Abstract
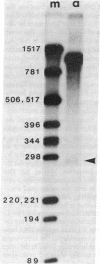
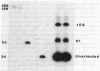
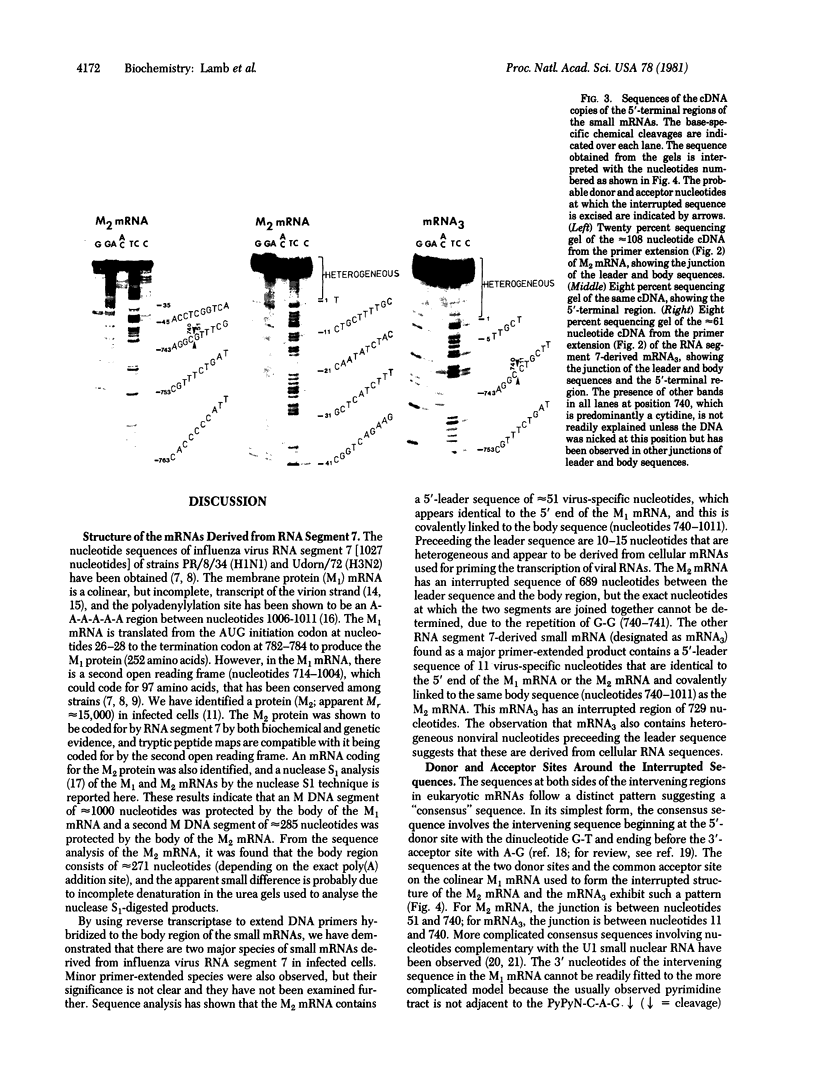
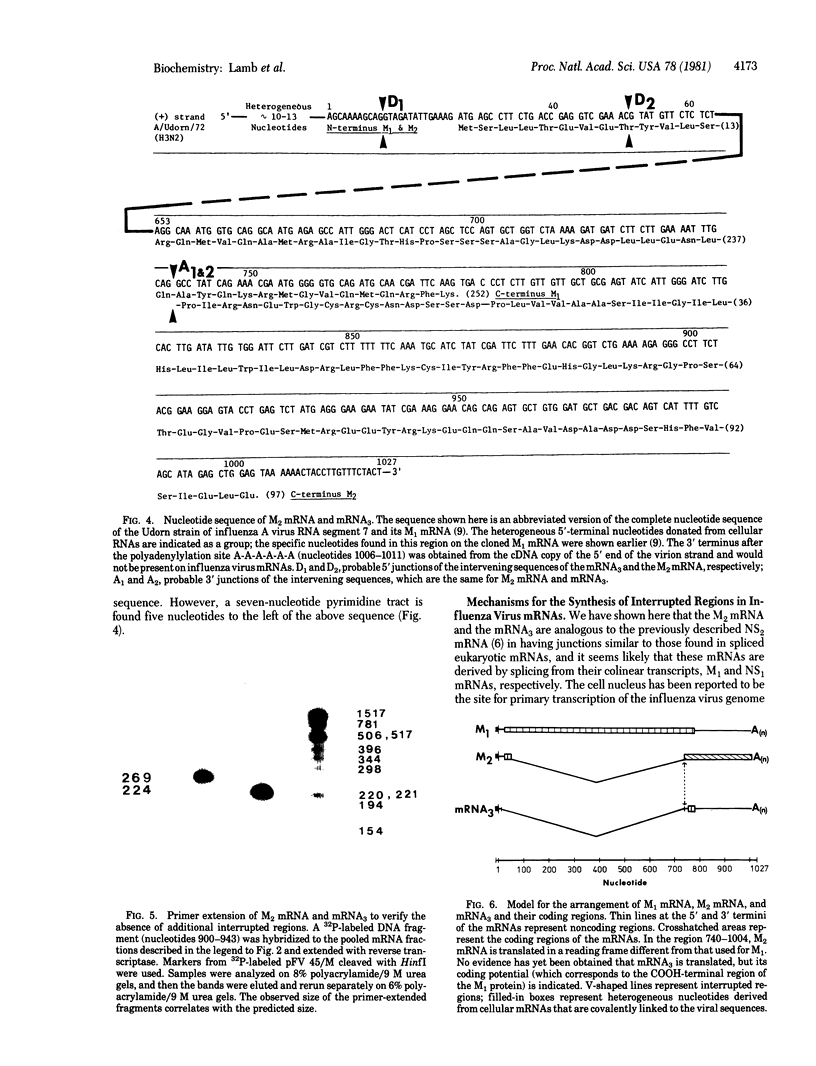
RNA segment 7 of the influenza A virus genome codes for at least two proteins, M1 and M2, which are synthesized from separate mRNA species. Sequence analysis of the M2 mRNA has shown that it contains an interrupted sequence of 689 nucleotides. The approximately 51 virus-specific nucleotides comprising the 5'-end leader sequence of the M2 mRNA are the same as those found at the 5' end of the colinear M1 mRNA. Following the leader sequence of the M2 mRNA, where is a 271-nucleotide body region that is 3' coterminal with the M1 mRNA. Another small potential mRNA (mRNA3) related to RNA segment 7 has been found. mRNA3 has a leader sequence of approximately 11 virus-specific nucleotides that are the same as the 5' end of the M1 and M2 mRNAs, followed by an interrupted sequence of 729 nucleotides, and then a body region of approximately 271 nucleotides that is the same as that of the M2 mRNA. The nucleotide sequences found at the junctions of the interrupted sequences in M2 mRNA and mRNA3 are similar to those found at the splicing points of intervening sequences in eukaryotic mRNAs. In addition, both mRNAs contain 10-15 heterogeneous nonviral nucleotides at their 5' ends that appear to be derived from cellular RNAs used for priming the transcription of viral RNAs. Because the 5'-end sequences of the M1 mRNA and the M2 mRNA are the same and share the 5'-proximal initiation codon for protein synthesis, the first nine amino acids would be the same in the M1 and M2 protein and then the sequences would diverge. The approximately 271-nucleotide body region of the M2 mRNA can be translated in the +1 reading frame, and the sequence indicates that M1 and M2 overlap by 14 amino acids. The coding potential of the mRNA3 is for only nine amino acids, and these would be identical to the COOH-terminal region of the membrane protein (M1).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., McCauley J., Waterfield M., Gething M. J. Influenza virus RNA segment 7 has the coding capacity for two polypeptides. Virology. 1980 Dec;107(2):548–551. doi: 10.1016/0042-6822(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Both the 7-methyl and the 2'-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Robertson J. S. Structure of the host-derived sequences present at the 5' ends of influenza virus mRNA. Nucleic Acids Res. 1980 Jun 25;8(12):2591–2603. doi: 10.1093/nar/8.12.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Chanock R. M., Lai C. J. Nonviral oligonucleotides at the 5' terminus of cytoplasmic influenza viral mRNA deduced from cloned complete genomic sequences. Cell. 1980 Sep;21(2):495–500. doi: 10.1016/0092-8674(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Barrett T., Brown C. M., Almond J. W. The smallest genome RNA segment of influenza virus contains two genes that may overlap. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3790–3794. doi: 10.1073/pnas.76.8.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Markoff L. J., Zimmerman S., Cohen B., Berndt J. A., Chanock R. M. Cloning DNA sequences from influenza viral RNA segments. Proc Natl Acad Sci U S A. 1980 Jan;77(1):210–214. doi: 10.1073/pnas.77.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W., Chanock R. M., Lai C. J. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1857–1861. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Taylor J. M., Broni B., Krug R. M. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J Virol. 1979 Feb;29(2):744–752. doi: 10.1128/jvi.29.2.744-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Processing of late adenovirus nuclear RNA to mRNA. Kinetics of formation of intermediates and demonstration that all events are nuclear. J Mol Biol. 1979 Jun 5;130(4):493–506. doi: 10.1016/0022-2836(79)90436-4. [DOI] [PubMed] [Google Scholar]
- Piper P., Wardale J., Crew F. Splicing of the late mRNAs of polyoma virus does not occur in the cytoplasm of the infected cell. Nature. 1979 Dec 13;282(5740):686–691. doi: 10.1038/282686a0. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Plotch S. J., Krug R. M. Identification of the RNA region transferred from a representative primer, beta-globin mRNA, to influenza mRNA during in vitro transcription. Nucleic Acids Res. 1980 Mar 11;8(5):925–942. doi: 10.1093/nar/8.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]