Slimestad R, Lea US, et al. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant, Cell and Environment. 2009;32:286–299. doi: 10.1111/j.1365-3040.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- Olsson LC, Veit M, Bornman JF. Epidermal transmittance and phenolic composition of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiologia Plantarum. 1999;107:259–266. [Google Scholar]
- Peer WA, Murphy AS, Brown DE, Tague BW, Muday GK, Taiz L. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiology. 2001;126:536–548. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. Flavonoids as signal molecules. In: Grotewold E, editor. The science of flavonoids. New York: Springer; 2006. pp. 239–267. [Google Scholar]
- Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends in Plant Science. 2007;12:556–563. doi: 10.1016/j.tplants.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. The Plant Cell. 2004;16:898–911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster J, Dithmar H, Burgemeister R, Friedemann G, Feucht W. Flavonoids in plant nuclei: detection by laser microdissection and pressure catapulting (LMPC), in vivo staining, and UV-visible spectroscopic titration. Physiologia Plantarum. 2006;126:163–174. [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of the trouble? Trends in Plant Science. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell and Environment. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Pritzschke A, Hirt H. Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiology. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowicz PD, Brau EL, Wolfe AD, Bowen B, Grotewold E. Maize R2R3 Myb genes: sequence analysis reveals amplification in the higher plants. Genetics. 1999;153:427–444. doi: 10.1093/genetics/153.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD. The evolution of flavonoids and their genes. In: Grotewold E, editor. The science of flavonoids. New York: Springer; 2006. pp. 175–211. [Google Scholar]
- Rice-Evans CA, Miller N, Papanga G. Structure–antioxidant relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Ringli C, Bilger L, Kuhn B, et al. The modified flavonol glycosylation profile in the Arabidopsis rol1 mutants results in alterations in plant growth and cell shape formation. The Plant Cell. 2008;20:1470–1481. doi: 10.1105/tpc.107.053249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Paul ND. Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytologist. 2006;170:677–699. doi: 10.1111/j.1469-8137.2006.01707.x. [DOI] [PubMed] [Google Scholar]
- Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- Ryan KG, Swinny EE, Winefield C, Markham KR. Flavonoids and UV photoprotection in Arabidopsis mutants. Zeitschrift für Naturforschung C. 2001;56:745–754. doi: 10.1515/znc-2001-9-1013. [DOI] [PubMed] [Google Scholar]
- Ryan KG, Swinny EE, Markham KR, Winefield Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry. 2002;59:23–32. doi: 10.1016/s0031-9422(01)00404-6. [DOI] [PubMed] [Google Scholar]
- Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H. Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochemical and Biophysical Research Communications. 2000;279:949–954. doi: 10.1006/bbrc.2000.4053. [DOI] [PubMed] [Google Scholar]
- Saslowsky DE, Warek U, Winjel BSJ. Nuclear localization of flavonoid enzymes in Arabidopsis. Journal of Biological Chemistry. 2005;280:23735–23740. doi: 10.1074/jbc.M413506200. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hoerner R, Weissenböck G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry. 2003;64:243–255. doi: 10.1016/s0031-9422(03)00203-6. [DOI] [PubMed] [Google Scholar]
- Semerdjieva SI, Sheffield E, Phoenix GK, Gwynn-Jones D, Callaghan TV, Johnson GN. Contrasting strategies for UV-B screening in sub-Arctic dwarf shrubs. Plant, Cell and Environment. 2003;26:957–964. doi: 10.1046/j.1365-3040.2003.01029.x. [DOI] [PubMed] [Google Scholar]
- Stafford HA. Flavonoid evolution: an enzymic approach. Plant Physiology. 1991;96:680–685. doi: 10.1104/pp.96.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack D, Heilemann J, Momken M, Wray V. Cell-wall conjugated phenolics from coniferae leaves. Phytochemistry. 1988;27:3517–3521. [Google Scholar]
- Streb PF, Feierabend J, Bigney R. Resistance to photoinhibition of photosystem II and catalase and antioxidative protection in high mountain plants. Plant, Cell and Environment. 1997;20:1030–1040. [Google Scholar]
- Swain T. Plant flavonoids in biology and medicine. In: Cody V, Middleton E Jr, Harborne JB, editors. Progress in clinical and biological research. New York: Liss; 1986. pp. 1–14. [Google Scholar]
- Takahama U. Hydrogen peroxide-dependent oxidation of flavonols by intact spinach chloroplasts. Plant Physiology. 1984;74:852–855. doi: 10.1104/pp.74.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama U. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidases: physiological significance of the oxidation reactions. Phytochemistry Reviews. 2004;3:207–219. [Google Scholar]
- Tattini M, Gravano E, Pinelli P, Mulinacci N, Romani A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytologist. 2000;148:69–77. doi: 10.1046/j.1469-8137.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytologist. 2004;163:547–561. doi: 10.1111/j.1469-8137.2004.01126.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Guidi L, Morassi-Bonzi L, Pinelli P, et al. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to excess solar radiation. New Phytologist. 2005;167:457–470. doi: 10.1111/j.1469-8137.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Remorini D, Pinelli P, et al. Morpho-anatomical, physiological and biochemical adjustments in response to root salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytologist. 2006;170:779–794. doi: 10.1111/j.1469-8137.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Tattini M, Matteini P, Saracini E, Traversi ML, Giordano C, Agati G. Morphology and biochemistry of non-glandular trichomes in Cistus salvifolius L. leaves growing in extreme habitats of the Mediterranean basin. Plant Biology. 2007;9:411–419. doi: 10.1055/s-2006-924662. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Grotewold E. Flavonoids as developmental regulators. Current Opinion in Plant Biology. 2005;8:317–323. doi: 10.1016/j.pbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiology. 2003;132:118–136. doi: 10.1104/pp.103.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakum B, Blakslee JJ, Bandyopadhyay A, et al. ABCB/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. The Plant Journal. 2009;57:27–44. doi: 10.1111/j.1365-313X.2008.03668.x. [DOI] [PubMed] [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, et al. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiology. 2005;139:806–821. doi: 10.1104/pp.105.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tunen AJ, Koes RE, Spelt CE, et al. Cloning of two chalcone flavanone isomerase genes from Petunia hybrida: coordinated, light-regulated and differential expression of flavonoid genes. EMBO Journal. 1988;7:1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia H, Wilson C, Condamine P, et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiology. 2005;139:822–835. doi: 10.1104/pp.105.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Spencer JPE, Rice-Evans CA. Flavonoids: antioxidants or signalling molecules. Free Radical Biology and Medicine. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley BJ. Biosynthesis of flavonoids and effect of stress. Current Opinion in Plant Biology. 2002;8:317–323. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakiyama Y, Ikehara N. Flavonoid–peroxidase reaction as a detoxification mechanism of plant cell against H2O2. Plant Physiology. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaprometov MN, Nikolaeva TN. Chloroplasts isolated from kidney bean leaves are capable of phenolic compound biosynthesis. Russian Journal of Plant Physiology. 2003;50:623–626. [Google Scholar]
- Zechmann B, Stumpe M, Mauch F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta. 2011;233:1–12. doi: 10.1007/s00425-010-1275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]