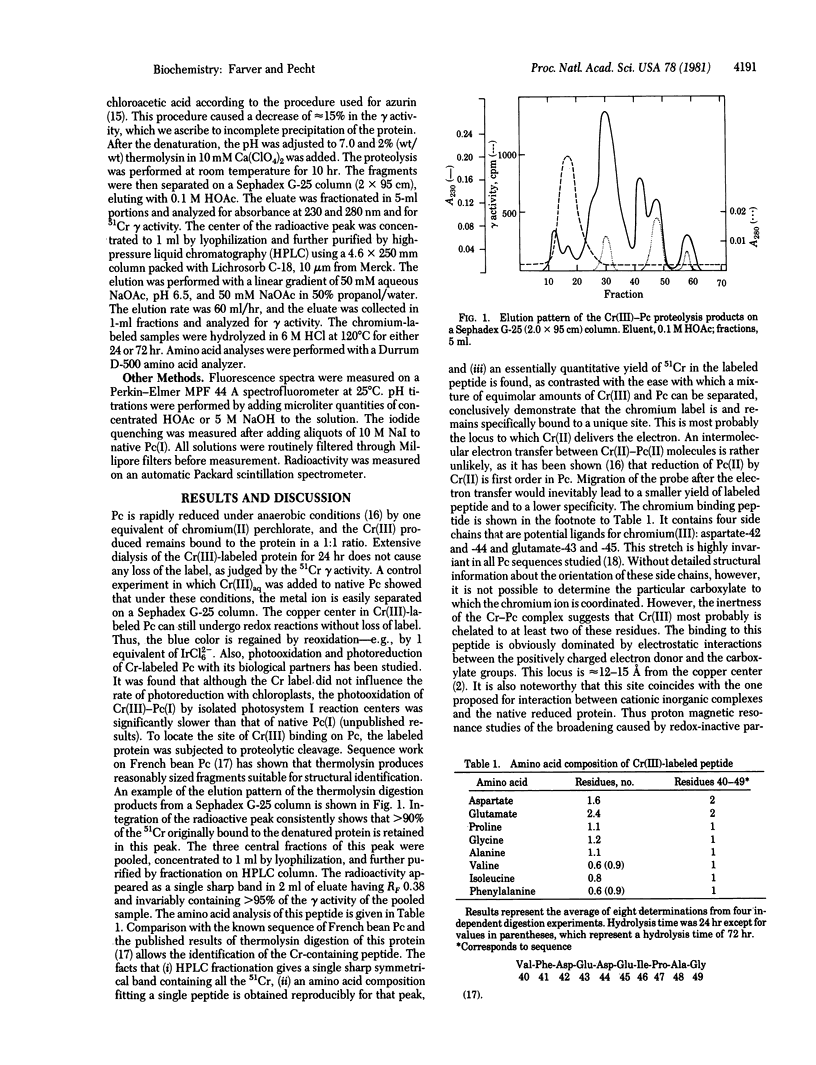
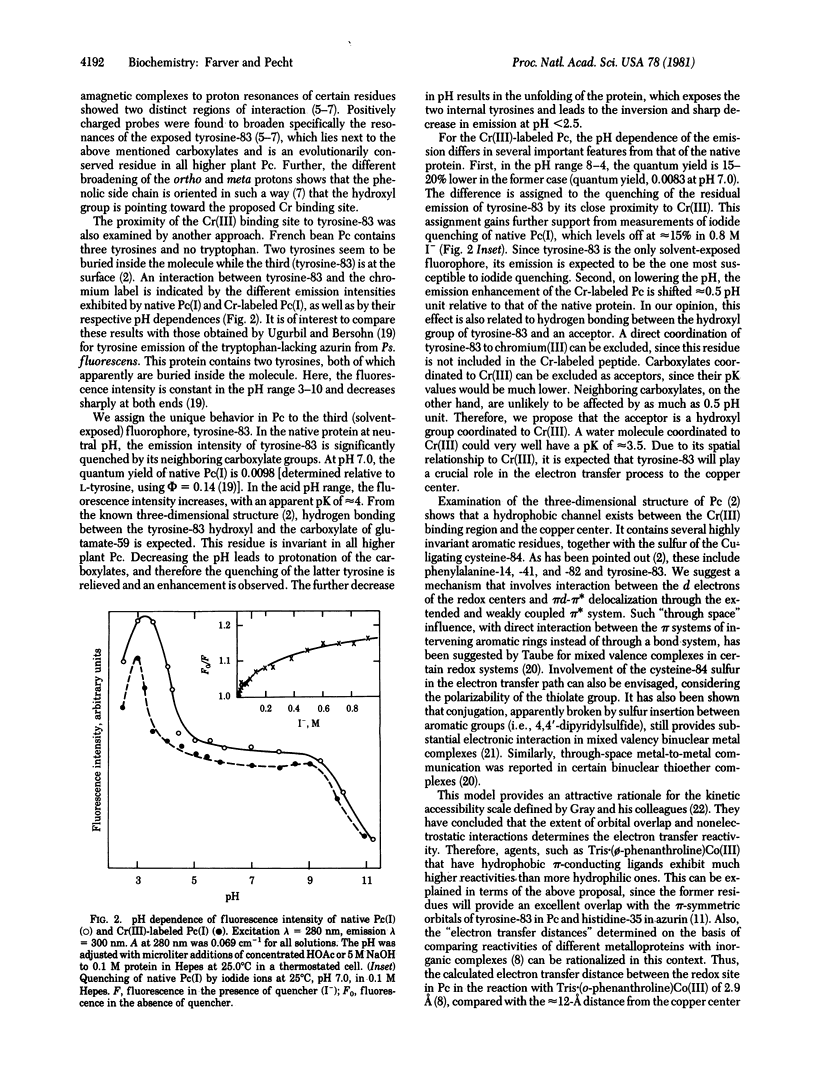
Abstract
Cu(II)--plastocyanin from French beans (Phaseolus vulgaris) is reduced quantitatively by Cr(II)aq ions to give a substitution-inert Cr(III) adduct of Cu(I)--plastocyanin. Enzymatic proteolysis of this derivative by thermolysin led to the identification of the Cr(III) binding peptide. This contains four potential ligands for the metal ion: aspartate-42 and -44 and glutamate-43 and -45. In the three-dimensional fold of plastocyanin, this stretch is very close to tyrosine-83. The emission intensity and its pH dependence observed for the tyrosines in this tryptophan-devoid protein differ markedly in the Cr(III) adduct. That difference is interpreted as reflecting proximity and interaction between the latter metal ion and tyrosine-83. The distance between the copper center and the suggested Cr(III) binding site is approximately 12 A. The intervening region contains an array of highly invariant aromatic residues. These are proposed to be involved in the electron transfer process. A mechanism for that process is presented that involves interaction between the d electrons of the metal ions with d pi-pi* delocalization through a weakly coupled pi* system. The rationale of this electron transfer pathway for the reactivity of plastocyanin with inorganic redox agents is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson D. J., Hayes M. T., Wright P. E. NMR study of the interaction of plastocyanin with chromium(II) analogues of inorganic electron transfer reagents. Biochim Biophys Acta. 1980 Jun 10;591(1):162–176. doi: 10.1016/0005-2728(80)90230-3. [DOI] [PubMed] [Google Scholar]
- Dawson J. W., Gray H. B., Holwerda R. A., Westhead E. W. Kinetics of the reduction of metalloproteins by chromous ion (laccase-cytochrome c-plastocyanins-temperature-rate constants). Proc Natl Acad Sci U S A. 1972 Jan;69(1):30–33. doi: 10.1073/pnas.69.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Pecht I. Kinetics and equilibria of the electron transfer between azurin and the hexacyanoiron (II/III) couple. Biochemistry. 1976 Sep 21;15(19):4197–4208. doi: 10.1021/bi00664a011. [DOI] [PubMed] [Google Scholar]
- Grimes C. J., Piszkiewicz D., Fleischer E. B. Electron transfer reactions in biological systems: the reduction of ferricytochrome c by chromous ions. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1408–1412. doi: 10.1073/pnas.71.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalsky A. A study of the mechanism of electron transfer in cytochrome c. Chromium as a probe. J Biol Chem. 1969 Dec 25;244(24):6619–6625. [PubMed] [Google Scholar]
- Maki A. W., Rubin A. J., Sykes R. M., Shank R. L. Reduction of nonionic surfactant toxicity following secondary treatment. J Water Pollut Control Fed. 1979 Sep;51(9):2301–2313. [PubMed] [Google Scholar]
- Milne P. R., Wells J. R., Ambler R. P. The amino acid sequence of plastocyanin from French bean (Phaseolus vulgaris). Biochem J. 1974 Dec;143(3):691–701. doi: 10.1042/bj1430691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne P. R., Wells J. R. Structural and molecular weight studies on the small copper protein, plastocyanin. J Biol Chem. 1970 Apr 10;245(7):1566–1574. [PubMed] [Google Scholar]
- Rosen P., Pecht I. Conformational equilibria accompanying the electron transfer between cytochrome c (P551) and azurin from Pseudomonas aeruginosa. Biochemistry. 1976 Feb 24;15(4):775–786. doi: 10.1021/bi00649a008. [DOI] [PubMed] [Google Scholar]
- Ryden L., Lundgren J. Homology relationships among the small blue proteins. Nature. 1976 May 27;261(5558):344–346. doi: 10.1038/261344a0. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Bersohn R. Tyrosine emission in the tryptophanless azurin from Pseudomonas fluorescens. Biochemistry. 1977 Mar 8;16(5):895–901. doi: 10.1021/bi00624a013. [DOI] [PubMed] [Google Scholar]



