Abstract
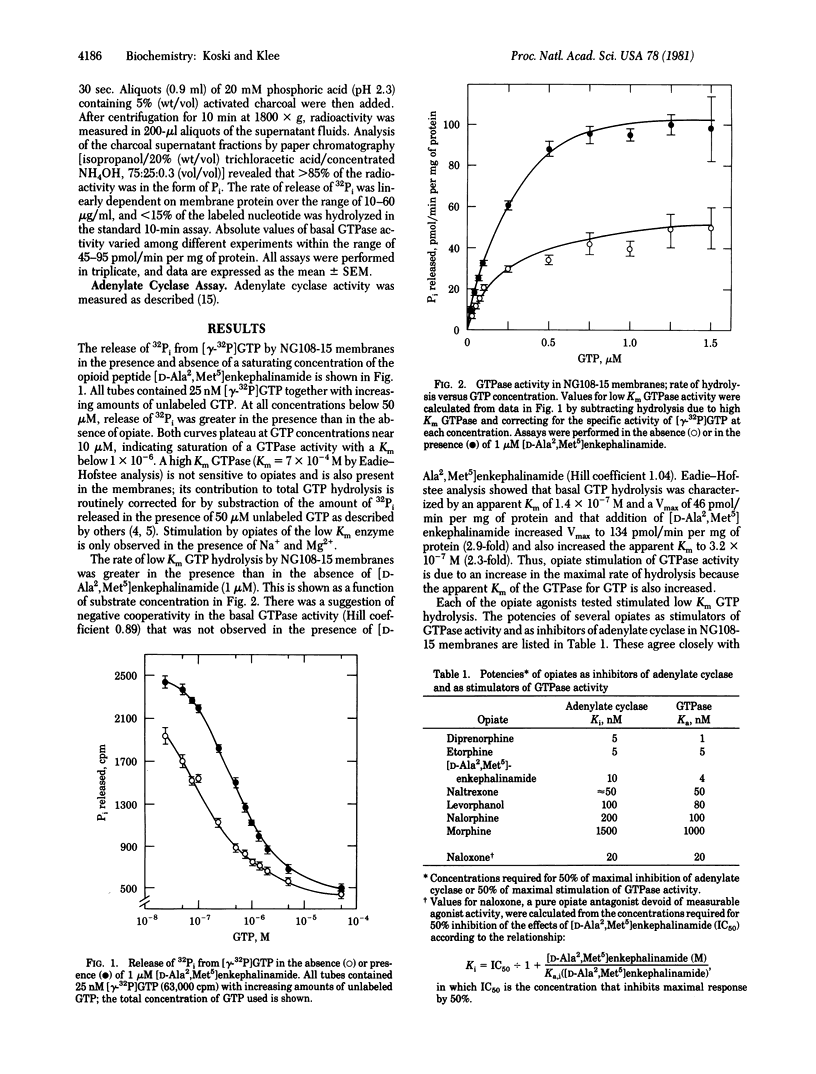
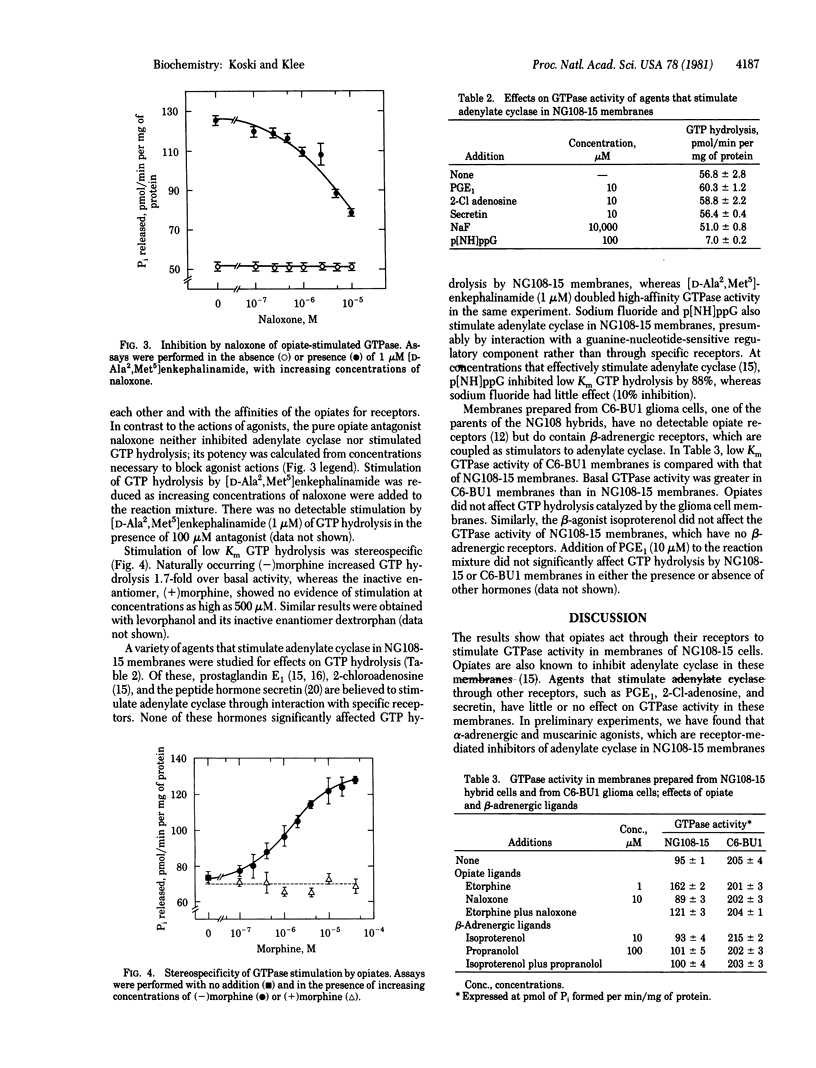
Specific, GTP hydrolysis catalyzed by membranes prepared from neuroblastoma--glioma (NG108-15) hybrid cells can be measured in the presence of adenosine-5'-[beta, gamma-imido] triphosphate (p[NH]ppA), ATP, and a nucleotide triphosphate-regenerating system. Opiates and opioid peptides stimulate low Km GTP hydrolysis when measured in the presence of Na+ and Mg2+. Opiate stimulation is rapid, stereospecific, and reserved by the antagonist naloxone. Potencies of opiates as stimulators of GTP hydrolysis and as inhibitors of adenylate cyclase are closely correlated. Agents that stimulate adenylate cyclase, including prostaglandin E1, 2-Cl-adenosine, secretin, and NaF, have little or no effect upon the rate of GTP hydrolysis. Opiates have no effect upon either adenylate cyclase or GTPase activity in membranes prepared from C6-BU1 glioma cells, which lack opiate receptors. In view of the pivotal role of GTP in the activation of adenylate cyclase, we conclude that receptor-mediated stimulation of GTP hydrolysis is the mechanism by which opiates and other inhibitory hormones lower adenylate cyclase activity in NG108-15 cell membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitonti A. J., Moss J., Tandon N. N., Vaughan M. Prostaglandins increase GTP hydrolysis by membranes from human mononuclear cells. J Biol Chem. 1980 Mar 10;255(5):2026–2029. [PubMed] [Google Scholar]
- Blume A. J., Lichtshtein D., Boone G. Coupling of opiate receptors to adenylate cyclase: requirement for Na+ and GTP. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5626–5630. doi: 10.1073/pnas.76.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J. Opiate binding to membrane preparations of neuroblastoma x glioma hybrid cells NG108-15: effects of ions and nucleotides. Life Sci. 1978 May 22;22(20):1843–1852. doi: 10.1016/0024-3205(78)90602-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Levkovitz H., Selinger Z. The regulatory GTPase cycle of turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):393–406. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Activation of turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5'-(gamma-thio) triphosphate. Biochem Biophys Res Commun. 1977 Aug 8;77(3):868–873. doi: 10.1016/s0006-291x(77)80058-2. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. W., Jr, Spiegel A. M., Singer M., Reen S., Aurbach G. D. Fluoride stimulation of adenylate cyclase is dependent on the guanine nucleotide regulatory protein. J Biol Chem. 1980 Feb 10;255(3):949–954. [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Kimura N., Shimada N. Glucagon-stimulated GTP hydrolysis in rat liver plasma membranes. FEBS Lett. 1980 Aug 11;117(1):172–174. doi: 10.1016/0014-5793(80)80938-0. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski G., Simonds W. F., Klee W. A. Guanine nucleotides inhibit binding of agonists and antagonists to soluble opiate receptors. J Biol Chem. 1981 Feb 25;256(4):1536–1538. [PubMed] [Google Scholar]
- Lad P. M., Nielsen T. B., Preston M. S., Rodbell M. The role of the guanine nucleotide exchange reaction in the regulation of the beta-adrenergic receptor and in the actions of catecholamines and cholera toxin on adenylate cyclase in turkey erythrocyte membranes. J Biol Chem. 1980 Feb 10;255(3):988–995. [PubMed] [Google Scholar]
- Lambert M., Svoboda M., Christophe J. Hormone-stimulated GTPase activity in rat pancreatic plasma membranes. FEBS Lett. 1979 Mar 15;99(2):303–307. doi: 10.1016/0014-5793(79)80978-3. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Lin M. C., Welton A. F., Lad P. M., Rodbell M. Reversible activation of hepatic adenylate cyclase by guanyl-5'-yl-(alpha,beta-methylene)diphosphonate and guanyl-5'-yl imidodiphosphate. J Biol Chem. 1977 Aug 10;252(15):5180–5182. [PubMed] [Google Scholar]
- Nathanson N. M., Klein W. L., Nirenberg M. Regulation of adenylate cyclase activity mediated by muscarinic acetylcholine receptors. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1788–1791. doi: 10.1073/pnas.75.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Lefkowitz R. J. Activation and desensitization of beta-adrenergic receptor-coupled GTPase and adenylate cyclase of frog and turkey erythrocyte membranes. J Biol Chem. 1980 Jul 25;255(14):6860–6867. [PubMed] [Google Scholar]
- Propst F., Moroder L., Wünsch E., Hamprecht B. The influence of secretin, glucagon and other peptides, of amino acids, prostaglandin endoperoxide analogues and diazepam on the level of adenosine 3',5'-cyclic monophosphate in neuroblastoma x glioma hybrid cells. J Neurochem. 1979 May;32(5):1495–1500. doi: 10.1111/j.1471-4159.1979.tb11090.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Sabol S. L., Nirenberg M. Regulation of adenylate cyclase of neuroblastoma x glioma hybrid cells by alpha-adrenergic receptors. I. Inhibition of adenylate cyclase mediated by alpha receptors. J Biol Chem. 1979 Mar 25;254(6):1913–1920. [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Koski G., Streaty R. A., Hjelmeland L. M., Klee W. A. Solubilization of active opiate receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4623–4627. doi: 10.1073/pnas.77.8.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]



